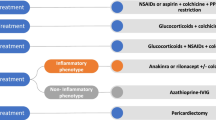
Abstract
Purpose
We describe the manifestations and course of patients with pleuropericarditis (PP). Serum parameters were analyzed to evaluate the contribution of autoimmune and autoinflammatory mechanisms to PP pathogenesis. Finally, we outline risk factors for recurrent PP attacks.
Methods
Electronic medical records of the University Hospital Heidelberg were screened for PP diagnosis between the years 2009 and 2021. A total of 164 patients were detected and compared to patients suffering from systemic lupus erythematosus (SLE)-associated PP. Follow-up data were collected until January 2023.
Results
In 57.3% of a total of 164 PP cases, no trigger was identified (idiopathic PP). The clinical manifestations were similar in subgroups with different triggers (idiopathic, post-cardiac injury and post-infectious). None of the patients in the idiopathic-PP (i-PP) group fulfilled the diagnostic criteria of an autoimmune disease and the i-PP group could be clearly discriminated by clinical, epidemiological and serological means from the control cohort of SLE-associated PP. After a median follow-up of 1048 days, the majority of PP patients (72.7%) had at least one PP relapse. Univariate analyses showed that CRP, SAA (serum amyloid A), troponin T, NT-BNP and post-cardiac injury were negatively correlated, while the presence of fever and an idiopathic trigger were positively correlated with recurrence of PP. Multivariate analyses showed that fever, an idiopathic trigger and low SAA values were risk factors for PP recurrence.
Conclusion
This study highlights that most cases of PP are idiopathic and PP cases with various triggers have an identical clinical phenotype. Our data suggest that the clinical, epidemiological and serological characteristics of idiopathic PP considerably differ from patients with PP caused by autoimmune disease like SLE. We further demonstrate that PP has a high risk of recurrence and identify factors associated with this risk, allowing for a targeted secondary prophylaxis.
Graphical Abstract

Similar content being viewed by others
Introduction
Acute pleuropericarditis (PP) is the most common inflammatory heart disorder and accounts for about 5% of all emergency department admissions for acute chest pain [1, 2]. Although acute PP usually is a mild and self-limiting disease, about 30% of patients experience complications, e.g. cardiac tamponade or recurrent PP [1, 3]. Recurrent episodes can profoundly impact the quality of life, leading to repeated hospital admissions and adverse effects related to long-term pharmacological therapy [1, 4,5,6,7].
While various triggers for PP have been described including viral infections, mycobacterial infections, vaccinations with mRNA vaccines against SARS-CoV-2, or cardiac tissue injury [8,9,10,11,12,13,14], the exact mechanism of inflammation remains elusive. An autoimmune aetiology was hypothesized because of the association of PP with various systemic rheumatic diseases like systemic lupus erythematosus (SLE), rheumatoid arthritis or vasculitis [15]. On the other hand, PP is also a typical feature of autoinflammatory syndromes like familial Mediterranean fever, TNF receptor-associated periodic syndrome, systemic juvenile idiopathic arthritis or adult onset Still’s disease [15]. In addition, mouse models suggested a role for inflammasome activation and IL-1β. Genetic analyses showed an increased frequency of rare MEFV (MEditerranean FeVer) mutations in PP patients, again suggesting an association of autoinflammatory mechanisms in the pathogenesis of PP [3, 16, 17].
Importantly, the risk factors for recurrence of PP remain unclear. Patients at risk for recurrent PP may require a more intense treatment of the first attack and more frequent follow-up visits. Previous studies claimed that an increased neutrophil/lymphocyte ratio (NLR) and higher age are risk factors for recurrent PP [4].
In order to better understand PP pathogenesis and identify risk factors for recurrence, we performed a retrospective analysis of PP patients treated at the University Hospital Heidelberg over the course of more than 10 years. Clinical phenotypes were compared according to the presence or absence of PP trigger events. The contribution of autoinflammatory versus autoimmune mechanisms to PP pathogenesis was assessed by comparing clinical, epidemiological and serological parameters in idiopathic PP with SLE-associated PP.
Methods
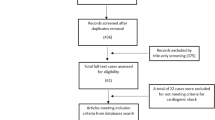
The electronic medical records of the University Hospital Heidelberg were screened for PP diagnosis between the years 2009 and 2021. In order to avoid wrong diagnoses, only patients who were additionally discussed with a rheumatologist were included in the further analysis. We further included all patients who were identified by interdisciplinary counseling between cardiologists and rheumatologists between 2009 and 2021 at our institution. Patients with known autoinflammatory diseases or with effusions due to heart, kidney or liver failure or effusions classified as transudates were excluded from this analysis. Follow-up data were collected until January 2023. This study was performed in line with the principles of the Declaration of Helsinki and was approved by the University of Heidelberg Ethics committee (S-550/2023).
Pericarditis was diagnosed according to the European Society of Cardiology guidelines when at least two of the following criteria were met: typical chest pain, pericardial friction rub, characteristic electrocardiogram changes or pericardial effusion [18]. Pericardial and pleural effusions were classified according to the Light criteria [19, 20].
Patients were assigned to three different categories of triggers: Patients with a current infection or a history of an infection within 4 weeks before the PP diagnosis were considered having post-infectious PP (pi-PP). Patients who had a myocardial ischemia or required invasive cardiovascular interventions and developed PP within 4 weeks after the intervention were diagnosed with post-cardiac injury PP (pc-PP). PP without associated infections, vaccinations, rheumatic diseases, myocardial ischemia or previous cardiac diseases was considered idiopathic PP (i-PP).
The clinical course was assigned as monocyclic, polycyclic or chronic PP according to the ESC recommendations [18]. Polycyclic PP was defined as the occurrence of symptoms and signs of PP after a period of complete resolution of symptoms. Chronic PP was defined as the persistence of symptoms for at least 3 months regardless of anti-inflammatory treatment. Due to the small number, chronic courses were excluded from further analyses.
To address the question of autoimmunity vs. autoinflammation, i-PP patients were compared to a control group of 15 patients with SLE and autoimmune polyserositis treated at our institution between the years 2009 and 2021. The diagnosis of systemic lupus erythematosus (SLE) was consistent with the recently published classification criteria by EULAR and ACR [21]. These criteria use an ANA (anti-nuclear antibodies) titre of ≥ 1:80 on Hep-2 cells as an entry criterion and subsequently evaluate clinical symptoms including pericarditis, pleural and pericardial effusions as signs of polyserositis.
All patients underwent routine diagnostic procedures, including clinical history, vital signs, electrocardiography, echocardiography, pleura sonography and routine laboratory analysis. The presence of fever was defined as a temperature of at least 38.0 °C. In a select set of patients with clinical indication, additional data was collected such as cardiac MRI (68 patients) or aspiration of effusion (35 patients). An exact overview on the number of patients with these clinical characteristics is shown in Suppl. Table 1. The median follow-up time for patients was 1048 days as of January 31, 2023. A total of 32 patients were lost to follow-up and were excluded from the analysis of recurrence.
Additionally, we registered the treatments received to evaluate how frequently treatment beyond the standard medication of colchicine and prednisolone is needed. Due to the retrospective nature of the study and a lack of randomization, we did not perform further analyses of the effectiveness of the treatments.
Statistical analyses
Numeric variables were summarized using medians, 25% and 75% quantiles, and categorical variables were reported via absolute and relative frequencies. For numeric variables, Kruskal–Wallis-one-way analyses of variance and two-sided Wilcoxon rank sum tests were used to derive p-values for descriptive comparisons. The latter test was also used as post hoc test in case of statistical significance in the Kruskal–Wallis test. Categorical variables were compared by chi-squared and Fisher-Boschloo tests [22]. The threshold for statistical significance was defined as 0.05 for p-values derived from two-sided tests. As the nature of our study was exploratory, no corrections for multiple testing were performed. Those values meeting statistical significance in the univariate model (p < 0.05) were included as candidate variables for a multivariable logistic regression model to investigate whether the observed effects persist when adjusting for other factors. Model selection was performed based on the Akaike information criterion. Cutoff points were determined using the cutpointr package maximizing the sum of sensitivity and specificity. All statistical analyses were conducted using R software version 4.2.2 [23]. In the further analysis of the data, patients with chronic pleuropericarditis were excluded as the statistical analysis was limited due to small patient numbers in this group.
Results
Patient characteristics
A total of 164 patients were included in the analysis. These patients were treated for monocyclic, recurrent or chronic PP in our tertiary single centre University Hospital Heidelberg between the years 2009 and 2021. The median follow-up time was 1048 days as of January 31, 2023. An overview of patients’ characteristics is presented in Table 1. Most patients presented with both pleural and pericardial effusions (n = 106 (64.6%)). Only a small percentage showed an isolated pericardial (n = 29 (17.7%)) or pleural (n = 24 (14.6%)) effusion. Twenty-seven patients required pericardiocentesis and 17 patients required pleurocentesis. According to the Light criteria, these effusions were classified as exudates, fitting to the concept of PP as an inflammatory disease. Five patients (3.0%) presented with the typical clinical symptoms of PP but were diagnosed with pleuropericarditis sicca; i.e. no pleural or pericardial effusion was detected.
Myocarditis was detected in 11.0% (18/68) of cardiac MRIs. In the group of patients with the diagnosis of myocarditis in the MRI, 64.7% (11/17) of patients with evaluable troponin had elevated troponin levels (median 173 pg/ml [6–428]) and 56.3% (9/16) of patients with evaluable NT-BNP had elevated NT-BNP levels (median 161 ng/l [57–1360]). We did not observe any cases of chronic myocarditis in our cohort.
The troponin levels were generally low, with a median value of 10.7 ng/l. During the first episode of PP, we saw serological signs of acute inflammation with elevated CRP levels in 78.8% and elevated SAA (serum amyloid A) levels in 71.2%, with a median CRP value of 148.7 mg/l (upper limit of normal: 5) and a median SAA value of 64.6 mg/l (upper limit of normal: 6.4).
Out of 132 patients with follow-up, the majority (88 patients) experienced at least one relapse after initial complete resolution of pleural or pericardial effusion, while only few cases of chronic effusion (8.3%) were seen in our cohort. Patients with a chronic course showed only mildly elevated inflammation markers (median CRP 14.9 mg/l, median SAA 4.3 mg/l), thus maybe explaining the low response to anti-inflammatory therapy.
Patients were treated according to the ESC guidelines [18]. Unless there were contraindications, all patients were treated with NSAIDs and colchicine (0.5 mg twice daily). Colchicine therapy was continued for at least 6 months. If severe manifestations were present or if this treatment was not sufficient, prednisolone (0.5mg/kg/day) with continuous tapering was added to the therapy. For most patients in our cohort, treatment with colchicine or prednisolone was sufficient to achieve a symptom-free condition. Additional medication such as azathioprine was only given in 25 out of 164 patients. Only 20 patients (12.2%) received further treatment with IL-1-inhibitors such as anakinra.
Underlying autoimmunity is not detected in patients with idiopathic PP
Some authors have suggested that PP is the symptom of an underlying autoimmune disease, while others have proposed autoinflammation as the underlying pathomechanism.
To further investigate these hypotheses, we screened our patients for clinical and serological parameters typical for autoimmunity. None of the patients in our PP cohort fulfilled the ACR criteria for the classification of SLE. We searched for anti-nuclear antibodies (ANA) that could hint at an underlying autoimmune process. In 85% of patients in our cohort, we did not find relevantly elevated ANA levels (> 1:320) and only six patients showed specificity for extracted nuclear antigens. High levels of ANA (> 1:2560) were exclusively seen in older patients above the age of 50 years (Fig. 1) and unlike in autoimmune diseases, women were not overrepresented in this group (51.2% female vs. 48.8% male).
To further discriminate the PP cohort from patients with PP caused by autoimmune disease, we compared our idiopathic PP group with a cohort of SLE patients with symptomatic polyserositis (SLE-PP) (Table 2). SLE has a clear predilection for women between the age of 15 and 44 years [24]. Elevated ANA titres are characteristic and an ANA titre of ≥ 1:80 is used as an entry criterion in the classification criteria [21]. In our cohort, we saw that the ANA values were significantly higher (p < 3.2 × 10−8) in the SLE-PP group, where an ANA value above 1:320 with a homogenous fluorescence pattern was detected in all patients. The average age was significantly lower in the SLE-PP group (37.0 years SLE vs. 52.0 years i-PP, p = 0.021). Unlike in the i-PP group, where the gender distribution was equal, 86.7% of patients in the SLE-PP group were female. We saw that the highest CRP value during the first episode (median 77.5 mg/l SLE-PP vs. 150.5 mg/l i-PP) was on average lower in the SLE group, though no statistically significant difference was detected (p = 0.189).
Taken together, the clinical, epidemiological and serological characteristics of our cohort of PP patients considerably differ from patients with PP caused by autoimmune disease like SLE.
Different triggers converge on a common pathway
To determine the influence of the trigger on the manifestations and course of the disease, we assigned our cohort to three groups: idiopathic PP (i-PP), post-cardiac injury PP (pc-PP) and post-infectious PP (pi-PP).
Out of 164 patients, 49 patients (29.9%) developed PP after cardiac injury, 21 patients (12.8%) after an infection and 94 patients (57.3%) developed PP without any defined trigger. Between the three triggers, we did not see significant differences regarding the clinical phenotype (Table 1).
However, we saw significant differences regarding the serological alterations between the groups. As expected, the troponin T levels were highest in the post-cardiac injury group (median 18.0 pg/ml vs. i-PP 8.0 pg/ml, Wilcoxon p = 0.002). The SAA levels were lowest in the group with an idiopathic trigger (i-PP median 25.2 pg/ml vs. pc-PP 187.0 mg/l, Wilcoxon p = 0.019) and the highest ferritin levels were seen in the post-infectious group (median 600.5 µg/l vs. pc-PP 268.0 µg/l, Wilcoxon p = 0.031; and i-PP 193.0 µg/l, Wilcoxon p = 0.003) with procalcitonin also showing a trend for higher levels in this subgroup (Wilcoxon p = 0.051).
In summary, while different triggers of PP lead to differences in specific laboratory values, no differences with regard to clinical presentation or general routine laboratory markers are observed.
An idiopathic trigger is strongly associated with the risk of recurrence
Recurrent episodes of PP have a profound impact on patients’ quality of life and morbidity. Therefore, patients with a high likelihood of recurrence may benefit from stricter surveillance and earlier and/or more intense treatment. Out of 121 patients with follow-up (median follow-up 1048 days, excluding chronic PP), the majority (72.7%) experienced at least one relapse after initial complete resolution of pleural or pericardial effusion. In this group, 47.7% of patients experienced further episodes of PP after the first relapse. Notably, the trigger was strongly associated with the risk of recurrence (Table 3). 82.9% of patients in the idiopathic group experienced recurrence (p = 0.003) compared to only 55.3% of patients in the post-cardiac injury group (p = 0.006) and 69.2% in the post-infectious group (p = 0.684).
Low SAA values and fever are risk factors for recurrence
We additionally performed a univariate analysis (Table 3) to identify clinical risk factors as well as serological biomarkers to anticipate the risk of recurrence. We identified significant differences between the patients with a monocyclic course (m-PP) versus a recurrent course (r-PP). The inflammatory markers at presentation were significantly lower in patients who experienced recurrence (CRP at presentation (median r-PP 20.2 mg/l vs. m-PP 95.7 mg/l, p = 0.002) and SAA (median r-PP 26.4 mg/l vs. m-PP 202.5 mg/l, p = 0.007)). Regarding cardiac parameters, we saw significantly lower values in the polycyclic group (troponin T median r-PP 10.7 pg/ml vs. m-PP 17.5 pg/ml, p = 0.032 and NT-BNP median r-PP 307.0 ng/l vs. m-PP 1137.0 ng/l, p = 0.015).
In addition, the number of patients presenting with fever was significantly higher in the polycyclic group (r-PP 53/88 (60.2%) vs. m-PP 11/33 (33.3%), p = 0.011).
Multivariate analysis identifies fever as the strongest predictor for recurrence
As a next step, we performed a multivariate analysis with the factors that showed significance in the univariate analysis and selected the best fitting model using the Akaike information criterion as a fitting criterion (Table 4). The best model contained SAA, TnT, NT-BNP, the presence of fever and idiopathic genesis as the most important predictors. Fever was the strongest predictor for recurrence (OR 42.2, p = 0.003). An idiopathic trigger was significantly associated with an increased risk of recurrence (OR 9.8, p = 0.035), while high SAA values negatively correlated with recurrence (OR 0.7/100 units, p = 0.017). In order to better interpret SAA levels, we performed a cut point analysis which identified the level of 63.9 mg/l as the cut point maximizing the sum of sensitivity (0.61) and specificity (0.81) in predicting a polycyclic course (see supplementary Fig. 1). However, prospective studies are required to better assess the suitability of SAA as a biomarker for relapse. The correlation of NT-BNP and TnT values (OR 1.03/100 units, p = 0.202 and OR 0.737/100 units, p = 0.086) was less profound. However, this correlation might be due to the confounding effect caused by the higher NT-BNP and TnT values in the post-cardiac injury group compared to the other groups.
In summary, our data indicate that higher levels of inflammatory markers, such as CRP and SAA, are associated with a decreased risk of recurrence, as are cardiac damage markers such as troponin T and NT-BNP. The most important risk factors for recurrent PP according to the multivariate analysis are fever at diagnosis, an idiopathic trigger and low SAA values.
Discussion
In this study, we present the analysis of a large cohort of 164 patients with PP who were treated at the University Hospital Heidelberg over the course of more than 10 years. We investigate whether idiopathic PP could be differentiated from PP in the context of autoimmune disease. When we compared patients with SLE-associated PP with idiopathic PP, we found that the clinical, epidemiological and serological characteristics considerably differ from patients with PP caused by autoimmune disease like SLE. We further observed that various trigger factors (post-cardiac injury, infectious, idiopathic) result in a similar phenotype. However, trigger factors are relevant for the risk of recurrent PP. We show that fever at PP diagnosis, an idiopathic trigger for PP and low SAA are risk factors for recurrent PP.
Although PP is relatively common, the exact pathogenesis is not well understood. An autoimmune mechanism provoked by different triggers has been proposed by some authors. It has been suggested that viral infections may stimulate autoimmune responses by molecular mimicry, and after cardiac injury, the release of cardiac autoantigens may activate T and B lymphocytes [25, 26]. Additionally, PP can be a complication of autoimmune disorders. In SLE patients, pericarditis can occur in up to 50% of cases, although often asymptomatically [26]. In our analysis, we found that autoantibodies in patients’ sera of our PP cohort were significantly different compared to patients with SLE-associated PP. In patients with SLE-associated PP, we detected high ANA titres with a homogeneous fluorescence pattern. Patients with idiopathic PP had either negative ANA or significantly lower ANA titres with an in most cases speckled fluorescence pattern and ENA were negative. None of the patients fulfilled the ACR criteria for the diagnosis of SLE. On the other hand, previous analyses have shown that rare deleterious MEFV variants were more prevalent in PP when compared with ancestry-matched controls [16, 17], pointing towards an autoinflammatory origin of the disease. PP patients usually respond well to colchicine and anti-IL1-targeted medication which is suggestive for an activation of inflammasomes and the role of IL-1 in the pathogenesis of PP [27]. The efficacy of IL-1 antagonists especially anakinra and rilonacept has been shown in large clinical trials. Therefore, this specific treatment should be strongly considered in patients with recurrent PP [28, 29]. Our results show that PP has many features in common with an autoinflammatory disease. The equal gender distribution, the occurrence at various ages, the elevation of CRP and SAA, the absence of specific ANA titres and the efficacy of colchicine and IL-1 inhibition suggest an autoinflammatory, rather than an autoimmune background. Thus, PP appears to be a new manifestation of autoinflammation and should be treated accordingly.
The majority of patients developed PP without any obvious trigger and thus were diagnosed with idiopathic PP. Post-cardiac injury PP and post-infectious PP were less common. We did not see significant differences between the clinical phenotypes resulting from different triggers consistent with previous reports [30, 31]. This suggests that autonomous autoinflammation, tissue damage and viral infections at the end trigger a common inflammatory pathway employing the activation of inflammasomes and the activation of IL-1β. Our data show that a distinction between the triggers is relevant for the prognosis as flare of PP are more frequent in idiopathic PP.
In our study, we further highlight a high risk of recurrence and outline risk factors for recurrence. We observed recurrent PP in 72.7% in our cohort, which is higher than previous estimates, such as the 30% estimate risk of relapse in the 2015 ESC guideline [18, 31]. The discrepancy could be explained by the fact that more severe cases might be overrepresented at the tertiary care setting at the University Hospital Heidelberg. The use of an increased neutrophil/lymphocyte ratio (NLR) and higher age was suggested previously to indicate a risk for PP recurrence [4, 32]. However, in our cohort, we could not confirm neither an association with age or with the NLR nor the absolute leukocyte count in the blood. Another report showed post-cardiac injury PP to be negatively associated with recurrence, which is consistent with our observations [33]. A multivariate analysis of our data showed that fever at diagnosis was the strongest risk factor for recurrence, followed by an idiopathic trigger and low SAA values. A possible interpretation could be that infections or cardiac damage might be transient triggers and the proinflammatory drivers might disappear in the course of the disease. Furthermore, a strong inflammatory reaction could also induce anti-inflammatory mechanisms which contribute to terminate a PP attack. However, an autoinflammatory predisposition might provide chronic or recurrent stimuli for a persistent autoinflammatory reaction. Further investigations are required to prove these hypotheses.
This study has limitations due to the single-centre cohort, the retrospective analyses and non-standardized assessment of laboratory parameters. The retrospective analysis of treatment response limited conclusions about the treatment efficacy. In addition, the risk factors identified in our cohort may not apply to patients in countries where infections like tuberculosis are more prevalent and common differential diagnoses for PP. As we only included patients who were discussed with a rheumatologist, there might be a selection bias towards more severe PP cases. Additionally, more severe cases might be overrepresented at the tertiary care setting. As this is a retrospective analysis, no randomization to different treatment groups was performed. Therefore, patients with more severe manifestations that did not resolve with colchicine alone were more likely to receive additional therapy. We did not include the different therapies into the multivariate model since this question has to be addressed in a prospective randomized trial.
Conclusions
In conclusion, this study provides evidence that various triggers for PP lead to an identical phenotype implying a common inflammatory pathomechanism. It supports the concept of PP as an autoinflammatory disease and discriminates idiopathic PP from SLE-associated PP. Our analysis shows that the majority of PP patients will experience flares, and that fever, an idiopathic trigger and low SAA values are the most relevant risk factors for recurrence of PP.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACR:
-
American College of Rheumatology
- ANA:
-
Anti-nuclear antibody
- CRP:
-
C-reactive protein
- ENA:
-
Extractable nuclear antigens
- EULAR:
-
European League Against Rheumatism
- N/L-ratio:
-
Neutrophil/leukocyte-ratio
- NSAR:
-
Non-steroidal anti-inflammatory drugs
- NT-BNP:
-
N-terminal-brain natriuretic peptide
- MRI:
-
Magnetic Resonance Imaging
- PP:
-
Pleuropericarditis
- SAA:
-
Serum amyloid A
- SLE:
-
Systemic lupus erythematosus
References
Lazarou E et al (2022) Acute pericarditis: update. Curr Cardiol Rep 24(8):905–913
Andreis A et al (2021) Recurrent pericarditis: an update on diagnosis and management. Intern Emerg Med 16(3):551–558
Mauro AG et al (2021) The role of NLRP3 inflammasome in pericarditis: potential for therapeutic approaches. JACC Basic Transl Sci 6(2):137–150
Lazarou E et al (2021) A risk score for pericarditis recurrence. Eur J Clin Invest 51(11):e13602
Lee HJ et al (2023) Sacubitril/valsartan and the risk of incident dementia in heart failure: a nationwide propensity-matched cohort study. Clin Res Cardiol. https://doi.org/10.1007/s00392-023-02322-0
Ney S et al (2023) Epidemiology of cardiac amyloidosis in Germany: a retrospective analysis from 2009 to 2018. Clin Res Cardiol 112(3):401–408
Koenig W et al (2023) Retrospective real-world analysis of adherence and persistence to lipid-lowering therapy in Germany. Clin Res Cardiol. https://doi.org/10.1007/s00392-023-02257-6
Lal A et al (2018) Unusual cause of chest pain, Bornholm disease, a forgotten entity; case report and review of literature. Respir Med Case Rep 25:270–273
Mayosi BM, Burgess LJ, Doubell AF (2005) Tuberculous pericarditis. Circulation 112(23):3608–3616
Husby A et al (2021) SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ 375:e068665
Verma BR et al (2020) Pericarditis and post-cardiac injury syndrome as a sequelae of acute myocardial infarction. Curr Cardiol Rep 22(10):127
Chou OHI et al (2022) COVID-19 vaccination and carditis in children and adolescents: a systematic review and meta-analysis. Clin Res Cardiol 111(10):1161–1173
Chou OHI et al (2022) Comparisons of the risk of myopericarditis between COVID-19 patients and individuals receiving COVID-19 vaccines: a population-based study. Clin Res Cardiol 111(10):1098–1103
Pastor Pueyo P et al (2024) Vaccine-carditis study: Spanish multicenter registry of inflammatory heart disease after COVID-19 vaccination. Clin Res Cardiol 113(2):223–234. https://doi.org/10.1007/s00392-023-02225-0
Kontzias A, Barkhodari A, Yao Q (2020) Pericarditis in systemic rheumatologic diseases. Curr Cardiol Rep 22(11):142
Peet CJ et al (2022) Pericarditis and autoinflammation: a clinical and genetic analysis of patients with idiopathic recurrent pericarditis and monogenic autoinflammatory diseases at a national referral center. J Am Heart Assoc 11(11):e024931
Roubille F, Delmas C, Roubille C (2022) Idiopathic Recurrent pericarditis: not really so idiopathic? J Am Heart Assoc 11(11):e026218
Adler Y et al (2015) 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 36(42):2921–2964
Light RW et al (1972) Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 77(4):507–513
Porcel JM, Light RW (2021) Pleural fluid analysis: are light’s criteria still relevant after half a century? Clin Chest Med 42(4):599–609
Aringer M et al (2019) 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 78(9):1151–1159
Boschloo RD (1970) Raised conditional level of significance for the 2 × 2-table when testing the equality of two probabilities. Stat Neerl 24(1):1–9
Team RC (2021) A language and environment for statistical computing. Vienna, Austria. Available at: https://www.R-project.org/. Accessed 01 Sep 2023
Fava A, Petri M (2019) Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun 96:1–13
Maestroni S et al (2012) Recurrent pericarditis: autoimmune or autoinflammatory? Autoimmun Rev 12(1):60–65
Bizzi E et al (2021) Autoimmune and autoinflammatory pericarditis: definitions and new treatments. Curr Cardiol Rep 23(9):128
Blank N, Lorenz HM (2019) Idiopathic pericarditis-an autoinflammatory disease? Curr Rheumatol Rep 21(5):18
Abadie BQ, Cremer PC (2022) Interleukin-1 antagonists for the treatment of recurrent pericarditis. BioDrugs 36(4):459–472
Affas ZR et al (2022) Rilonacept and Anakinra in recurrent pericarditis: a systematic review and meta-analysis. Cureus 14(11):e31226
Brucato A et al (2008) Recurrent pericarditis: infectious or autoimmune? Autoimmun Rev 8(1):44–47
Imazio M, Gaita F, LeWinter M (2015) Evaluation and treatment of pericarditis: a systematic review. JAMA 314(14):1498–1506
Yılmaz F et al (2022) Usefulness of neutrophil-to-lymphocyte ratio for predicting acute pericarditis outcomes. Acta Cardiol 77(5):422–430
Vecchié A et al (2020) Clinical presentation and outcomes of acute pericarditis in a large urban hospital in the United States of America. Chest 158(6):2556–2567
Acknowledgements
The graphical abstract was created with BioRender.com.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by Dorothee Kaudewitz, Norbert Blank, Jan Meis, and Lukas John. The first draft of the manuscript was written by Dorothee Kaudewitz, Norbert Blank, and Lukas John. Florian Leuschner, Norbert Frey, and Hanns-Martin Lorenz revised the manuscript and provided intellectual input. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the University of Heidelberg (S-550/2023).
Consent to participate
Not applicable, approved by the Ethics Committee of the University of Heidelberg (S-550/2023).
Consent for publication
Not applicable, approved by the Ethics Committee of the University of Heidelberg (S-550/2023).
Competing interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaudewitz, D., John, L., Meis, J. et al. Clinical and serological characterization of acute pleuropericarditis suggests an autoinflammatory pathogenesis and highlights risk factors for recurrent attacks. Clin Res Cardiol (2024). https://doi.org/10.1007/s00392-024-02390-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00392-024-02390-w