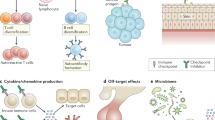
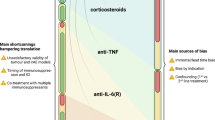
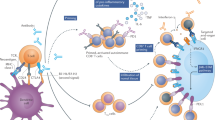
Abstract
With immune checkpoint inhibitors (ICIs) becoming the mainstay of treatment for many cancers, managing their immune-related adverse events (irAEs) has become an important part of oncological care. This Review covers the clinical presentation of irAEs and crucial aspects of reversibility, fatality and long-term sequelae, with special attention to irAEs in specific patient populations, such as those with autoimmune diseases. In addition, the genetic basis of irAEs, along with cellular and humoral responses to ICI therapy, are discussed. Detrimental effects of empirically used high-dose steroids and second-line immunosuppression, including impaired ICI effectiveness, call for more tailored irAE-treatment strategies. We discuss open therapeutic challenges and propose potential avenues to accelerate personalized management strategies and optimize outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Haslam, A., Gill, J. & Prasad, V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw. Open 3, e200423 (2020).
Luke, J. J. et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet 399, 1718–1729 (2022).
Cascone, T. et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat. Med. 29, 593–604 (2023).
Scott, E. C. et al. Trends in the approval of cancer therapies by the FDA in the twenty-first century. Nat. Rev. Drug Discov. 22, 625–640 (2023).
Yu, Y. et al. Coverage evaluation of CTCAE for capturing the immune-related adverse events leveraging text mining technologies. AMIA Jt Summits Transl. Sci. Proc. 2019, 771–778 (2019).
Naidoo, J. et al. Society for Immunotherapy of Cancer (SITC) consensus definitions for immune checkpoint inhibitor-associated immune-related adverse events (irAEs) terminology. J. Immunother. Cancer 11, e006398 (2023).
Hsiehchen, D., Watters, M. K., Lu, R., Xie, Y. & Gerber, D. E. Variation in the assessment of immune-related adverse event occurrence, grade, and timing in patients receiving immune checkpoint inhibitors. JAMA Netw. Open 2, e1911519 (2019).
Gault, A. et al. Cutaneous immune-related adverse events in patients with melanoma treated with checkpoint inhibitors. Br. J. Dermatol. 185, 263–271 (2021).
Khoja, L., Day, D., Wei-Wu Chen, T., Siu, L. L. & Hansen, A. R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann. Oncol. 28, 2377–2385 (2017).
Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532 (2015).
Wolchok, J. D. et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J. Clin. Oncol. 40, 127–137 (2022).
Tarhini, A. A. et al. Phase III study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma: North American Intergroup E1609. J. Clin. Oncol. 38, 567–575 (2020).
Lebbé, C. et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV CheckMate 511 trial. J. Clin. Oncol. 37, 867–875 (2019).
Tawbi, H. A. et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N. Engl. J. Med. 386, 24–34 (2022).
Versluis, J. M., Long, G. V. & Blank, C. U. Learning from clinical trials of neoadjuvant checkpoint blockade. Nat. Med. 26, 475–484 (2020).
Verheijden, R. J. et al. Lower risk of severe checkpoint inhibitor toxicity in more advanced disease. ESMO Open 5, e000945 (2020).
Blank, C. U. et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 24, 1655–1661 (2018).
Rozeman, E. A. et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nat. Med. 27, 256–263 (2021).
Amaria, R. N. et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 24, 1649–1654 (2018).
Reijers, I. L. M. et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: the PRADO trial. Nat. Med. 28, 1178–1188 (2022).
Eggermont, A. M. M. et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage III melanoma: updated results from the EORTC 1325-MG/KEYNOTE-054 trial. J. Clin. Oncol. 38, 3925–3936 (2020).
Patel, S. P. et al. Neoadjuvant–adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N. Engl. J. Med. 388, 813–823 (2023).
Amaria, R. N. et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature 611, 155–160 (2022).
Nguyen, N. et al. Influence of melanoma type on incidence and downstream implications of cutaneous immune-related adverse events in the setting of immune checkpoint inhibitor therapy. J. Am. Acad. Dermatol. 88, 1308–1316 (2023).
Castaneda, C. A. et al. Tumor infiltrating lymphocytes in acral lentiginous melanoma: a study of a large cohort of cases from Latin America. Clin. Transl. Oncol. 19, 1478–1488 (2017).
Shankar, B. et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 6, 1952–1956 (2020).
Kichenadasse, G. et al. Multiorgan immune-related adverse events during treatment with atezolizumab. J. Natl Compr. Canc. Netw. 18, 1191–1199 (2020).
Laparra, A. et al. Multiple immune-related toxicities in cancer patients treated with anti-programmed cell death protein 1 immunotherapies: a new surrogate marker for clinical trials? Ann. Oncol. 32, 936–937 (2021).
Abu-Sbeih, H. et al. The impact of immune checkpoint inhibitor-related adverse events and their immunosuppressive treatment on patients’ outcomes. J. Immunother. Precis. Oncol. 1, 7–18 (2018).
Li, Y., Pond, G. R. & McWhirter, E. Multisystem immune-related adverse events from dual agent immunotherapy use. J. Clin. Oncol. 41, 2635 (2023).
Weber, J. S. et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 35, 785–792 (2017).
Tang, S.-Q. et al. The pattern of time to onset and resolution of immune-related adverse events caused by immune checkpoint inhibitors in cancer: a pooled analysis of 23 clinical trials and 8,436 patients. Cancer Res. Treat. 53, 339–354 (2021).
Ascierto, P. A. et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB–C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 21, 1465–1477 (2020).
Owen, C. N. et al. Delayed immune-related adverse events with anti-PD-1-based immunotherapy in melanoma. Ann. Oncol. 32, 917–925 (2021).
Robert, C. et al. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: a landmark analysis in patients with advanced melanoma. Eur. J. Cancer 144, 182–191 (2021).
Weiss, S. A. & Kluger, H. CheckMate-067: raising the bar for the next decade in oncology. J. Clin. Oncol. 40, 111–113 (2022).
Johnson, D. B., Nebhan, C. A., Moslehi, J. J. & Balko, J. M. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat. Rev. Clin. Oncol. 19, 254–267 (2022).
Patrinely, J. R. et al. Chronic immune-related adverse events following adjuvant anti-PD-1 therapy for high-risk resected melanoma. JAMA Oncol. 7, 744–748 (2021).
Goodman, R. S. et al. Long-term outcomes of chronic immune-related adverse events from adjuvant anti-PD-1 therapy for high-risk resected melanoma. J. Clin. Oncol. 41, 9591 (2023).
Min, L. et al. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin. Cancer Res. 21, 749–755 (2015).
Schulz, T. U. et al. Persistent immune-related adverse events after cessation of checkpoint inhibitor therapy: prevalence and impact on patients’ health-related quality of life. Eur. J. Cancer 176, 88–99 (2022).
Martins, F. et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16, 563–580 (2019).
Ruste, V. et al. The determinants of very severe immune-related adverse events associated with immune checkpoint inhibitors: a prospective study of the French REISAMIC registry. Eur. J. Cancer 158, 217–224 (2021).
Wang, D. Y. et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4, 1721–1728 (2018).
Drobni, Z. D. et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation 142, 2299–2311 (2020).
Laenens, D. et al. Incidence of cardiovascular events in patients treated with immune checkpoint inhibitors. J. Clin. Oncol. 40, 3430–3438 (2022).
Newman, J. L. & Stone, J. R. Immune checkpoint inhibition alters the inflammatory cell composition of human coronary artery atherosclerosis. Cardiovasc. Pathol. 43, 107148 (2019).
Suero-Abreu, G. A., Zanni, M. V. & Neilan, T. G. Atherosclerosis with immune checkpoint inhibitor therapy: evidence, diagnosis, and management: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. 4, 598–615 (2022).
Poels, K. et al. Immune checkpoint inhibitor therapy aggravates T cell-driven plaque inflammation in atherosclerosis. JACC CardioOncol. 2, 599–610 (2020).
Garutti, M., Lambertini, M. & Puglisi, F. Checkpoint inhibitors, fertility, pregnancy, and sexual life: a systematic review. ESMO Open 6, 100276 (2021).
Scovell, J. M. et al. Association of impaired spermatogenesis with the use of immune checkpoint inhibitors in patients with metastatic melanoma. JAMA Oncol. 6, 1297–1299 (2020).
Salzmann, M. et al. Male fertility during and after immune checkpoint inhibitor therapy: a cross-sectional pilot study. Eur. J. Cancer 152, 41–48 (2021).
Winship, A. L. et al. Checkpoint inhibitor immunotherapy diminishes oocyte number and quality in mice. Nat. Cancer 3, 1–13 (2022).
Barroso-Sousa, R. et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 4, 173–182 (2018).
Bai, X. et al. Mapping endocrine toxicity spectrum of immune checkpoint inhibitors: a disproportionality analysis using the WHO adverse drug reaction database, VigiBase. Endocrine 69, 670–681 (2020).
Jessel, S. et al. Immune checkpoint inhibitor-induced hypophysitis and patterns of loss of pituitary function. Front. Oncol. 12, 836859 (2022).
Nguyen, H. et al. Immune checkpoint inhibitor related hypophysitis: diagnostic criteria and recovery patterns. Endocr. Relat. Cancer 28, 419–431 (2021).
Keogh, R. J. et al. A prospective observational study investigating the impact of immune checkpoint inhibitors for cancer on fertility in males and females of child-bearing age (immuno-fertility). J. Clin. Oncol. 41, TPS2679 (2023).
Cha, E. et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci. Transl. Med. 6, 238ra70 (2014).
Gangaev, A. et al. Differential effects of PD-1 and CTLA-4 blockade on the melanoma-reactive CD8 T cell response. Proc. Natl Acad. Sci. USA 118, e2102849118 (2021).
Kvistborg, P. et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci. Transl. Med. 6, 254ra128 (2014).
Jiao, S. et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell 179, 1177–1190 (2019).
Wei, S. C. et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 170, 1120–1133 (2017).
Luoma, A. M. et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell 185, 2918–2935 (2022).
Yost, K. E. et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 25, 1251–1259 (2019).
Chow, A., Perica, K., Klebanoff, C. A. & Wolchok, J. D. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 19, 775–790 (2022).
Wei, S. C. et al. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc. Natl Acad. Sci. USA 116, 22699–22709 (2019).
Huuhtanen, J. et al. Single-cell characterization of anti-LAG-3 and anti-PD-1 combination treatment in patients with melanoma. J. Clin. Invest. 133, e164809 (2023).
Sharma, A. et al. Anti-CTLA-4 immunotherapy does not deplete FOXP3+ regulatory T cells (Tregs) in human cancers—response. Clin. Cancer Res. 25, 3469–3470 (2019).
Huang, A. C. et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat. Med. 25, 454–461 (2019).
Blomberg, O. S. et al. Neoadjuvant immune checkpoint blockade triggers persistent and systemic Treg activation which blunts therapeutic efficacy against metastatic spread of breast tumors. Oncoimmunology 12, 2201147 (2023).
Waterhouse, P. et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988 (1995).
Nishimura, H. et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319–322 (2001).
Schwab, C. et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4-insufficient subjects. J. Allergy Clin. Immunol. 142, 1932–1946 (2018).
Ogishi, M. et al. Inherited PD-1 deficiency underlies tuberculosis and autoimmunity in a child. Nat. Med. 27, 1646–1654 (2021).
Bins, S. et al. Association between single-nucleotide polymorphisms and adverse events in nivolumab-treated non-small cell lung cancer patients. Br. J. Cancer 118, 1296–1301 (2018).
Weidhaas, J. et al. Germline biomarkers predict toxicity to anti-PD1/PDL1 checkpoint therapy. J. Immunother. Cancer 10, e003625 (2022).
Khan, Z. et al. Polygenic risk for skin autoimmunity impacts immune checkpoint blockade in bladder cancer. Proc. Natl Acad. Sci. USA 117, 12288–12294 (2020).
Hasan Ali, O. et al. Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur. J. Cancer 107, 8–14 (2019).
Akturk, H. K. et al. Immune checkpoint inhibitor-induced type 1 diabetes: a systematic review and meta-analysis. Diabet. Med. 36, 1075–1081 (2019).
Abed, A. et al. Human leucocyte antigen genotype association with the development of immune-related adverse events in patients with non-small cell lung cancer treated with single agent immunotherapy. Eur. J. Cancer 172, 98–106 (2022).
Marschner, D. et al. MicroRNA-146a regulates immune-related adverse events caused by immune checkpoint inhibitors. JCI Insight 5, e132334 (2020).
Groha, S. et al. Germline variants associated with toxicity to immune checkpoint blockade. Nat. Med. 28, 2584–2591 (2022).
Taylor, C. A. et al. IL7 genetic variation and toxicity to immune checkpoint blockade in patients with melanoma. Nat. Med. 28, 2592–2600 (2022).
Berner, F. et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 5, 1043–1047 (2019).
Johnson, D. B. et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 375, 1749–1755 (2016).
Läubli, H. et al. The T cell repertoire in tumors overlaps with pulmonary inflammatory lesions in patients treated with checkpoint inhibitors. Oncoimmunology 7, e1386362 (2018).
Johnson, D. B. et al. A case report of clonal EBV-like memory CD4+ T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat. Med. 25, 1243–1250 (2019).
Hutchinson, J. A. et al. Virus-specific memory T cell responses unmasked by immune checkpoint blockade cause hepatitis. Nat. Commun. 12, 1439 (2021).
Bomze, D., Hasan Ali, O., Bate, A. & Flatz, L. Association between immune-related adverse events during anti-PD-1 therapy and tumor mutational burden. JAMA Oncol. 5, 1633–1635 (2019).
Andrews, M. C. et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 27, 1432–1441 (2021).
Simpson, R. C., Shanahan, E. R., Scolyer, R. A. & Long, G. V. Towards modulating the gut microbiota to enhance the efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 20, 697–715 (2023).
Kerepesi, C., Bakacs, T., Moss, R. W., Slavin, S. & Anderson, C. C. Significant association between tumor mutational burden and immune-related adverse events during immune checkpoint inhibition therapies. Cancer Immunol. Immunother. 69, 683–687 (2020).
Berner, F. et al. Autoreactive napsin A-specific T cells are enriched in lung tumors and inflammatory lung lesions during immune checkpoint blockade. Sci. Immunol. 7, eabn9644 (2022).
Axelrod, M. L. et al. T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature 611, 818–826 (2022).
Won, T. et al. Cardiac myosin-specific autoimmune T cells contribute to immune-checkpoint-inhibitor-associated myocarditis. Cell Rep. 41, 111611 (2022).
Hu, Z. I. et al. Immune checkpoint inhibitors unleash pathogenic immune responses against the microbiota. Proc. Natl Acad. Sci. USA 119, e2200348119 (2022).
Han, J. et al. Resident and circulating memory T cells persist for years in melanoma patients with durable responses to immunotherapy. Nat. Cancer 2, 300–311 (2021).
Meier, S. L., Satpathy, A. T. & Wells, D. K. Bystander T cells in cancer immunology and therapy. Nat. Cancer 3, 143–155 (2022).
Subudhi, S. K. et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc. Natl Acad. Sci. USA 113, 11919–11924 (2016).
Robert, L. et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin. Cancer Res. 20, 2424–2432 (2014).
Oh, D. Y. et al. Immune toxicities elicted by CTLA-4 blockade in cancer patients are associated with early diversification of the T-cell repertoire. Cancer Res. 77, 1322–1330 (2017).
Lozano, A. X. et al. T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat. Med. 28, 353–362 (2022).
Jing, Y. et al. Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Nat. Commun. 11, 4946 (2020).
Hommes, J. W., Verheijden, R. J., Suijkerbuijk, K. P. M. & Hamann, D. Biomarkers of checkpoint inhibitor induced immune-related adverse events—a comprehensive review. Front. Oncol. 10, 585311 (2020).
Earland, N. et al. CD4 T cells and toxicity from immune checkpoint blockade. Immunol. Rev. 318, 96–109 (2023).
van Eijs, M. J. M. et al. Toxicity-specific peripheral blood T and B cell dynamics in anti-PD-1 and combined immune checkpoint inhibition. Cancer Immunol. Immunother. 72, 4049–4064 (2023).
Kim, K. H. et al. The first-week proliferative response of peripheral blood PD-1+CD8+ T cells predicts the response to anti-PD-1 therapy in solid tumors. Clin. Cancer Res. 25, 2144–2154 (2019).
Nuñez, N. G. et al. Immune signatures predict development of autoimmune toxicity in patients with cancer treated with immune checkpoint inhibitors. Med 4, 113–129 (2023).
Kim, K. H. et al. Immune-related adverse events are clustered into distinct subtypes by T-cell profiling before and early after anti-PD-1 treatment. Oncoimmunology 9, 1722023 (2020).
Bukhari, S. et al. Single-cell RNA sequencing reveals distinct T cell populations in immune-related adverse events of checkpoint inhibitors. Cell Rep. Med. 4, 100868 (2023).
Kumar, B. V. et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 20, 2921–2934 (2017).
Szabo, P. A., Miron, M. & Farber, D. L. Location, location, location: tissue resident memory T cells in mice and humans. Sci. Immunol. 4, eaas9673 (2019).
Damo, M. et al. PD-1 maintains CD8 T cell tolerance towards cutaneous neoantigens. Nature 619, 151–159 (2023).
Weisberg, S. P. et al. Tissue-resident memory T cells mediate immune homeostasis in the human pancreas through the PD-1/PD-L1 pathway. Cell Rep. 29, 3916–3932 (2019).
Luoma, A. M. et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell 182, 655–671 (2020).
Sasson, S. C. et al. Interferon-γ-producing CD8+ tissue resident memory T cells are a targetable hallmark of immune checkpoint inhibitor-colitis. Gastroenterology 161, 1229–1244 (2021).
Thomas, M. et al. Altered interactions between circulating and tissue-resident CD8 T cells with the colonic mucosa define colitis associated with immune checkpoint inhibitors. Preprint at bioRxiv https://doi.org/10.1101/2021.09.17.460868 (2021).
van Eijs, M. J. M. et al. Highly multiplexed spatial analysis identifies tissue-resident memory T cells as drivers of ulcerative and immune checkpoint inhibitor colitis. iScience 26, 107891 (2023).
Reschke, R. et al. Checkpoint blockade-induced dermatitis and colitis are dominated by tissue-resident memory T cells and TH1/TC1 cytokines. Cancer Immunol. Res. 10, 1167–1174 (2022).
Lechner, M. G. et al. Clonally expanded, thyrotoxic effector CD8+ T cells driven by IL-21 contribute to checkpoint inhibitor thyroiditis. Sci. Transl. Med. 15, eadg0675 (2023).
Franken, A. et al. Single-cell transcriptomics identifies pathogenic T-helper 17.1 cells and pro-inflammatory monocytes in immune checkpoint inhibitor-related pneumonitis. J. Immunother. Cancer 10, e005323 (2022).
Suresh, K. et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J. Clin. Invest. 129, 4305–4315 (2019).
Kim, S. T. et al. Distinct molecular and immune hallmarks of inflammatory arthritis induced by immune checkpoint inhibitors for cancer therapy. Nat. Commun. 13, 1970 (2022).
Wang, R. et al. Clonally expanded CD38hi cytotoxic CD8 T cells define the T cell infiltrate in checkpoint inhibitor-associated arthritis. Sci. Immunol. 8, eadd1591 (2023).
Chang, M. H. et al. Arthritis flares mediated by tissue-resident memory T cells in the joint. Cell Rep. 37, 109902 (2021).
Flores-Chávez, A. et al. Using autoantibodies to diagnose systemic autoimmune diseases triggered by immune checkpoint inhibitors: a clinical perspective. Crit. Rev. Immunol. 42, 21–36 (2022).
Johannet, P. et al. Baseline serum autoantibody signatures predict recurrence and toxicity in melanoma patients receiving adjuvant immune checkpoint blockade. Clin. Cancer Res. 28, 4121–4130 (2022).
Velasco, R. et al. Encephalitis induced by immune checkpoint inhibitors: a systematic review. JAMA Neurol. 78, 864–873 (2021).
Ghosh, N., Chan, K. K., Jivanelli, B. & Bass, A. R. Autoantibodies in patients with immune-related adverse events from checkpoint inhibitors: a systematic literature review. J. Clin. Rheumatol. 28, e498–e505 (2022).
Patel, A. J. et al. Regulatory B cell repertoire defects predispose lung cancer patients to immune-related toxicity following checkpoint blockade. Nat. Commun. 13, 3148 (2022).
Das, R. et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Invest. 128, 715–720 (2018).
Fridman, W. H. et al. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat. Rev. Clin. Oncol. 19, 441–457 (2022).
Singh, S. et al. Tertiary lymphoid structure signatures are associated with immune checkpoint inhibitor related acute interstitial nephritis. JCI Insight https://doi.org/10.1172/jci.insight.165108 (2022).
Tsukamoto, H. et al. Aging-associated and CD4 T-cell-dependent ectopic CXCL13 activation predisposes to anti-PD-1 therapy-induced adverse events. Proc. Natl Acad. Sci. USA 119, e2205378119 (2022).
Liu, B., Zhang, Y., Wang, D., Hu, X. & Zhang, Z. Single-cell meta-analyses reveal responses of tumor-reactive CXCL13+ T cells to immune-checkpoint blockade. Nat. Cancer 3, 1123–1136 (2022).
Zhou, J., Du, Z., Fu, J. & Yi, X. Blood cell counts can predict adverse events of immune checkpoint inhibitors: a systematic review and meta-analysis. Front. Immunol. 14, 1117447 (2023).
Niemantsverdriet, M. S. A. et al. Added diagnostic value of routinely measured hematology variables in diagnosing immune checkpoint inhibitor mediated toxicity in the emergency department. Cancer Med. 12, 12462–12469 (2023).
Scanvion, Q. et al. Moderate-to-severe eosinophilia induced by treatment with immune checkpoint inhibitors: 37 cases from a national reference center for hypereosinophilic syndromes and the French pharmacovigilance database. Oncoimmunology 9, 1722022 (2020).
Blomberg, O. S. et al. IL-5-producing CD4+ T cells and eosinophils cooperate to enhance response to immune checkpoint blockade in breast cancer. Cancer Cell 41, 106–123 (2023).
Zheng, X. et al. CTLA4 blockade promotes vessel normalization in breast tumors via the accumulation of eosinophils. Int. J. Cancer 146, 1730–1740 (2020).
Sung, C. et al. Integrative analysis of risk factors for immune-related adverse events of checkpoint blockade therapy in cancer. Nat. Cancer 4, 844–859 (2023).
Iwama, S. et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 6, 230ra45 (2014).
Haanen, J. et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 33, 1217–1238 (2022).
Brahmer, J. R. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 9, e002435 (2021).
Schneider, B. J. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J. Clin. Oncol. 39, 4073–4126 (2021).
Ma, C. et al. Recommendations for standardizing biopsy acquisition and histological assessment of immune checkpoint inhibitor-associated colitis. J. Immunother. Cancer 10, e004560 (2022).
Hughes, M. S. et al. Budesonide treatment for microscopic colitis from immune checkpoint inhibitors. J. Immunother. Cancer 7, 292 (2019).
Zou, F. et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: a two-center observational study. J. Immunother. Cancer 9, e003277 (2021).
Liu, D. et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 9, 30 (2013).
Bass, A. R. et al. Comparative safety and effectiveness of TNF inhibitors, IL6 inhibitors and methotrexate for the treatment of immune checkpoint inhibitor-associated arthritis. Ann. Rheum. Dis. 82, 920–926 (2023).
Mann, J. E. et al. scRNA-seq defines dynamic T-cell subsets in longitudinal colon and peripheral blood samples in immune checkpoint inhibitor-induced colitis. J. Immunother. Cancer 11, e007358 (2023).
Dall’Olio, F. G. et al. Immortal time bias in the association between toxicity and response for immune checkpoint inhibitors: a meta-analysis. Immunotherapy 13, 257–270 (2021).
Hussaini, S. et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors — a systematic review and meta-analysis. Cancer Treat. Rev. 92, 102134 (2021).
Verheijden, R. J., van Eijs, M. J. M., May, A. M., van Wijk, F. & Suijkerbuijk, K. P. M. Immunosuppression for immune-related adverse events during checkpoint inhibition: an intricate balance. NPJ Precis. Oncol. 7, 41 (2023).
Eggermont, A. M. M. et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 6, 519–527 (2020).
Walsh, M. J. et al. Blockade of innate inflammatory cytokines TNFα, IL-1β, or IL-6 overcomes virotherapy-induced cancer equilibrium to promote tumor regression. Immunother. Adv. 3, ltad011 (2023).
Perez-Ruiz, E. et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 569, 428–432 (2019).
Bertrand, F. et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat. Commun. 8, 2256 (2017).
Montfort, A. et al. Combining nivolumab and ipilimumab with infliximab or certolizumab in patients with advanced melanoma: first results of a phase Ib clinical trial. Clin. Cancer Res. 27, 1037–1047 (2021).
Fa’ak, F. et al. Selective immune suppression using interleukin-6 receptor inhibitors for management of immune-related adverse events. J. Immunother. Cancer 11, e006814 (2023).
Hailemichael, Y. et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 40, 509–523 (2022).
Verheijden, R. J. et al. Association of anti-TNF with decreased survival in steroid refractory ipilimumab and anti-PD1-treated patients in the Dutch Melanoma Treatment Registry. Clin. Cancer Res. 26, 2268–2274 (2020).
van Not, O. J. et al. Association of immune-related adverse event management with survival in patients with advanced melanoma. JAMA Oncol. 8, 1794–1801 (2022).
van der Kooij, M. K. et al. Safety and efficacy of checkpoint inhibition in patients with melanoma and preexisting autoimmune disease: a cohort study. Ann. Intern. Med. 174, 641–648 (2021).
Abu-Sbeih, H. et al. Immune checkpoint inhibitor therapy in patients with preexisting inflammatory bowel disease. J. Clin. Oncol. 38, 576–583 (2020).
Leonardi, G. C. et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J. Clin. Oncol. 36, 1905–1912 (2018).
McCarter, K. R. et al. Mortality and immune-related adverse events after immune checkpoint inhibitor initiation for cancer among patients with pre-existing rheumatoid arthritis: a retrospective, comparative, cohort study. Lancet Rheumatol. 5, e274–e283 (2023).
de Glas, N. A. et al. Toxicity, response and survival in older patients with metastatic melanoma treated with checkpoint inhibitors. Cancers 13, 2826 (2021).
Nebhan, C. A. et al. Clinical outcomes and toxic effects of single-agent immune checkpoint inhibitors among patients aged 80 years or older with cancer: a multicenter international cohort study. JAMA Oncol. 7, 1856–1861 (2021).
Bruijnen, C. P. et al. Frailty and checkpoint inhibitor toxicity in older patients with melanoma. Cancer 128, 2746–2752 (2022).
van der Kooij, M. K. et al. Age does matter in adolescents and young adults versus older adults with advanced melanoma; a national cohort study comparing tumor characteristics, treatment pattern, toxicity and response. Cancers 12, 2072 (2020).
Wong, S. K. et al. Efficacy and safety of immune checkpoint inhibitors in young adults with metastatic melanoma. Eur. J. Cancer 181, 188–197 (2023).
Cluxton, C. & Naidoo, J. Prospective clinical trials to advance the study of immune checkpoint inhibitor toxicity. Curr. Oncol. 30, 6862–6871 (2023).
Bolte, L. A. et al. Association of a Mediterranean diet with outcomes for patients treated with immune checkpoint blockade for advanced melanoma. JAMA Oncol. 9, 705–709 (2023).
Verheijden, R. J. et al. Physical activity and checkpoint inhibition: association with toxicity and survival. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djad245 (2023).
Acknowledgements
All figures were created with https://www.biorender.com. Owing to space and reference limitations, we were unable to discuss and cite all interesting studies performed in this field.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
K.P.M.S. has advisory relationships with Bristol Myers Squibb, Novartis, MSD, Pierre Fabre and AbbVie and has received honoraria from Novartis, MSD and Sairopa and research funding from BMS, Philips and TigaTx, all paid to the institution. F.v.W. has received advisory and/or speaker fees from Takeda and Johnson & Johnson and research funding from BMS, Takeda, Sanofi, Pfizer, Galapagos and Leo Pharma. A.M.M.E. has received honoraria for advisory relationships from Agenus, Boehringer Ingelheim, BioInvent, BioNTech, Brenus, CatalYm, Epics, GenOway, IO Biotech, IQVIA, ISA Pharmaceuticals, Merck & Co/MSD, Oncimmune, Pfizer, Pierre Fabre, Sairopa, Sellas, Scorpion, SkylineDX, TigaTx and Trained Therapeutics and moreover speaker fees from BMS and MSD and has equity in IO Biotech, Sairopa and SkylineDX. No potential conflicts of interest were disclosed by M.J.M.v.E.
Peer review information
Nature Cancer thanks Michael Dougan and Michael Postow for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suijkerbuijk, K.P.M., van Eijs, M.J.M., van Wijk, F. et al. Clinical and translational attributes of immune-related adverse events. Nat Cancer 5, 557–571 (2024). https://doi.org/10.1038/s43018-024-00730-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-024-00730-3