Abstract
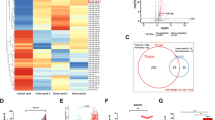
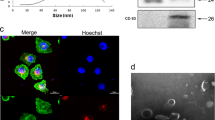
Numerous studies have demonstrated that regulatory T (Treg) cells play an important role in the tumour microenvironment (TME). The aim of this study was to investigate whether VEGFR2 affects the expression of miR-3200-3p in exosomes secreted by tumour cells, thereby influencing Treg senescence in the TME. The results showed that VEGFR2 expression level was the highest in Calu-1 cells, and after transfection with si-VEGFR2, the exosomes secreted from Calu-1 cells were extracted and characterised with no significant difference from the exosomes of the untransfected group, but the expression of miR-3200-3p in the exosomes of the transfected si-VEGFR2 group was elevated. The Cell Counting Kit-8 (CCK-8) and flow cytometry (FCM) results suggested that exosomes highly expressing miR-3200-3p could inhibit Treg cell viability and promote apoptosis levels when treated with Treg cells. Detection of the senescence-associated proteins p16 INK4A and MMP3 by western blot (WB) revealed that exosomes highly expressing miR-3200-3p were able to elevate their protein expression levels. Tumour xenograft experiments demonstrated that exosomes with high miR-3200-3p expression promoted Treg cell senescence and inhibited subcutaneous tumour growth in nude mice. Dual-luciferase reporter assays and RNA pull-down assays showed that miR-3200-3p could be linked with DDB1. Overexpression of DDB1 reverses changes in DCAF1/GSTP1/ROS protein expression caused by exosomes with high miR-3200-3p expression. In conclusion, inhibition of VEGFR2 expression in tumour cells promotes the expression of miR-3200-3p in exosomes secreted by tumour cells. miR-3200-3p enters the TME through exosomes and acts on DDB1 in Treg cells to promote senescence of Treg cells to inhibit tumour progression.





Similar content being viewed by others
Data availability
The dataset used and/or analysed in this study is available from the corresponding author on reasonable request.
References
Chen L, Heikkinen L, Wang C, Yang Y, Sun H, Wong G (2019) Trends in the development of miRNA bioinformatics tools. Brief Bioinform 20:1836–1852. https://doi.org/10.1093/bib/bby054
Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK (2014) Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 14:535–546. https://doi.org/10.1038/nrc3775
Fisher SA, Aston WJ, Chee J et al (2016) Transient Treg depletion enhances therapeutic anti-cancer vaccination. Immun Inflamm Dis 5:16–28. https://doi.org/10.1002/iid3.136
Guo H, Chang Z, Wu J, Li W (2019) Air pollution and lung cancer incidence in China: who are faced with a greater effect? Environ Int 132:105077. https://doi.org/10.1016/j.envint.2019.105077
He C, Zheng S, Luo Y, Wang B (2018) Exosome theranostics: biology and translational medicine. Theranostics 8:237–255. https://doi.org/10.7150/thno.21945
He Z, Wang J, Zhu C, Xu J, Chen P, Jiang X, Chen Y, Jiang J, Sun C (2022) Exosome-derived FGD5-AS1 promotes tumor-associated macrophage M2 polarization-mediated pancreatic cancer cell proliferation and metastasis. Cancer Lett 548:215751. https://doi.org/10.1016/j.canlet.2022.215751
Hu Z, Chen G, Zhao Y, Gao H, Li L, Yin Y, Jiang J, Wang L, Mang Y, Gao Y, Zhang S, Ran J, Li L (2023) Exosome-derived circCCAR1 promotes CD8 + T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Mol Cancer 22:55. https://doi.org/10.1186/s12943-023-01759-1
Ji D, Song C, Li Y, Xia J, Wu Y, Jia J, Cui X, Yu S, Gu J (2020) Combination of radiotherapy and suppression of Tregs enhances abscopal antitumor effect and inhibits metastasis in rectal cancer. J Immunother Cancer 8:e000826. https://doi.org/10.1136/jitc-2020-000826
Jin RH, Yu DJ, Zhong M (2018) MiR-1269a acts as an onco-miRNA in non-small cell lung cancer via down-regulating SOX6. Eur Rev Med Pharmacol Sci 22:4888–4897. https://doi.org/10.26355/eurrev
Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science 367:eaau6977. https://doi.org/10.1126/science.aau6977
Lainé A, Labiad O, Hernandez-Vargas H, This S, Sanlaville A, Léon S, Dalle S, Sheppard D, Travis MA, Paidassi H, Marie JC (2021) Regulatory T cells promote cancer immune-escape through integrin αvβ8-mediated TGF-β activation. Nat Commun 12:6228. https://doi.org/10.1038/s41467-021-26352-2
Lee YJ, Shin KJ, Jang HJ, Ryu JS, Lee CY, Yoon JH, Seo JK, Park S, Lee S, Je AR, Huh YH, Kong SY, Kwon T, Suh PG, Chae YC (2023) GPR143 controls ESCRT-dependent exosome biogenesis and promotes cancer metastasis. Dev Cell 58:320–334. https://doi.org/10.1016/j.devcel.2023.01.006
Liang X, Shangguan W, Zhang M, Mei S, Wang L, Yang R (2017) miR-128 enhances dendritic cell-mediated anti-tumor immunity via targeting of p38. Mol Med Rep 16:1307–1313. https://doi.org/10.3892/mmr.2017.6717
Liu X, Si F, Bagley D, Ma F, Zhang Y, Tao Y, Shaw E, Peng G (2022) Blockades of effector T cell senescence and exhaustion synergistically enhance antitumor immunity and immunotherapy. J Immunother Cancer 10:e005020. https://doi.org/10.1136/jitc-2022-005020
Moreno Ayala MA, Campbell TF, Zhang C, Dahan N, Bockman A, Prakash V, Feng L, Sher T, DuPage M (2023) CXCR3 expression in regulatory T cells drives interactions with type I dendritic cells in tumors to restrict CD8+ T cell antitumor immunity. Immunity 56:1613–1630. https://doi.org/10.1016/j.immuni.2023.06.003
Nishii K, Ohashi K, Tomida S, Nakasuka T et al (2022) CD8+ T-cell responses are boosted by dual PD-1/VEGFR2 blockade after EGFR inhibition in EGFR-mutant lung cancer. Cancer Immunol Res 10:1111–1126. https://doi.org/10.1158/2326-6066.cir-21-0751
Qi M, Xia Y, Wu Y, Zhang Z, Wang X, Lu L, Dai C, Song Y, Xu K, Ji W, Zhan L (2022) Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat Commun 13:897. https://doi.org/10.1038/s41467-022-28438-x
Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J, Xuan Z et al (2022) Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res 41:296. https://doi.org/10.1186/s13046-022-02499-8
Relli V, Trerotola M, Guerra E, Alberti S (2019) Abandoning the notion of non-small cell lung cancer. Trends Mol Med 25:585–594. https://doi.org/10.1016/j.molmed.2019.04.012
Russo A, Franchina T, Ricciardi GRR, Smiroldo V, Picciotto M, Zanghì M, Rolfo C, Adamo V (2017) Third generation EGFR TKIs in EGFR-mutated NSCLC: where are we now and where are we going. Crit Rev Oncol Hematol 117:38–47. https://doi.org/10.1016/j.critrevonc.2017.07.003
Sharma A, Johnson A (2020) Exosome DNA: critical regulator of tumor immunity and a diagnostic biomarker. J Cell Physiol 235:1921–1932. https://doi.org/10.1002/jcp.29153
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33. https://doi.org/10.3322/caac.21708
Speiser DE, Ho PC, Verdeil G (2016) Regulatory circuits of T cell function in cancer. Nat Rev Immunol 16:599–611. https://doi.org/10.1038/nri.2016.80
Thommen DS, Schumacher TN (2018) T cell dysfunction in cancer. Cancer Cell 33:547–562. https://doi.org/10.1016/j.ccell.2018.03.012
Wang H, Zeng Z, Yi R, Luo J, Chen J, Lou J (2022) MicroRNA-3200-3p targeting CAMK2A modulates the proliferation and metastasis of glioma in vitro. Bioengineered 13:7785–7797. https://doi.org/10.1080/21655979.2022.2048995
Yan JB, Luo MM, Chen ZY, He BH (2020) The function and role of the Th17/Treg cell balance in inflammatory bowel disease. J Immunol Res 15:2020. https://doi.org/10.1155/2020/8813558
Yang C, Wu S, Mou Z, Zhou Q, Dai X, Ou Y, Chen X, Chen Y, Xu C, Hu Y, Zhang L, Zou L, Jin S, Hu J, Mao S, Jiang H (2022) Exosome-derived circTRPS1 promotes malignant phenotype and CD8+ T cell exhaustion in bladder cancer microenvironments. Mol Ther 30:1054–1070. https://doi.org/10.1016/j.ymthe.2022.01.022
Yu JX, Upadhaya S, Tatake R, Barkalow F, Hubbard-Lucey VM (2020) Cancer cell therapies: the clinical trial landscape. Nat Rev Drug Discov 19:583–584. https://doi.org/10.1038/d41573-020-00099-9
Zhang A, Ren Z, Tseng KF et al (2021) Dual targeting of CTLA-4 and CD47 on T(reg) cells promotes immunity against solid tumors. Sci Transl Med 13:eabg8693. https://doi.org/10.1126/scitranslmed.abg8693
Zhang M, Shi H, Zhang C, Zhang SQ (2019) MiRNA-621 inhibits the malignant progression of non-small cell lung cancer via targeting SIX4. Eur Rev Med Pharmacol Sci 23:4807–4814. https://doi.org/10.26355/eurrev
Zhang X, Xu Y, Ma L et al (2022) Essential roles of exosome and circRNA_101093 on ferroptosis desensitization in lung adenocarcinoma. Cancer Commun (Lond) 42:287–313. https://doi.org/10.1002/cac2.12275
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P (2020) Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine 15:6917–6934. https://doi.org/10.2147/ijn.S264498
Zhang Z, Xing T, Chen Y, Xiao J (2018) Exosome-mediated miR-200b promotes colorectal cancer proliferation upon TGF-β1 exposure. Biomed Pharmacother 106:1135–1143. https://doi.org/10.1016/j.biopha.2018.07.042
Zhao S, Li W, Yu W, Rao T, Li H, Ruan Y, Yuan R, Li C, Ning J, Li S, Chen W, Cheng F, Zhou X (2021) Exosomal miR-21 from tubular cells contributes to renal fibrosis by activating fibroblasts via targeting PTEN in obstructed kidneys. Theranostics 11:8660–8673. https://doi.org/10.7150/thno.62820
Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, Zhang Z, Cai S, Xu Y, Li X, He X, Zhong X, Li G, Chen Z, Li D (2020) Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol 13:156. https://doi.org/10.1186/s13045-020-00991-2
Zhao Y, Guo S, Deng J, Shen J, Du F, Wu X, Chen Y, Li M, Chen M, Li X, Li W, Gu L, Sun Y, Wen Q, Li J, Xiao Z (2022) VEGF/VEGFR-targeted therapy and immunotherapy in non-small cell lung cancer: targeting the tumor microenvironment. Int J Biol Sci 18:3845–3858. https://doi.org/10.7150/ijbs.70958
Zheng JB et al (2022) Glucose metabolism inhibitor PFK-015 combined with immune checkpoint inhibitor is an effective treatment regimen in cancer. Oncoimmunology 11:2079182. https://doi.org/10.1080/2162402x.2022.2079182
Zhu J, Liu B, Wang Z, Wang D, Ni H, Zhang L, Wang Y (2019) Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 9:6901–6919. https://doi.org/10.7150/thno.37357
Funding
This work was supported by Xuzhou Medical University Affiliated Hospital Science and Technology Development Fund (XYFM2021047).
Author information
Authors and Affiliations
Contributions
XJ designed the study. KH, CD, and CH carried out the experiment and wrote the paper. KH and DY analysed the data and discussed the result. JL, KH, and XJ revised the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to participate
The study protocol was proved by the Animal Ethics Committee of The Affiliated Lianyungang Hospital of Xuzhou Medical University. No patients were involved in this study.
Animal ethics
The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985). The study protocol was approved by the Animal Ethics Committee of The Affiliated Lianyungang Hospital of Xuzhou Medical University
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hui, K., Dong, C., Hu, C. et al. VEGFR affects miR-3200-3p-mediated regulatory T cell senescence in tumour-derived exosomes in non-small cell lung cancer. Funct Integr Genomics 24, 31 (2024). https://doi.org/10.1007/s10142-024-01305-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10142-024-01305-2