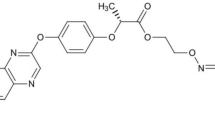
Abstract
Purpose
The decline in organic matter content in many agricultural soils results in a dramatic decrease in their ability to retain xenobiotics. Due to its carbon-rich nature and sorptive behaviour, digestate used as soil amendment can counteract this trend. This study investigated the sorption efficiency of the herbicide oxyfluorfen and the fungicide boscalid by a digestate from olive pomace only, and by a loamy calcareous agricultural soil before and after its amendment with 2 and 5% (w/w) digestate.
Methods
To investigate the surface micromorphology and the functional groups of the digestate, scanning electron microscopy (SEM-EDX) and Fourier-transform infrared (FTIR) spectroscopy were employed, respectively. Adsorption kinetics and adsorption/desorption isotherms of the compounds on the digestate and the soil were performed. Adsorption data were described using the Henry, Freundlich, Langmuir and Temkin equations.
Results
Both oxyfluorfen and boscalid reached the steady state on both substrates in approximately 2 h according to a pseudo-second order model, thus denoting a prevalent chemisorptive interaction. The Freundlich model was generally the best fit for both molecules on any substrate. The KFads values for oxyfluorfen on the digestate, soil, soil + 2% digestate, and soil + 5% digestate were, respectively, 7158, 19, 60 and 170 L kg−1, while for boscalid, in the same order, they were 3700, 11, 37 and 31 L kg−1, at a temperature of 20 °C. The desorption of both compounds from the non-amended and amended soil and, especially, from the digestate was quite slow and incomplete, indicating the occurrence of a hysteretic process. Highly significant correlations were found for both molecules between the adsorption and desorption parameters of all adsorbents and their organic carbon content.
Conclusion
This study confirms the prominent role of organic matter in the retention/release of pesticides in soil. It is expected that the addition of digestate to soil can reduce the risk of transport of toxic compounds in natural waters and/or limit their uptake in edible plant organs.
Similar content being viewed by others
1 Introduction
It is widely recognized that a significant contribution to global warming comes from the intensive and continuous use of fossil fuels such as oil and coal. Therefore, alternative sources of renewable energy, including bioenergy, are currently researched and developed. Waste biomass is certainly an alternative and renewable source for green energy production. The REPowerEU Plan of the European Commission has the goal to substitute fossil fuels and accelerate clean energy transition, sustaining annual increases of biomethane production and use in European Countries in order to reach 35 billion cubic meters of biomethane by 2030 (EC 2022). The wide availability of biowaste from agriculture, agri-food industry and civil activities makes its recycling sustainable both from an environmental and an economic point of view. Nowadays, several thermochemical and biological technologies are adopted to transform waste biomass into energy, such as pyrolysis, gasification, hydrothermal carbonization and anaerobic digestion (AD) (Singh and Kalia 2017). The AD is a biomass conversion process conducted by microorganisms under anaerobic conditions to produce biogas, which is a mixture of CH4, CO2, and small quantities of other gases (Ugwu et al. 2022). By means of this process, it is possible to satisfy both the need for new energy sources and the demand for organic waste recycling and carbon sequestration. The by-product of AD is a heterogeneous semi-solid material containing approximately 60–80% (on d.w.) of undecomposed organic fraction which after solid/liquid separation originates a clarified liquid component called ‘liquid digestate’ and a solid fraction called ‘solid digestate’ or simply ‘digestate’ (Wang and Lee 2021). Digestate can be subjected to further technological processes, such as pyrolysis, composting or vermicomposting, or used directly as soil amendment (Quan et al. 2023).
The use of by-products of bioenergy to improve soil quality and fertility seems very attractive for the perfect compliance with the principles of circular economy, which is a need increasingly invoked by scientists and widely accepted by the population. The Apulia Region has a strong vocation and tradition for the cultivation of olive trees and contributes approximately one-third of the national production of olives to be transformed into virgin olive oil for human consumption (Valenti et al. 2017). Pomace is the main by-product of olive pressing to produce olive oil, commonly a two-phase procedure. Pomace can have several uses including animal feed or non-food oil extraction. In most cases, pomace is mixed with plant and animal wastes and addressed to AD plants for biogas production. The physicochemical characteristics of pomace and its very high moisture content make it very well suited for microbiological conversion.
Recently, in oil mill industry, the practice of directing pomace alone to AD plants is becoming more and more spread, as it appears to be the most economically convenient choice. This practice releases large quantities of a particular type of digestate quite different from the more common ones obtained from mixed ingestate. The properties of this novel digestate are very poorly investigated.
The high content of organic carbon and macro- and micronutrients make this material particularly suitable as soil fertilizer (Cristina et al. 2020). In general, digestate can represent an important tool for the development of sustainable agricultural practices as it allows the reuse of organic waste and provides significant quantities of organic matter as well as nutritional elements in the soil. Furthermore, digestate produced exclusively from plant residues does not present a significant load of inorganic and organic contaminants which could instead be present in digestates obtained from urban organic waste or sewage sludge. However, the direct spreading of digestate on the soil has also raised concern for the emission of bad odours and nitrogen oxides into the atmosphere, for the presence of plant and animal pathogens (Peng and Pivato 2019) and for the transport of excessive nitrogen and mineral elements in natural waters with consequent eutrophication (Wang and Lee 2021).
The quality and the optimum utilization of digestate depend mainly on its physicochemical characteristics, which in turn depend on the feedstock used and the operating conditions of the AD process, including biomass moisture, temperature, and permanence into the digester. The common use of mixed biomass to feed AD is the reason for the wide variability of digestate properties, while the use of only one type of biomass, such as olive pomace alone, ensures more stable characteristics of the digestate. The reactive functional groups present on digestate surface, its microstructure and porosity, along with large surface area, are essential for a good performance as adsorbent of organic compounds (Loffredo 2022). Hence, it is expected that when digestate is incorporated into soil, it can act as efficient biosorbent of organic xenobiotics, thus regulating their bioavailability for plants and soil microorganisms, and preventing their entry into edible plant organs.
The extensive or improper use of pesticides that has characterized conventional agriculture in recent decades has endangered the environment and wildlife. If highly soluble pesticides are particularly dangerous for the quality of natural waters, hydrophobic ones are especially dangerous for animals and humans as they can accumulate in vital organs and cause diseases and death. Furthermore, hydrophobic compounds are by far the most persistent in the environment as they can bind strongly to organic matter and be recalcitrant to biodegradation. Oxyfluorfen and boscalid are two pesticides widely used for the protection of olive groves and vineyards which are among the most typical and economically important crops in the Mediterranean area. Oxyfluorfen [2-chloro-1-(3 ethoxy–4-nitrophenoxy)-4-(triflouromethyl) benzene] is a diphenyl-ether used as broad-spectrum herbicide for the control of annual broadleaf and grassy weeds in pre- and post-emergence of a great variety of crops (Sondhia 2010). Oxyfluorfen is persistent and relatively immobile in soil; however, under certain conditions, such as sandy soils with low organic matter content or when incorrectly used, it can contaminate surface waters through spray drift and runoff. Boscalid [2-chloro-N-[2-(4-chlorophenyl)phenyl]pyridine-3-carboxamide] is a broad-spectrum fungicide used particularly against pathogens in specialized high-end crops, such as fruit and horticultural plants (Chen and Zhang 2010). Boscalid is persistent in soil and especially in aquatic systems (USEPA 2010). In 2016, the European Commission has included boscalid in the list of suspected endocrine disrupting chemicals (EC 2016).
Adsorption is one of the most effective and inexpensive physical processes for the removal of pollutants from soil and wastewater (De Wilde et al. 2009; Loffredo 2022). Adsorption is the first process pesticides undergo soon after their distribution in soil; therefore, it is of primary importance in controlling the overall fate of pesticides in the soil-plant system. Adsorbed compounds are not bioavailable in the short period for plants and microorganisms, which guarantees their limited accumulation in ecosystems and prevents their entry into the human and animal food chain.
To date, the adsorbent properties of digestate towards organic pollutants have been poorly studied (Loffredo et al. 2021). Yao et al. (2020) used raw and modified digestate to remove by adsorption numerous dyes from wastewater; they concluded that digestate could represent an innovative and inexpensive adsorbent for that type of pollutants. Mukherjee et al. (2016) prepared soil/digestate biomixtures at 5 and 30% (w/w) digestate and used them to remove various pesticides. In addition to being used for the adsorption of contaminants, this material is also used to adsorb micronutrients in soil (Diana et al. 2009).
Giving the importance of this process and the high cost of synthetic adsorbents, there is an increasing demand for low-cost and readily available bioadsorbents able to remove or control contaminants in both liquid and solid environmental matrices (Loffredo et al. 2021; Mukherjee et al. 2016). In this work, we quantitatively and qualitatively evaluated and modelled the adsorption/desorption of the pesticides oxyfluorfen and boscalid on/from a new anaerobic digestate obtained from the sole olive pomace. Furthermore, we estimated the contribution of digestate supplemented at doses of 2 and 5% (w/w) to the overall sorption capacity of the two compounds by an agricultural loamy soil.
2 Materials and methods
2.1 Chemicals, digestate and soil
Oxyfluorfen and boscalid at purity ≥ 98% and 99%, respectively, were purchased from Sigma Aldrich s.r.l., Milano, Italy. The molecular mass, water solubility and log Kow of oxyfluorfen are 361.7 g mol−1, about 1 mg L−1 at 25 °C and 4.73, respectively, while they are 343.2 g mol−1, 4.6 mg L−1 and 2.96 for boscalid (PubChem 2023). Chemical structures of the compounds are depicted in Fig. 1. All other chemicals used in the experiments were of extra pure grade. Methanol (HPLC grade) solutions of oxyfluorfen and boscalid were prepared individually at a concentration of 2000 mg L−1; subsequently working solutions were prepared by combining appropriate aliquots of each methanol solution and diluting with a mixture of double distilled water/methanol (95:5, v/v).
The digestate sample was obtained exclusively from two-phase olive pomace in the Agrolio s.r.l. company, Andria, Italy. Before use, the digestate was air-dried, ground with mortar and pestle and 0.5-mm sieved.
The soil used was a loamy calcareous agricultural soil sampled at 0–20 cm depth at an experimental station belonging to the University of Bari and located at Valenzano, South Italy. The soil was air-dried, sieved at particle size < 2 mm to remove the coarser fraction and thoroughly homogenized. Soil properties were determined according to conventional methods (Carnimeo et al. 2022). Some soil properties are: 4% moisture, pH value of 8.0, 3.3% total organic C (on dry weight), 15.4% total CaCO3 (on dry weight). Other soil properties are reported in Carnimeo et al. (2022). Due to its overall characteristics, particularly its organic matter content, this soil can be considered representative of many agricultural soils in Southern Italy and, in general, in the Mediterranean area.
2.2 Digestate characterization
Basic characterization of the digestate, including moisture, pH, electrical conductivity and ash content, was carried out according to conventional procedures. The ash content was determined by burning the sample in a muffle furnace at a temperature of 550 °C for 6 h. Elemental analysis was determined in triplicate using a CHNS-O Elemental Analyser Flash 2000 (Thermo Scientific) calibrated with a BBOT [2,5-bis-(5-tert-butyl-benzoxazol-2-yl)-thiophene] standard (ThermoQuest Italia s.p.a.). The specific surface area of the digestate was determined according to the Brunauer, Emmett and Teller (BET) methodology (Brunauer et al. 1938). The BET analysis is based on the measurement of N2 adsorption/desorption at a fix temperature and within a selected relative pressure range. A previously degassed 0.27 g sample at 110 °C for 16 h was analysed by a QuantaChrome Autosorb AS-1 analyser (Quantachrome Instrumentes, Boynton Beach, FL, USA), varying the N2 relative pressure from 0.05 to 0.30 at 77.3 K. Some properties of the air-dried digestate are referred in Table 1.
Scanning electron microscopy (SEM) analysis was performed to investigate the surface micromorphology of the digestate. A little amount of sample was fixed with an adhesive carbon tape, metallized with Au/Pd and analysed using a Hitachi TM3000 microscope (Hitachi, Tokyo, Japan) equipped with an energy-dispersive X-ray (EDX) Oxford Swift ED3000 microanalysis system. By adopting an accelerating potential of 15 kV, backscattered electrons were detected, and SEM micrographs were obtained at both 600× and 1800× magnifications.
Fourier transform infrared (FTIR) spectroscopy was adopted to evaluate the surface functional groups of the digestate before and after 16-h interaction with an aqueous solution of oxyfluorfen and boscalid at the individual concentration of 2 mg L−1. For the purpose, a mixture of 2 mg of air-dried digestate (before and after interaction) and 400 mg of dried KBr (FTIR grade) was homogenized using an agate mortar and pestle. The mixture was kept under vacuum at a pressure of 6000 kg cm−1 for 10 min to obtain a thin pellet. The sample was then analysed using a Thermo Nicolet iS50 FTIR spectrophotometer equipped with Nicolet Omnic 6.0 software. The FTIR spectra were recorded in transmittance mode in the range of 4000–400 cm−1 wavenumber with a resolution of 2 cm−1 and 64 scans min−1.
2.3 Sorption and desorption
2.3.1 Effects of the solution/adsorbent ratio
The efficiency of different solution/digestate ratios to remove the two compounds was evaluated in slurry-type experiments. For the purpose, aliquots of 20, 40, 50, 100 and 200 mg of digestate were interacted with a volume of 20 mL of a mixture of oxyfluorfen and boscalid at the individual concentration of 2 mg L−1, thus obtaining solution/adsorbent ratios equal to 1000, 500, 400, 200 and 100, respectively. All samples were mechanically shaken at 350 g for 16 h at a temperature of 20 ± 1 °C to reach the steady state. Previous experiments showed that both compounds reached sorption equilibrium in few hours. The suspensions were then centrifuged at 10,000 g for 10 min and a volume of 15 mL of supernatant solution was collected from each sample to determine the equilibrium concentration of the compounds using ultra-high performance liquid chromatography (UHPLC) analysis (see Section 2.4).
The compound concentration on the adsorbent, qt (mg kg−1), was calculated from the equation: qt = (C0 – Ct) V/m, being C0 (mg L−1) and Ct (mg L−1) the initial and the final (16 h in these experiments) concentration of the compound in solution, respectively, V (mL) the solution volume and m (g) the adsorbent mass.
2.3.2 Sorption kinetics and isotherms
Sorption kinetics were carried out in batch mode to evaluate the concentration of adsorbed oxyfluorfen and boscalid on the digestate and on the soil at different times of the sorption process and to establish the equilibrium time. Hence, volumes of 20 mL of an aqueous solution of the two compounds at individual concentration of 2 mg L−1 and pH equal to 7.37 were interacted with 20 mg of digestate (solution/adsorbent ratio equal to 1000) in glass centrifuge tubes. In the experiments with the soil, volumes of 10 mL of the above solution were interacted with 2.5 g of soil (solution/adsorbent ratio equal to 4). Blank control samples of digestate and soil (not interacted with the compounds) were included in the experiments to check for artifacts and matrix effects eventually occurring in the analytical procedure. No significant effects were observed in these experiments.
All samples were then stirred for 0.1, 0.25, 0.5, 1, 2, 4, 8 and 16 h in the dark at a temperature of 20 ± 1 °C. At each time, the samples were centrifuged at 10,000 g for 15 min at 10 °C and the supernatants were analysed by UHPLC to determine the solution concentration of each compound as described in Section 2.4. A volume of 20 mL of the pesticide solution alone (without adsorbents) was stirred for 16 h and subjected to the same procedure adopted for digestate and soil samples to test the stability of the two compounds and their possible adsorption to the batch container surface. Neither degradation nor adsorption was detected in these trials. All experiments were triplicated. The amount of compound adsorbed on the unit of substrate at time t, qt (mg kg−1), was calculated according to the equation described in Section 2.3.1. Comparing two by two the concentrations of the adsorbed compound at any time with the Student’s t test at P ≤ 0.05, the equilibrium time of each compound was settled when there was no significant difference between the values at two successive times.
Adsorption isotherms of oxyfluorfen and boscalid onto digestate, non-amended soil and soil amended with digestate at 2% and 5% (w/w) were studied. Aliquots of 20 mg of digestate or 2.5 g of non-amended or amended soil were added, respectively, with 20 mL or 10 mL of aqueous mixtures of the two compounds at concentrations of 0.1, 0.2, 0.5, 1 and 2 mg L−1, in glass containers. The suspensions were then stirred for 16 h at 20 ± 1 °C, in the dark, to reach the equilibrium condition. Subsequently, samples were centrifuged at 10,000 g for 10 min at 10 °C and the equilibrium concentration of each compound in the supernatant solution was measured using the UHPLC protocol described in Section 2.4. All experiments were conducted in triplicate.
Desorption of oxyfluorfen and boscalid from any adsorbent was started immediately after the adsorption trials using the samples added with the maximum concentration of the two compounds (2 mg L−1). At each desorption step, an aliquot of 16 mL (in the case of digestate) or 7 mL (in the case of soil) of supernatant solution was replaced with double distilled water; the sample was stirred again for 16 h at 20 ± 1 °C and centrifuged in the conditions described above. Four desorption steps were carried out for each adsorbent. Residual compounds in the supernatants were analysed using the UHPLC procedure reported in the next section.
2.4 UHPLC analysis
Before chromatographic analysis, samples were filtered through 0.45 μm Millipore™ cellulose acetate filters. The concentration of the two compounds in solution was measured using a UHPLC (Dionex Ultimate 3000 RSLC, Waltham, MA, USA) instrument equipped with an HPG-3200 RS pump, a WPS-3000 autosampler and a TCC-3000 column compartment connected to a Supelco™ LC-18 column (250 mm × 4.6 mm × 5 μm). The mobile phase, which flowed at 0.8 mL min−1 was water/methanol (30/70, v/v). A DAD-3000 RS diode array detector (Dionex Ultimate 3000 RSLC, Waltham MA, USA) at wavelengths of 210 and 207 nm for oxyfluorfen and boscalid, respectively, was used; retention times of the compounds were, in the order, 2.8 and 6.2 min. The external standard method was used to quantify the two molecules.
2.5 Theoretical sorption models
Two theoretical models were used to describe sorption kinetics data, determine the kinetic rate constants, and investigate on sorption mechanisms. The pseudo-first-order (PFO) and the pseudo-second-order (PSO) equations were used. The non-linear form of the PFO model of Lagergreen (1907) is based on the adsorbent capacity and is given by: qt = qe (1 – exp– k1t) (Kumar 2006), where qe and qt are, respectively, the concentration of the adsorbed compound (mg kg−1) at equilibrium and at time t, and k1 (h−1) is the PFO rate constant. The PSO kinetic model attributes a prominent role to the sorption at the equilibrium condition; the non-linear form is expressed as: \({{\text{q}}}_{{\text{t}}}= \frac{{{\text{q}}}_{{\text{e}}}^{2}{{\text{k}}}_{2}{\text{t}}}{1\;+\; {{\text{k}}}_{2}{{\text{q}}}_{{\text{e}}}{\text{t}}}\) (Ho 2006), where qt and qe have the same meaning of the PFO model and k2 (kg mg−1 h−1) is the PSO rate constant. Using the solver add-in component of Microsoft® Excel®, a trial-and-error procedure was adopted to estimate the PFO and PSO kinetic parameters with the non-linear regression method (Ho 2006). The accordance between the experimental data and each model was estimated by the correlation coefficient: \({\text{r}}=\sqrt{\frac{\sum {\left({{\text{q}}}_{{\text{t}}}{\text{m}}\;-\;\overline{{{\varvec{q}} }_{{\varvec{t}}}}\right)}^{2}}{\sum {\left({{\text{q}}}_{{\text{t}}}{\text{m}}\;-\;\overline{{{\text{q}} }_{{\text{t}}}}\right)}^{2}\;+\; \sum {\left({{\text{q}}}_{{\text{t}}}{\text{m}}\;-\;{{\text{q}}}_{{\text{t}}}\right)}^{2}}}\), where qtm is the theoretical adsorbed concentration of the compound (mg kg−1) at time t, qt is the experimental concentration (mg kg−1), and \(\overline{{{\text{q}} }_{{\text{t}}}}\) is the average qt.
To describe the sorption and desorption isotherm data obtained from the experiments, the two-parameter non-linear equations of Freundlich, Langmuir and Temkin, and the linear Henry equation were applied. The Freundlich equation is given by: qe = KF Ce1/n, where qe (mg g−1) and Ce are, respectively, the concentration of the compound on the adsorbent and in solution at the equilibrium condition, 1/n expresses the degree of nonlinearity of the sorption process, the reciprocal n indicates the sorption intensity, and KF (L kg−1) is the Freundlich sorption constant which estimates the efficacy of the adsorbent. The Freundlich model fits well when the sorbate forms a multilayer on the adsorbent having surface heterogeneity. The Langmuir model is described by the equation: qe = (KLCeb)/(1 + KLCe), where qe and Ce have been previously defined, b (mg kg−1) is the maximum adsorption, and KL (L kg−1) is the Langmuir constant which expresses the energy of the process which, in turn, estimates the affinity of the solute for the adsorbent. The Langmuir model is proper when the adsorbent surface is homogeneous, the solute molecules form a monolayer on the adsorbent without interacting with each other. The Temkin isotherm predicts a logarithmic reduction of the adsorptive sites and energy during the process and is given by: qe = Bln (ATCe), where qe and Ce have been already defined, AT is the Temkin equilibrium constant (L kg−1), and B (J mol−1) is the Temkin coefficient quantifying the enthalpy of the process. The B values are calculated from: B = RT/bT, where bT is a constant related to the heat of adsorption, T is the absolute temperature (K) and R is the universal gas constant (8.314 J mol−1 K−1). In the present study, the Freundlich (KF and 1/n), Langmuir (b and KL) and Temkin (B and AT) parameters were all calculated by the non-linear regression method using the solver add-in component of Microsoft® Excel® and a trial-and-error procedure which allowed to minimize the sum of squared residuals (SSR) between experimental and theoretical data. The appropriateness of the theoretical model was established on the basis of both the correlation coefficient, r, and SSR value. Finally, the linear Henry equation: qe = Kd Ce, where qe and Ce have the same meaning of the previous equations, was also employed to calculate the distribution coefficient, Kd (L kg−1), from the slope, and the organic-carbon-partition coefficient, KOC, by: KOC = (Kd × 100)/(%OC)) which expresses the quantity of solute adsorbed per unit of organic carbon of the adsorbent.
The desorption process, i.e., the decrease of the adsorbed compound due to the progressive dilution of the solution, was also theoretically described using the Freundlich and the Henry equations. This allowed to calculate KFdes, 1/ndes, Kddes and KOCdes as described for the corresponding sorption parameters. The adsorption and desorption parameters obtained from the Henry and Freundlich models were correlated to the carbon content (%) of the corresponding adsorbent used. Finally, the hysteresis coefficient, H, was calculated from the expression: H = (1/ndes)/(1/nads) (Barriuso et al. 1994). A H value < 1 denotes a hysteretic condition, i.e., a difficult, slow, and incomplete desorption.
3 Results and discussion
3.1 Digestate characterization
3.1.1 Physicochemical characterization
As a result of the microbial activity during the AD process, readily degradable organic compounds of the ingestate are converted to biogas, while a substantial part of larger and more complex molecules, such as cellulose, hemicellulose and lignin, remain in the digestate. It is the lignocellulosic component, as well as macro- and micronutrients, that make the digestate an appreciable soil amendment.
Table 1 shows the main characteristics of the digestate used in this study. The pH value was slightly higher, and the N and ash contents were much lower, than those reported in the literature for digestates obtained from mixed plant and animal residues (Mukherjee et al. 2016). Also the electrical conductivity of our digestate was much lower than that measured for other digestates obtained from animal waste (Valentinuzzi et al. 2020). These results were expected considering the different origin and composition of the feedstock used. The alkaline reaction of our digestate suggests a valuable use for pH correction of acidic soils. Furthermore, when incorporated into soil, it might control important processes, such as sorption/desorption of organic and inorganic compounds, which is generally more effective at non-neutral pH. Although there is a certain variability for the total N content, typical values for digestate obtained from animal manure or mixed biomass are around 2–4% (Wang and Lee 2021), while lower values (~ 1%) are usually reported for digestates obtained exclusively from vegetable materials (Cao et al. 2020). On the other hand, the organic carbon content measured in our sample is comparable to those reported for digestates obtained from composite biomass (Mukherjee et al. 2016) or manure-based feedstock (Valentinuzzi et al. 2020). A high C content of the material is essential when it is used as soil improver for both C storage and adsorption of contaminants.
The elemental analysis performed provided the elemental composition of digestate and allowed to calculate the atomic ratios of elements (Table 1). During the biochemical conversion of biomass, anaerobic conditions determine an increase of C content and a decrease of O and H contents, compared to the original biomass (Wang and Lee 2021). The atomic H/C, O/C and (O+N)/C ratio express, respectively, the degree of aromaticity, hydrophilicity and polarity index of the material, which depends on the nature of the raw biomass feeding AD and on the operating conditions adopted. The relatively high H/C ratios is indicative of low aromaticity of the material and consequently of low recalcitrance to biodegradation when added to the soil.
3.1.2 Morphological analysis: Scanning electron microscopy (SEM)
Sorptive properties of an adsorbent are related to its surface characteristics and to the abundance and type of porosity. Therefore, SEM analysis is an important tool to visually evaluate the micro-morphological aspects of the surface and the physical state of the material. The micrographs of the digestate obtained at 600× (Fig. 2A, C) and 1800× (Fig. 2B) magnifications showed surface details that are typical of material produced through AD of plant residues. The scans evidenced a heterogeneous and porous surface with cavities and incrustations as well as a diffuse roughness. A porous structure and a large surface extension are essential features for adsorbents, as they assure the diffusion of sorbate to inner surfaces and sites. The digestate micrographs also revealed the presence of microparticles of few µm, irregular ridges and channels, fibers and pores originating from vascular tissue (Fig. 2A, B). SEM images of digestate are scarce in the literature. A rather different microstructure was observed in a digestate obtained from mixed feedstock (Loffredo et al. 2021).
The EDX spectrum evidenced the presence on digestate surface of various elements, such as Ca, Mg, K, P, Al, Si and so on, that are typical of a plant-based material (Fig. 2D). During AD, some alkali and alkaline earth metals such as Ca, Mg, K and Na are retained by the solid fraction causing an increase of pH and EC values.
3.1.3 Fourier transform infrared spectroscopy (FT-IR)
The FTIR spectroscopy provided information on the surface functional groups of the digestate and on their modifications after the adsorption of the two compounds (Fig. 3). This technique, therefore, is useful to understand the type of binding reactions between the material and the solute molecules. The FTIR spectra of the digestate and its interaction products with oxyfluorfen and boscalid are presented, respectively, in Fig. 3a–c. The digestate spectrum (Fig. 3a) was characterized by several peaks, strictly related to the composition of the starting biomass, including kernels, peel, and parts of olive pulp. In particular, the peaks observed and their attributions were as follows: (i) 3419 cm−1, typical of O–H and N–H stretching, also hydrogen bonded; (ii) 2922 and 2852 cm−1, C-H asym and sym stretching of aliphatic CH2 groups; (iii) 1736 cm−1, due to the stretching of C=O of esters; (iv) 1650 cm−1, possibly ascribed to asymmetric COO− and C=O stretching of ketones, conjugated to aromatic rings of lignin, and/or C=O stretching of primary amides; (v) 1544 and 1512 cm−1, respectively, N–H bending of secondary amides and associated N-H and C-N bending of secondary amides; (vi) 1463 cm−1, asymmetric bending of C-H of CH3 groups of lignin and xylan; (vii) 1424 cm−1, possibly ascribed to conjugated C=N systems and amino functionalities, and OH deformation and C-O stretching of phenolic groups; (viii) 1384 cm−1, can be attributed C–H deformation of CH2 and CH3 groups and COO− asymmetric stretching; (ix) 1264 cm−1, C-O stretching of aromatic ethers; (x) 1161 and 1114 cm−1, sym C-O-C stretching vibrations in cellulose and hemicellulose and/or aliphatic -OH, as well as C-O stretching of primary and secondary alcohols, in-plane strain of aromatic CH; (xi) 1046 cm−1, due to O-H stretching of polysaccharide structures such as cellulose and hemicellulose; and, finally, (xii) 897 cm−1, likely attributable to the presence of vinyl radicals.
The FTIR spectra obtained after the interaction of the digestate with the two pesticides (Fig. 3b, c) generally showed the same absorption bands, evidencing some modifications especially in relation to the band intensity. In general, in both interaction products, a reduction in the intensity of the aliphatic bands (2922 and 2852 cm−1) could be observed. In detail, the FTIR spectrum of the digestate-oxyfluorfen interaction product showed a reduction of the bands at 1650 and 1509 cm−1, and a marked inversion of the intensity of the bands around 1460 and 1423 cm−1 (Fig. 3b). More pronounced changes were observed in the digestate-boscalid interaction product (Fig. 3c). In particular, the bands around 1736 and 1509 cm−1 were more intense, denoting the involvement of these groups in the adsorption of the fungicide molecule on the digestate. Furthermore, compared to the digestate spectrum (Fig. 3a), the digestate-boscalid spectrum showed an inversion of the intensity of the bands around 1460 and 1424 cm−1, as well as a sharp reduction in the absorption intensity of the band at 1384 cm−1 (Fig. 3b).
Overall, the FTIR analysis of the digestate indicated a high content of ligno-cellulosic residues and recalcitrant aromatic units. The O- and N-containing groups of both the digestate and the two molecules examined likely allowed the formation of hydrogen bonds with complementary groups. Furthermore, we can assume that hydrophobic partitioning mechanisms occurred between the aromatic structures of the digestate and the structural phenolic units of both pesticides (Lou et al. 2017; Provenzano et al. 2018).
3.2 Sorption and desorption
3.2.1 Effects of the solution/adsorbent ratio
Five different solution/digestate ratios (100, 200, 400, 500 and 1000) were chosen to evaluate the effects of digestate mass on the retention efficiency of the compounds and to select the most suitable ratio in subsequent adsorption experiments. As clearly illustrated in Fig. 4, increasing the solution/adsorbent ratio, the quantities of adsorbed compounds at equilibrium per unit mass of digestate increased significantly. In particular, doubling the solution/digestate ratio from 500 to 1000, the quantities of adsorbed compounds approximately doubled (from 808 to 1623 µg g−1 and from 773 to 1596 µg g−1 for oxyfluorfen and boscalid, respectively). Similar findings were reported in a previous study by Loffredo et al. (2021). We can assume that a higher solution/digestate ratio increases the availability of reactive sites in the adsorbent, especially the less accessible ones located in the innermost pores. The results obtained in this study are in agreement with the findings of Yao et al. (2020) who observed an increasing solute (dye) adsorption with increasing solution/adsorbent ratio; the authors concluded that at low ratios fewer sites of the adsorbent were available and the monolayer condition was more likely.
3.2.2 Adsorption kinetics
Adsorption kinetics data of the two compounds on both digestate and unamended soil showed that the stationary state was reached in about 2 h (Fig. 5), which is a much shorter time than that recently reported for boscalid on various soils (Bhatt et al. 2023) (up to 12 h). At equilibrium, the amounts of oxyfluorfen and boscalid retained by the digestate were about 1600 mg kg−1 for both, while they were 6.44 and 5.96 mg kg−1, respectively, on the unamended soil (Table 2). The shape of the kinetics curves suggests that adsorption occurred at different rates, being the first phase (adsorption on more external sites) almost instantaneous, followed by a much slower phase of intraparticle diffusion of the solute and physico-chemical interaction with appropriate functional groups of the substrate (Mukherjee et al. 2016). Experimental data obtained from kinetics experiments were described with the PFO and PSO models (Ho 2006; Ho and Mckay 1999; Kumar 2006). Applying the non-linear regression method and fitting the data of each compound into the PFO and PSO models, the r values were higher and the SSR values were lower with the PSO equation, indicating a better accordance of experimental data with the latter model (Table 2). The PSO model assumes that the limiting stage of adsorption is the type of interaction (adsorptive mechanism) rather than the mass transfer from the solution to the adsorbent surface (Yao et al. 2020). Furthermore, the PSO model is indicative of chemisorption, which assumes the formation of valency forces between the interacting species with sharing or exchange of electrons (Ho and Mckay 1999; Yao et al. 2020).
Few studies are available in the literature on the adsorption of oxyfluorfen and boscalid on soil, and even less on digestate. Barbosa et al. (2021) investigated the relationship between the structure of stable organic matter (humic acids) in forest and agricultural soils and the accumulation of oxyfluorfen in soil. Those authors attributed the strong retention of oxyfluorfen on humic acids of agricultural soils to the numerous possibilities of binding between organic matter and the herbicide; in particular, those researchers highlighted possible hydrogen bonds between -NH2 groups of humic acid and -NO2 groups of the herbicide, and/or bonds between the carbonyl and carboxyl groups of humic acid and H3C-CH2-O-R group of oxyfluorfen, suggesting the preference of this molecule for nitrogenated and oxygenated aliphatic structures of organic matter, as well as hydrophobic interactions with aliphatic chains. Studying the adsorption of some pesticides, including boscalid, on various soils, Bhatt et al. (2023) found very high qe values for boscalid onto some soils and ascribed their results to their high clay, silt and organic carbon contents. Recently, Loffredo et al. (2021) studied the adsorption of some endocrine disrupting chemicals, including boscalid, on a digestate obtained from plant and animal wastes and reported for all molecules the best fit of data in the PSO kinetic model.
3.3 Adsorption and desorption isotherms
3.3.1 Digestate
The adsorption isotherm describes the distribution of solute molecules between the liquid and the solid phase at different solute concentrations and at a fixed temperature. Adsorption and desorption data of oxyfluorfen and boscalid on and from digestate are shown in Fig. 6, while the values of the sorption parameters obtained interpreting the experimental data with the theoretical models are reported in Table 3. The fitting of experimental sorption data in the non-linear Freundlich, Langmuir and Temkin equations and the linear Henry equations allowed to evaluate quantitatively the process and provide indications on sorption mechanisms. The empirical Freundlich model describes multilayer adsorption of solute molecules on a heterogeneous adsorbent surface (Freundlich 1906). Differently, the Langmuir model is more appropriate for adsorbents having homogeneous surfaces, equivalent sorption energies, and when the solute forms a monolayer on the adsorbent, without interaction between the solute molecules (Langmuir 1918). The Temkin isotherm is best suited when the process occurs with logarithmic reduction of available sites and sorption energy and at intermediate solute concentrations. The simple Henry model assumes a linear partition of the solute between the adsorbent and the solution over the entire concentration range tested. To evaluate the consistency of the experimental data with the model, the correlation coefficient, r, and the sum of squared residuals (SSR) were considered. Based on these two parameters, the sorption of oxyfluorfen on the digestate was best described by the Freundlich model (r = 0.997 and lowest SSR), while for boscalid, although the Freundlich model was very well suited (lowest SSR), the best fit seemed to be the Henry model (r = 0.997). These results are consistent with the heterogeneous surface of the digestate evidenced by SEM analysis and with the chemical variability of the surface active sites investigated by FTIR spectroscopy. Based on the Freundlich parameter 1/n, adsorption of oxyfluorfen was S-type (1/n > 1), whereas that of boscalid was C-type (1/n ~ 1). The Giles classification of adsorption isotherms relates the shape of the sorption curve, in particular the slope of the initial portion of the curve, to sorption mechanisms and parameters and is valid both for homogeneous and heterogeneous adsorption surfaces (Giles et al. 1960). The S-type (sigmoidal shape) isotherm of Giles is indicative of vertical orientation of the solute on the reactive sites of the adsorbent, and occurs when, as adsorption proceeds, it is increasingly easier for the solute molecules to find available sites on the absorbent; it is termed ‘cooperative adsorption’. The C-type isotherm can be represented as a straight line and indicates that a proportional distribution of solute molecules occurs between the adsorbent and the solution. In the C-curve, the availability of sorption sites remains constant at all solute concentration up to saturation. Hence, the parameter 1/n is related to the strength and feasibility of adsorption, while the reciprocal n is the heterogeneity factor. When 1/n < 1 the process is predominantly physical, it is mainly chemical when 1/n > 1, and linear when 1/n = 1 (Prasannamedha et al. 2021). The 1/n value of oxyfluorfen (1.50) suggested the prevalent occurrence of chemisorption on the digestate. Furthermore, as the oxyfluorfen molecules were adsorbed onto the digestate, additional oxyfluorfen was more easily immobilized (S-type curve). This model suggests that the molecules are side-by-side associated and cooperate to remain held by the surface, a phenomenon usually observed for very hydrophobic compounds such as oxyfluorfen. The 1/n value of boscalid (0.99) suggested the occurrence of physical and chemical interactions to a similar extent. Huang et al. (2019) studied the changes of pesticide behaviour in soil following a fumigation treatment and reported 1/n value for oxyfluorfen very close to unity in fumigated soil. The authors commented that the change of the sorption curve of oxyfluorfen, which is normally S-shape, was due to some alterations of soil characteristics, including the structure and reactivity of organic matter, resulting the soil less adsorptive for those pesticides. The adsorption constants Kdads, KFads and KOCads indicated a higher adsorption of oxyfluorfen (more hydrophobic), compared to boscalid. As far as we know, there are no studies concerning the adsorption of oxyfluorfen on digestate, and therefore it is not possible to compare our results with those of the literature. The KFads value obtained in this study for boscalid was approximately 12 times higher than that observed for the same fungicide on a digestate from mixed plant and animal wastes (Loffredo et al. 2021).
The experimental desorption data and the Freundlich plots are depicted in Fig. 5, while desorption parameters obtained applying the Henry and the Freundlich equations are given in Table 3. For both molecules, r values were quite high, ranging from 0.91 to 1.00. Also in the desorption process, oxyfluorfen followed preferentially the Freundlich model (higher r value and lower SSR value) rather than the linear model, while both the Henry and the Freundlich models were appropriate for boscalid (Table 3), which was consistent with what was observed in the sorption study. However, the desorption curve of oxyfluorfen was definitely L-type, suggesting that the release of the compound occurred by mechanisms different from those of adsorption, possibly only the molecules physically bound were desorbed, while the desorption curve of boscalid was again very close to linearity. After three desorption steps, approximately 9.5 and 17.4% of the retained oxyfluorfen and boscalid were desorbed, respectively. The KFdes values of the two compounds were lower than the corresponding KFads values, indicating a not complete reversibility of the process. Comparing 1/nads and 1/ndes values, it appeared that for both molecules 1/ndes < 1/nads, which indicates a desorption process slower and incomplete with remarkable positive hysteresis (H < 1). In general, low H values are indicative of increased difficulty of the adsorbed compound to be released from the adsorbent (Barriuso et al. 1994). In general, the hysteresis phenomenon is an important condition for adsorbent materials because it guarantees effective trapping of pollutants which can subsequently be removed and eliminated in the regeneration phase of the adsorbent. The hysteresis phenomenon observed in this study for both compounds on digestate is consistent with the results of the sorption kinetic study which indicated a prevalent chemisorption mechanism.
The physicochemical properties of digestate largely influence its retention capacity of pesticides. Large surface area and porosity of the adsorbent are relevant in the sorption process, but also the type and abundance of chemical functional groups allocated on the adsorbent surface are definitely important. The presence of alcoholic and phenolic -OH, carboxylic -OH, ketonic C=O, amine, amide and ester groups, aromatic structure and aliphatic chains on hydrophilic and hydrophobic reactive sites of digestate has been demonstrated in this study and previous investigations (Pizzanelli et al. 2023; Provenzano et al. 2016). Based on our results, it is reasonable to argue that both oxyfluorfen and boscalid were retained on the digestate via high-energy bonds that hindered the reversibility of adsorption. Of course, this behaviour was expected on the basis of the chemical characteristics of the compounds, especially their hydrophobic nature and low water solubility. Our results are in agreement with those of Mukherjee et al. (2016) who reported a not complete reversibility of boscalid adsorption on a biomixture of 30% digestate and soil.
3.3.2 Soil
Although soil amendment with digestate has become a quite common agricultural practice, a very limited number of studies have concerned the contribution of this material to the overall retention capacity of soil towards pesticides. Hence, our interest and the contribution of this study. Sorption and desorption data of all treatments, along with the most suited theoretical models, are presented in Fig. 7, while the values of the adsorption and desorption parameters are given in Table 4. Considering both the r and SSR values obtained by adsorption data modeling, the Freundlich isotherm appeared to be the best fit for both molecules and all treatments, even if a good accordance was generally observed also for the other models considered (Table 4). In the case of the unamended soil, the constants Kdads, KFads and KOCads for oxyfluorfen (16.45, 18.83 and 498.34 mg kg−1, respectively) were all higher than those calculated for boscalid (12.20, 10.55 and 369.60 mg kg−1, respectively), which confirms the higher affinity of the more hydrophobic compound for the substrate. As already observed for oxyfluorfen adsorption on digestate (Section 3.3.1), the values of the Freundlich coefficient 1/nads were higher than the unit in all soil treatments. Differently, boscalid adsorption on soil showed variable 1/nads values, suggesting L-type, S-type and C-type adsorption on soil, soil + 2% digestate, and soil + 5% digestate, respectively (Table 4). The L-shaped isotherm is the most common for adsorption of organic compounds on heterogeneous matrices. It assumes that as more sites of the adsorbent are filled, it is increasingly difficult for solute molecules to find vacant sites, and that there is no strong competition by the solvent (Giles et al. 1960). Few information is available in the literature on this aspect. In a study of Sireesha et al. (2013), the adsorption isotherms of oxyfluorfen on various soils were of S-type.
Adsorption and desorption isotherms of the compounds on unamended soil (A) and soil amended with 2% digestate (B) and 5% digestate (C). Points indicate experimental data, while solid lines and dashed lines are plots of the Freundlich model for adsorption and desorption, respectively. The vertical bar on each point indicates the standard error (n = 3)
As expected, a relevant increase of the adsorption constants was obtained in digestate-amended soil, compared to unamended soil (Fig. 7B, C and Table 4). Based on Kdads values, the adsorption efficiency followed the trend: not amended soil < soil + 2% digestate < soil + 5% digestate. In the same order, the Kdads values were 16.45, 22.61 and 35.84 mg kg−1 for oxyfluorfen and 12.20, 22.64 and 32.52 mg kg−1 for boscalid. As expected, these constants were much lower (two orders of magnitude lower than those obtained for the digestate alone (Table 3). In not unamended soil, the KFads values of oxyfluorfen were lower than that reported by Wu et al. (2019) in soils with different textures (KFads values ranging from 62 to 116 mg kg−1), whereas the KFads value of boscalid was similar to that found by Mukherjee et al. (2016) in a loamy sand soil (KFads value equal to 19.3 mg kg−1). The significant rise of Kdads values of digestate-amended soil, compared to soil only, agrees with the results of Mukherjee et al. (2016) who observed that the application of 5% digestate to a loamy sand soil increased the Kdads value for boscalid from 19.3 (soil only) to 41.6 mg kg−1. In the same study, the authors found that by adding 5% biochar to 5%digestate-amended soil the Kdads value of boscalid increased by 55-fold, thus indicating a much higher efficiency of biochar, compared to digestate, in adsorb the fungicide. In our study, the KFads value of the soil for oxyfluorfen increased by 3.2 times after the addition of 2% digestate, which matches what Wu et al. (2019) reported after adding 2% biochar to the soil. However, the KFads values of oxyfluorfen on biochar and biochar-amended soil were much higher than those on digestate and digestate-amended soil. The larger surface area and extended porosity, higher C content and aromaticity of biochar may explain the difference. A significant increase in the KOC value of oxyfluorfen on a loam soil was reported by Perez-Lucas et al. (2020) after incorporation of composted agroforestry waste and manure waste into the soil.
The addition of digestate to the soil caused an increase in soil organic carbon which enhanced the sorption capacity of the two compounds. The organic matter content is the main property controlling the adsorption of organic pollutants in soil, especially the more hydrophobic ones, even if clay minerals may also have a significant role in the process (Parlavecchia et al. 2019). The adsorption of pesticides on soil organic matter depends not only on the amount of organic matter present but also on its origin and characteristics (Senesi and Loffredo 2018). The high affinity of boscalid for soil organic matter has been previously reported (Chen and Zhang 2010; Karlsson et al. 2016) and ascribed to the very low water solubility and the high hydrophobicity of this pesticide. Sireesha et al. (2013) highlighted the prominent role of organic matter in oxyfluorfen adsorption in soil. The addition of 2 and 5% digestate to the soil caused significant changes also of the normalized organic carbon coefficient, KOCads, which increased by 37 and 118% for oxyfluorfen and by 85 and 167% for boscalid, respectively (Table 4). Similar findings were reported by Loffredo et al. (2021) and Mukherjee et al. (2016).
The desorption study started soon after adsorption. The shapes of the desorption curves and the rates of release of the compounds from not amended soil and amended soil were different (Fig. 7 and Table 4). After three desorption steps, approximately 28, 21 and 15% of the initially adsorbed oxyfluorfen, and 40, 30 and 19% of adsorbed boscalid were desorbed from soil, soil + 2% digestate, and soil + 5% digestate, respectively. Based on these results, it is evident that, supplementing the soil with digestate, both the adsorption and the desorption process are modified. In general, experimental desorption data were well described by the Freundlich model, even if for soil + 5% digestate the r values were higher applying the Henry equation (Table 4). The KFdes and 1/ndes values of both compounds and for all soil treatments were lower than the corresponding KFads and 1/nads values, denoting a lower and incomplete release of the two pesticides. Similarly to what was observed for the sole digestate, all the hysteresis coefficients, H, were lower than unity, clearly denoting a strong hysteresis phenomenon. The hysteresis phenomenon observed for both compounds on soil can be related to the prevalent chemisorption mechanism indicated by the sorption kinetic study.
The lower release of the compounds from amended soil can be reasonably ascribed to the higher organic matter content in amended soils which makes desorption more difficult. The hysteresis phenomenon is quite common in the adsorption/desorption process of hydrophobic contaminants in soil, particularly in soil organic matter, and strictly depends on the affinity of the solute to the soil components. In general, the hysteretic desorption of pesticides slows down their dissipation so that residues can be found in the soil even after years. This could cause toxicity on sensitive crops following in the cultural rotation but, at the same time, guarantees long-term protection for tolerant plants. On the other hand, strong and persistent retention of a pesticide in soil prevents it from leaching into groundwater, transfer into natural surface waters, and uptake by plants.
3.3.3 Relationship between organic carbon content of adsorbents and their sorption/desorption efficiency
The values of the adsorption/desorption parameters of each compound on the various adsorbents, i.e., digestate, unamended soil, soil + 2% digestate and soil + 5% digestate, were correlated with the corresponding organic carbon contents by means of linear regressions. The probability levels obtained are shown in Table 5. Highly significant correlations were obtained for both compounds and all adsorption and desorption parameters when all adsorbents were considered. In contrast, when exclusively the soil treatments were considered, only Kddes of oxyfluorfen was significantly correlation with the percentage of organic carbon (P ≤ 0.05). These results confirm the prominent role of organic matter in the adsorption of pesticides.
4 Conclusions
A new type of anaerobic digestate obtained exclusively from olive pomace is currently produced and used as soil amendment. Its properties are supposed to be more stable and quite different from those of common digestates from mixed biowaste. However, no information is available in the literature either on its characterization or on its behaviour as adsorbent of pesticides, both when used alone and when incorporated into the soil. Therefore, we conducted this study with the aim of knowing digestate properties and investigating the quantitative and mechanistic aspects of its adsorption/desorption of two widely employed pesticides. The kinetic study showed that relevant amounts of the herbicide oxyfluorfen and the fungicide boscalid were adsorbed on the digestate very rapidly according to a PSO model, which suggested the formation of high-energy bonds between the interacting species (chemisorption). The adsorption constants of this digestate, calculated by interpreting the equilibrium sorption data with various theoretical equations, were much higher than those of other digestates obtained from mixed plant and animal wastes. By supplementing a loamy soil with 2 and 5% digestate, the values of the Freundlich isotherm constants increased significantly, being at least threefold compared to those of the unamended soil. Desorption of both compounds from digestate and unamended or amended soil was much slower than adsorption and incomplete, denoting the occurrence of a hysteresis phenomenon. In conclusion, this study provided evidence of good performance of this innovative digestate as adsorbent of hydrophobic pesticides, both when used directly and when incorporated into soil. In the latter case, this behaviour is particularly relevant because it guarantees the control of the bioavailability of pesticides and other organic chemicals in soil, avoiding their leaching or runoff in natural waters, and their excessive uptake by plants with consequent entry into the human and animal food chain. Furthermore, the overall results of this study are expected to be applicable to other pesticides having similar hydrophobicity and behaviour in soil. Finally, the satisfactory results obtained in this study encourage future investigations into the actions exerted by the digestate in the soil, also considering and comparing digestates from different types of starting biomass.
Data availability
Data generated during this study are included in this paper, further information is available from the corresponding author upon request.
References
Barbosa DR, García AC, de Souza CDCM, do Amaral Sobrinho NMB (2021) Influence of humic acid structure on the accumulation of oxyfluorfen in tropical soils of mountain agroecosystems. Environ Pollut 284:117380. https://doi.org/10.1016/j.envpol.2021.117380
Barriuso E, Laird DA, Koskinen WC, Dowdy RH (1994) Atrazine desorption from smectites. Soil Sci Soc Am J 58:1632–1638. https://doi.org/10.2136/sssaj1994.03615995005800060008x
Bhatt D, Srivastava A, Srivastava PC (2023) An insight into the sorption kinetics of boscalid onto soils: effect of general soil properties. Chemosphere. https://doi.org/10.1016/j.chemosphere.2023.138274
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319. https://doi.org/10.1021/ja01269a023
Cao ZB, Hulsemann B, Wust D, Illi L, Oechsner H, Kruse A (2020) Valorization of maize silage digestate from two-stage anaerobic digestion by hydrothermal carbonization. Energy Convers Manag 222:113218. https://doi.org/10.1016/j.enconman.2020.113218
Carnimeo C, Gelsomino A, Cirrottola G, Panuccio MR, Loffredo E (2022) Compost and vermicompost in cucumber rhizosphere promote plant growth and prevent the entry of anthropogenic organic pollutants. Sci Hortic 303:111250. https://doi.org/10.1016/j.scienta.2022.111250
Chen L, Zhang S (2010) Dissipation and residues of boscalid in strawberries and soils. Bull Environ Contam Toxicol 84:301–304. https://doi.org/10.1007/s00128-010-9934-y
Cristina G, Camelin E, Tommasi T, Fino D, Pugliese M (2020) Anaerobic digestates from sewage sludge used as fertilizer on a poor alkaline sandy soil and on a peat substrate: effects on tomato plants growth and on soil properties. J Environ Manag. https://doi.org/10.1016/j.jenvman.2020.110767
De Wilde T, Spanoghe P, Ryckeboer J, Jaeken P, Springael D (2009) Sorption characteristics of pesticides on matrix substrates used in biopurification systems. Chemosphere 75:100–108. https://doi.org/10.1016/j.chemosphere.2008.11.037
Diana G, Beni C, Marconi S (2009) Comparison of isotherm equations for boron adsorption and desorption on soils with fertilizer applications. Agrochimica 53:260–272
European Commission (EC) (2016) Defining Criteria for Identifying Endocrine Disruptors in the Context of the Implementation of the Plant Protection Products Regulation and Biocidal Products Regulation. https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A52016SC0211. Accessed 21 June 2023
European Commission (EC) (2022) REPowerEU Plan. https://energy.ec.europa.eu/system/files/2022-05/COM_2022_230_1_EN_ACT_part1_v5.pdf. Accessed14 June 2023
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470. https://doi.org/10.1016/S1001-0742(08)62146-4
Giles CH, MacEwan TH, Nakhwa SN, Smith D (1960) Studies in adsorption Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J Am Chem Soc 111:3973–3993. https://doi.org/10.1016/S2095-3119(13)60429-3
Ho YS (2006) Second-order kinetic model for the sorption of cadmium onto tree fern: a comparison of linear and non-linear methods. Water Res 40:119–125. https://doi.org/10.1016/j.watres.2005.10.040
Ho YS, Mckay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Huang B, Yan D, Wang X, Wang X, Fang W et al (2019) Soil fumigation alters adsorption and degradation behavior of pesticides in soil. Environ Pollut 246:264–273. https://doi.org/10.1016/j.envpol.2018.12.003
Karlsson AS, Weihermueller L, Tappe W, Mukherjee S, Spielvogel S (2016) Field scale boscalid residues and dissipation half-life estimation in a sandy soil. Chemosphere 145:163–173. https://doi.org/10.1016/j.chemosphere.2015.11.026
Kumar KV (2006) Linear and non-linear regression analysis for the sorption kinetics of methylene blue onto activated carbon. J Hazard Mater 137:1538–1544. https://doi.org/10.1016/j.jhazmat.2006.04.036
Lagergreen S (1907) Zur Theorie der sogenannten Adsorption gelöster Stoffe. Zeitschrift Für Chemie Und Industrie Der Kolloide 1907(2):15. https://doi.org/10.1007/BF01501332
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Loffredo E (2022) Recent advances on innovative materials from biowaste recycling for the removal of environmental estrogens from water and soil. Materials. https://doi.org/10.3390/ma15051894
Loffredo E, Carnimeo C, Silletti R, Summo C (2021) Use of the solid by-product of anaerobic digestion of biomass to remove anthropogenic organic pollutants with endocrine disruptive activity. Processes. https://doi.org/10.3390/pr9112018
Lou Y-Y, Zhou K, Shi J-H, Pan D-Q (2017) Characterizing the binding interaction of fungicide boscalid with bovine serum albumin (BSA): a spectroscopic study in combination with molecular docking approach. J Photochem Photobiol B Biol 173:589–597. https://doi.org/10.1016/j.jphotobiol.2017.06.037
Mukherjee S, Weihermüller L, Tappe W, Hofmann D, Köppchen S et al (2016) Sorption-desorption behaviour of bentazone, boscalid and pyrimethanil in biochar and digestate based soil mixtures for biopurification systems. Sci Total Environ 559:63–73. https://doi.org/10.1016/j.scitotenv.2016.03.145
Parlavecchia M, D’Orazio V, Loffredo E (2019) Wood biochars and vermicomposts from digestate modulate the extent of adsorption-desorption of the fungicide metalaxyl-m in a silty soil. Environ Sci Pollut Res 35:35924–35934. https://doi.org/10.1007/s11356-019-06729-z
Peng W, Pivato A (2019) Sustainable management of digestate from the organic fraction of municipal solid waste and food waste under the concepts of back to earth alternatives and circular economy. Waste Biomass Valorization 10:465–481. https://doi.org/10.1007/s12649-017-0071-2
Perez-Lucas G, Gambín M, Navarro S (2020) Leaching behaviour appraisal of eight persistent herbicides on a loam soil amended with different composted organic wastes using screening indices. J Environ Manage 273:111179. https://doi.org/10.1016/j.jenvman.2020.111179
Pizzanelli S, Calucci L, Forte C, Borsacchi S (2023) Studies of organic matter in composting, vermicomposting, and anaerobic digestion by 13C solid-state NMR spectroscopy. Appl Sci. https://doi.org/10.3390/app13052900
Prasannamedha G, Senthil Kumar P, Mehala R, Sharumitha TJ, Surendhar D (2021) Enhanced adsorptive removal of sulfamethoxazole from water using biochar derived from hydrothermal carbonization of sugarcane bagasse. J Hazard Mater 407:124825. https://doi.org/10.1016/j.jhazmat.2020.124825
Provenzano MR, Cavallo O, Malerba AD, Di Maria F, Cucina M, Massaccesi L, Gigliotti G (2016) Co-treatment of fruit and vegetable waste in sludge digesters: chemical and spectroscopic investigation by fluorescence and Fourier transform infrared spectroscopy. Waste Manag 50:283–289. https://doi.org/10.1016/j.wasman.2016.02.026
Provenzano MR, Cavallo O, Malerba A, Fabbri C, Zaccone C (2018) Unravelling (maize silage) digestate features throughout a full-scale plant: a spectroscopic and thermal approach. J Clean Prod 193:372–378. https://doi.org/10.1016/j.jclepro.2018.05.081
PubChem (2023) Open chemistry database at the National Institutes of Health (NIH), U. S. National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/8814. Accessed 20 June 2023
Quan C, Zhou Y, Wu C, Xu G, Feng D et al (2023) Valorization of solid digestate into activated carbon and its potential for CO2 capture. J Anal Appl Pyrolysis 169:105874. https://doi.org/10.1016/j.jaap.2023.105874
Senesi N, Loffredo E (2018) The chemistry of soil organic matter. In: Sparks DL (ed) Soil physical chemistry, 2nd edn. CRC Press, Boca Raton FL, pp 239–370. https://doi.org/10.1201/9780203739280
Singh L, Kalia VC (2017) Waste biomass management – a holistic approach. Springer International Publishing AG, Cham, Switzerland. https://doi.org/10.1007/978-3-319-49595-8
Sireesha A, Rao PC, Rao PV, Ramalakshmi CHS, Swapna G (2013) Adsorption desorption of pendimethalin and oxyfluorfen in soils of Andhra Pradesh. J Res ANGRAU 41:1–10
Sondhia S (2010) Persistence and bioaccumulation of oxyfluorfen residues in onion. Environ Monit Assess 162:163–168. https://doi.org/10.1007/s10661-009-0784-1
Ugwu SN, Harding K, Enweremadu CC (2022) Comparative life cycle assessment of enhanced anaerobic digestion of agro-industrial waste for biogas production. J Clean Prod. https://doi.org/10.1016/j.jclepro.2022.131178
USEPA Office of Pesticide Programs (2010) Environmental Fate and Ecological Risk Assessment for Boscalid New Use on Rapeseed, Including Canola (Seed Treatment). https://www3.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-128008_23-Dec10_a.pdf. Accessed 17 June 2023
Valenti F, Arcidiacono C, Chinnici G, Cascone G, Porto SMC (2017) Quantification of olive pomace availability for biogas production by using a GIS-based model. Biofuel Bioprod Biorefin 11:784–797. https://doi.org/10.1002/bbb.1784
Valentinuzzi F, Cavani L, Porfido C, Terzano R, Pii Y, Cesco S, Marzadori C, Mimmo T (2020) The fertilising potential of manure-based biogas fermentation residues: pelleted vs. liquid digestate. Heliyon. https://doi.org/10.1016/j.heliyon.2020.e03325
Wang W, Lee D-J (2021) Valorization of anaerobic digestion digestate: a prospect review. Biores Technol 323:124626. https://doi.org/10.1016/j.biortech.2020.124626
Wu C, Liu X, Wu X, Dong F, Xu J, Zheng Y (2019) Sorption, degradation and bioavailability of oxyfluorfen in biochar-amended soils. Sci Total Environ 658:87–94. https://doi.org/10.1016/j.scitotenv.2018.12.059
Yao S, Fabbricino M, Race M, Ferraro A, Pontoni L, Aimone O, Chen Y (2020) Study of the digestate as an innovative and low-cost adsorbent for the removal of dyes in wastewater. Processes. https://doi.org/10.3390/pr8070852
Acknowledgements
The authors thank AGROLIO s.r.l., Andria, Italy, for providing the digestate used in this study. The authors are also grateful to the anonymous reviewers for their valuable suggestions.
Funding
Open access funding provided by Università degli Studi di Bari Aldo Moro within the CRUI-CARE Agreement. This study was carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) – MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 – D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them. The study was also partly financed by University of Bari Aldo Moro.
Author information
Authors and Affiliations
Contributions
Elisabetta Loffredo analyzed and validated the data, was the major contributor to the writing of the manuscript and supervised the entire work. Claudia Carnimeo collected and analyzed the data. Valeria D’Orazio collected and analyzed the data and contributed to the writing of the manuscript. Nicola Colatorti collected and analyzed the data. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
The manuscript is approved by all authors who give consent to publish it.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Zhaoliang Song
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loffredo, E., Carnimeo, C., D’Orazio, V. et al. Sorption and release of the pesticides oxyfluorfen and boscalid in digestate from olive pomace and in digestate-amended soil. J Soils Sediments 24, 1489–1506 (2024). https://doi.org/10.1007/s11368-024-03748-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-024-03748-3