Opinion Statement
This paper shines a light on the exciting progress being made in using immunotherapy to treat advanced gastroesophageal cancers. The positive results from trials using drugs like Pembrolizumab and Nivolumab are certainly encouraging and open new possibilities for treating this challenging disease. However, it is clear that we still have a lot to learn about how to predict which patients will benefit most from these treatments. The exploration of combining therapies and using machine learning to guide treatment shows promise. Moving forward, it is crucial that researchers and healthcare professionals continue to work together, sharing knowledge and findings to continue the advancements in this important area.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistiCS 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Paydary K, Reizine N, Catenacci DVT. Immune-checkpoint inhibition in the treatment of gastro-esophageal cancer: a closer look at the emerging evidence. Cancers. 2021;13:5929.
Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol. 2022;29:3044–60.
• Zhou KI, Peterson B, Serritella A, Thomas J, Reizine N, Moya S, et al. Spatial and temporal heterogeneity of PD-L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clin Cancer Res. 2020;26:6453–63. This reference is of importance because it discusses the intricate challenges and complexities encountered with PD-L1 immunohistochemistry assays, notably issues related to reproducibility, heterogeneity, and subjectivity, in the context of gastroesophageal cancer. By highlighting the consistent correlation between higher CPS scores and an increased likelihood of clinical benefit, while also navigating through the debate on the efficacy of PD-L1 as a standalone biomarker, the reference provides a critical foundation. It thereby aids in exploring and validating alternative or complementary biomarkers, ensuring informed and nuanced discussions and investigations in future immunotherapy response studies across gastroesophageal cancer trials.
• Park R, Da Silva LL, Saeed A. Immunotherapy predictive molecular markers in advanced gastroesophageal cancer: MSI and beyond. Cancers. 2021;13:1715. This reference is of importance because it sheds light on the potential and early promise of various markers while emphasizing the necessity for their further prospective evaluations in the context of gastroesophageal cancer.
•• Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. The Lancet. 2021;398:27–40. This reference is of outstanding importance because it is the phase III trial that demonstrated the superiority of first-line nivolumab plus chemotherapy to chemotherapy alone and it led to FDA approval.
•• Sun J-M, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. The Lancet. 2021;398:759–71. This reference is of outstanding importance because is the phase III trial that demonstrated the superiority of pembrolizumab plus chemotherapy to chemotherapy alone and it led to FDA approval.
Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603:942–8.
• Yap DWT, Leone AG, Wong NZH, Zhao JJ, Tey JCS, Sundar R, et al. Effectiveness of immune checkpoint inhibitors in patients with advanced esophageal squamous cell carcinoma: a meta-analysis including low PD-L1 subgroups. JAMA Oncol. 2023;9:215–24. This reference is of importance because it reveals insightful yet sobering findings regarding the efficacy of adding pembrolizumab to chemotherapy in low PD-L1–expressing gastric, esophageal, or GE cancers, specifically those with PD-L1 CPS 1 to 9. This metanalysis indicates no significant benefit of the addition of pembrolizumab in certain patient cohorts. Thus, it becomes a cornerstone for future research, guiding investigations and discussions toward understanding the varied impacts of immunotherapy across different PD-L1 expression levels and ensuring that subsequent trials and treatment strategies are sculpted with a more tailored approach.
Park Y, Koh J, Na HY, Kwak Y, Lee K-W, Ahn S-H, et al. PD-L1 testing in gastric cancer by the combined positive score of the 22C3 PharmDx and SP263 assay with clinically relevant cut-offs. Cancer Res Treat. 2020;52:661–70.
Sajjadi E, Venetis K, Scatena C, Fusco N. Biomarkers for precision immunotherapy in the metastatic setting: hope or reality? Ecancermedicalscience. 2020;14:1150.
Bang Y-J, Cutsem EV, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
•• Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727–30. This reference is of outstanding importance because it clearly demonstrated the benefits of adding pembrolizumab to trastuzumab and chemotherapy which led to FDA approval.
KEYTRUDA® (pembrolizumab) plus trastuzumab and chemotherapy met primary endpoint of progression-free survival as first-line treatment in patients with HER2-positive advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma [Internet]. Merck.com. [cited 2023 Oct 2]. Available from: https://www.merck.com/news/keytruda-pembrolizumab-plus-trastuzumab-and-chemotherapy-met-primary-endpoint-of-progression-free-survival-as-first-line-treatment-in-patients-with-her2-positive-advanced-gastric-or-gastroesop/
•• Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386:449–62. This reference is of outstanding importance because it is a phase III trial that underscored the pivotal role of nivolumab-based treatments, particularly in combination with chemotherapy, for advanced ESCC revealing a notable overall survival benefit compared to chemotherapy alone. Subgroup analyses illuminated that patients with a PD-L1 tumor proportion score (TPS) of ≥ 1% manifested pronounced survival advantages, thereby not only bolstering the FDA approval of Nivolumab but also emphasizing the significant impact of PD-L1 expression levels on treatment efficacy and survival outcomes in advanced ESCC.
Kang Y-K, Chen L-T, Ryu M-H, Oh D-Y, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234–47.
•• Shitara K, Van Cutsem E, Bang Y-J, Fuchs C, Wyrwicz L, Lee K-W, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1571–80. This reference is of outstanding importance because it is a phase III trial that illuminated the efficacy of pembrolizumab, especially within the MSI-H subgroup of advanced gastric cancer patients, showcasing a substantial survival benefit and reduced risk of death compared to chemotherapy alone. Particularly, it showed striking outcomes in patients with MSI-H tumors and a PD-L1 CPS of 1 or greater.
Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu C-H, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138–48.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
•• Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord J-P, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. This reference is of outstanding importance because of spotlights the significance of tumor mutation burden (TMB) in immunotherapy response across varied tumor types. Despite its foundational role, the trial prompts critical inquiries regarding the universal applicability of the TMB cutoff of 10 mutations per megabase across diverse GI malignancies and underscores the necessity for further exploration and validation in steering management strategies, especially in gastro-esophageal cancers.
Samstein RM, Lee C-H, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202–6.
Hsiehchen D, Hsieh A, Samstein RM, Lu T, Beg MS, Gerber DE, Wang T, Morris LGT, Zhu H. DNA repair gene mutations as predictors of immune checkpoint inhibitor response beyond tumor mutation burden. Cell reports medicine. 2020;1(3): 100034. https://doi.org/10.1016/j.xcrm.2020.100034.
Nishikawa J, Iizasa H, Yoshiyama H, Shimokuri K, Kobayashi Y, Sasaki S, et al. Clinical importance of Epstein-Barr virus-associated gastric cancer. Cancers (Basel). 2018;10:167.
Kim ST, Cristescu R, Bass AJ, Kim K-M, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–58.
Saeed A, Park R, Dai J, Al-Rajabi R, Kasi A, Baranda J, et al. Cabozantinib plus durvalumab in advanced gastroesophageal cancer and other gastrointestinal malignancies: phase Ib CAMILLA trial results. Cell Rep Med. 2023;4(2):100916. https://doi.org/10.1016/j.xcrm.2023.100916.
Saeed A, Park R, Sun W. The integration of immune checkpoint inhibitors with VEGF targeted agents in advanced gastric and gastroesophageal adenocarcinoma: a review on the rationale and results of early phase trials. J Hematol Oncol. 2021;14:13.
Cutsem EV, di Bartolomeo M, Smyth E, Chau I, Park H, Siena S, et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 2023;24:744–56.
Qi C, Gong J, Li J, Liu D, Qin Y, Ge S, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med. 2022;28:1189–98.
Lu Z, Chen H, Jiao X, Zhou W, Han W, Li S, et al. Prediction of immune checkpoint inhibition with immune oncology-related gene expression in gastrointestinal cancer using a machine learning classifier. J Immunother Cancer. 2020;8:e000631.
Author information
Authors and Affiliations
Contributions
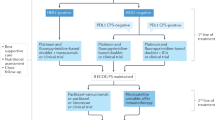
S.E.K. and A.S. conceptualized the review. S.E.K. wrote the original draft of the manuscript. N.A. prepared the figure and formulated the table. All authors reviewed and edited the manuscript. A.S. provided supervision throughout the project. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
We report the following conflicts of interest:
A.S. (Anwaar Saeed) reports research grants (to institution) from AstraZeneca, Bristol Myers Squibb, Merck, Clovis, Exelixis, Actuate Therapeutics, Incyte Corporation, Daiichi Sankyo, Five Prime Therapeutics, Amgen, Innovent Biologics, Dragonfly Therapeutics, KAHR Medical, and BioNTech and advisory board fees from Merck, AstraZeneca, Bristol Myers Squibb, Exelixis, Taiho, and Pfizer. The remaining authors have no relevant financial interests to disclose.
No external funding was received for the conduct of this research or preparation of this manuscript. The authors confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. The authors further confirm that the order of authors listed in the manuscript has been approved by all of us.
Competing interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khateeb, S., Cavalcante, L., Alnairat, N. et al. Who Should Receive Immunotherapy for Advanced Gastroesophageal Cancer?. Curr. Treat. Options in Oncol. 25, 496–509 (2024). https://doi.org/10.1007/s11864-024-01189-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-024-01189-1