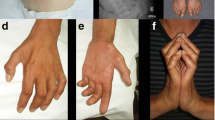
Abstract
Spinal muscular atrophy (SMA) is a neuromuscular, rare genetic disorder caused due to loss-of-function mutations in the survival motor neuron-1 (SMN1) gene, leading to deficiency of the SMN protein. The severity of the disease phenotype is inversely proportional to the copy number of another gene, SMN2, that differs from SMN1 by a few nucleotides. The current diagnostic methods for SMA include symptom-based diagnosis, biochemical methods like detection of serum creatine kinase, and molecular detection of disease-causing mutations using polymerase chain reaction (PCR), multiplex ligation-dependent probe amplification (MLPA), and exome or next-generation sequencing (NGS). Along with detection of the disease-causing mutation in the SMN1 gene, it is crucial to identify the copy number of the SMN2 gene, which is a disease modifier. Therapeutic options like gene therapy, antisense therapy, and small molecules are available for SMA, but, the costs are prohibitively high. This review discusses the prevalence, diagnosis, available therapeutic options for SMA, and their clinical trials in the Indian context, and highlights the need for measures to make indigenous diagnostic and therapeutic interventions.




Similar content being viewed by others
Notes
Without considering the effect of SMN2 on the phenotype and the 2/0 genotypes, explained in the following sections.
References
Alías L, Bernal S, Barceló MJ, et al. 2011 Accuracy of marker analysis, quantitative real-time polymerase chain reaction, and multiple ligation-dependent probe amplification to determine SMN2 copy number in patients with spinal muscular atrophy. Genet. Test. Mol. Biomarkers 15 587–594
Alías L, Bernal S, Calucho M, et al. 2018 Utility of two SMN1 variants to improve spinal muscular atrophy carrier diagnosis and genetic counselling. Eur. J. Hum. Genet. 26 1554–3557
Anhuf D, Eggermann T, Rudnik-Schöneborn S, et al. 2003 Determination of SMN1 and SMN2 copy number using TaqMan technology. Hum. Mutat. 22 74–78
Arkblad EL, Darin N, Berg K, et al. 2006 Multiplex ligation-dependent probe amplification improves diagnostics in spinal muscular atrophy. Neuromuscul. Disord. 16 830–838
Arslan D, Inan B, Kilinc M, et al. 2023 Nusinersen for adults with spinal muscular atrophy. Neurol. Sci. 44 2393–2400
Azad AK, Huang CK, Jin H, et al. 2020 Enhanced carrier screening for spinal muscular atrophy: detection of silent (SMN1: 2 + 0) carriers utilizing a novel TaqMan genotyping method. Lab. Med. 51 408–415
Bajaj A, Mathew S, Vellarikkal SK, et al. 2019 Genomics of rare genetic diseases—experiences from India. Hum. Genomics 13 52
Baranello G, Darras BT, Day JW, et al. 2021 Risdiplam in Type 1 Spinal Muscular Atrophy. N. Engl. J. Med. 384 915–923
Ben-Shachar S, Orr-Urtreger A, Bardugo E, et al. 2011 Large-scale population screening for spinal muscular atrophy: clinical implications. Genet. Med. 13 110–114
Blasco-Pérez L, Paramonov I, Leno J, et al. 2021 Beyond copy number: A new, rapid, and versatile method for sequencing the entire SMN2 gene in SMA patients. Hum. Mutat. 42 787–795
Bose M, Parab SD, Patil SM, et al. 2019 Exploring spinal muscular atrophy and its impact on functional status: Indian scenario. Indian. J. Public. Health 63 254–257
Brzustowicz LM, Lehner T, Castilla LH, et al. 1990 Genetic mapping of chronic childhood-onset spinal muscular atrophy to chromosome 5q11.2–33.3. Nature 344 540–541
Burghes AH 1997 When is a deletion not a deletion? When it is converted. Am. J. Hum. Genet. 61 9–15
Burghes AH and Beattie CE 2009 Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat. Rev. Neurosci. 10 597–609
Burnett BG, Muñoz E, Tandon A, et al. 2009 Regulation of SMN protein stability. Mol. Cell. Biol. 29 1107–1115
Burr P and Reddivari AKR 2022 Spinal muscle atrophy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA
Butchbach MER 2021 Genomic Variability in the Survival Motor Neuron Genes (SMN1 and SMN2): Implications for Spinal Muscular Atrophy Phenotype and Therapeutics Development. Int. J. Mol. Sci. 22 7896
Calucho M, Bernal S, Alías L, et al. 2018 Correlation between SMA type and SMN2 copy number revisited: An analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul. Disord. 28 208–215
Cancès C, Richelme C, Barnerias C, et al. 2020 Clinical features of spinal muscular atrophy (SMA) type 2. Arch. Pediatr. 27 7S18–7S22
Cartegni L and Krainer AR 2002 Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat. Genet. 30 377–384
Cartegni L, Hastings ML, Calarco JA, et al. 2006 Determinants of exon 7 splicing in the spinal muscular atrophy genes, SMN1 and SMN2. Am. J. Hum. Genet. 78 63–77
Ceylan AC, Erdem HB, Sahin I, et al. 2020 SMN1 gene copy number analysis for spinal muscular atrophy (SMA) in a Turkish cohort by CODE-SEQ technology, an integrated solution for detection of SMN1 and SMN2 copy numbers and the “2+0” genotype. Neurol. Sci. 41 2575–2584
Chen X, Sanchis-Juan A, French CE, et al. 2020 Spinal muscular atrophy diagnosis and carrier screening from genome sequencing data. Genet. Med. 22 945–953
Chencheri N, Alexander G, Nugud A, et al. 2023 Gene transfer therapy in children with spinal muscular atrophy: A single-center experience with a cohort of 25 children. Muscle Nerve 68 269–277
Chiriboga CA, Swoboda KJ, Darras BT, et al. 2016 Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology 86 890–897
Chiriboga C, Mercuri E, Fischer D, et al. 2019 P.363JEWELFISH: safety and pharmacodynamic data in patients with spinal muscular atrophy (SMA) receiving treatment with risdiplam (RG7916) that have previously been treated with nusinersen. Neuromuscul. Disord. 29 S187
Chiriboga CA, Bruno C, Duong T, et al. 2023 Risdiplam in patients previously treated with other therapies for spinal muscular atrophy: An interim analysis from the Jewelfish study. Neurol. Ther. 12 543–557
Cho S and Dreyfuss G 2010 A degron created by SMN2 exon 7 skipping is a principal contributor to spinal muscular atrophy severity. Genes. Dev. 24 438–442
Collén A, Bergenhem N, Carlsson L, et al. 2022 VEGFA mRNA for regenerative treatment of heart failure. Nat. Rev. Drug. Discov. 21 79–80
Costa-Roger M, Blasco-Pérez L, Cuscó I, et al. 2021 The importance of digging into the genetics of SMN genes in the therapeutic scenario of spinal muscular atrophy. Int. J. Mol. Sci. 22 9029
Crawford TO, Paushkin SV, Kobayashi DT, et al. 2012 Evaluation of SMN protein, transcript, and copy number in the biomarkers for spinal muscular atrophy (BforSMA) clinical study. PLoS One 7 e33572
Dangouloff T, Vrščaj E, Servais L, et al. 2021 Newborn screening programs for spinal muscular atrophy worldwide: Where we stand and where to go. Neuromuscul. Disord. 31 574–582
Darras BT, Masson R, Mazurkiewicz-Bełdzińska M, et al. 2021 Risdiplam-treated infants with type 1 spinal muscular atrophy versus historical controls. N. Engl. J. Med. 385 427–435
Darras B, Baranello G, Boespflug-Tanguy O, et al. 2023 FIREFISH Parts 1 and 2: 36-month safety and efficacy of risdiplam in Type 1 spinal muscular atrophy (SMA) (P7–3.009). Neurology 100 3928
Day JW, Finkel RS, Chiriboga CA, et al. 2021 Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 20 284–293
DiDonato CJ, Chen XN, Noya D, et al. 1997a Cloning, characterization, and copy number of the murine survival motor neuron gene: homolog of the spinal muscular atrophy-determining gene. Genome Res. 7 339–352
DiDonato CJ, Ingraham SE, Mendell JR, et al. 1997b Deletion and conversion in spinal muscular atrophy patients: is there a relationship to severity? Ann. Neurol. 41 230–237
Dobnik D, Štebih D, Blejec A, et al. 2016 Multiplex quantification of four DNA targets in one reaction with Bio-Rad droplet digital PCR system for GMO detection. Sci. Rep. 6 35451
Duong T, Wolford C, McDermott MP, et al. 2021 Nusinersen treatment in adults with spinal muscular atrophy. Neurol. Clin. Pract. 11 e317–e327
Eggermann K, Gläser D, Abicht A, et al. 2020 Spinal muscular atrophy (5qSMA): best practice of diagnostics, newborn screening and therapy. Med. Genet. 32 263–272
Elsheikh B, Prior T, Zhang X, et al. 2009 An analysis of disease severity based on SMN2 copy number in adults with spinal muscular atrophy. Muscle Nerve 40 652–656
Emmady PD and Bodle J 2022 Werdnig Hoffmann disease. StatPearls
Ertl HCJ 2022 Immunogenicity and toxicity of AAV gene therapy. Front. Immunol. 13 975803
Fainmesser Y, Drory VE, Ben-Shushan S, et al. 2022 Longer-term follow-up of nusinersen efficacy and safety in adult patients with spinal muscular atrophy types 2 and 3. Neuromuscul. Disord. 32 451–459
Fang P, Li L, Zeng J, et al. 2015 Molecular characterization and copy number of SMN1, SMN2 and NAIP in Chinese patients with spinal muscular atrophy and unrelated healthy controls. BMC Musculoskelet. Disord. 16 11
Fang YL, Li N, Zhi XF, et al. 2021 Discovery of specific mutations in spinal muscular atrophy patients by next-generation sequencing. Neurol. Sci. 42 1827–1833
Federici T, Taub JS, Baum GR, et al. 2012 Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 19 852–859
Feldkötter M, Schwarzer V, Wirth R, et al. 2002 Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am. J. Hum. Genet. 70 358–368
Finkel RS, Chiriboga CA, Vajsar J, et al. 2016 Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 388 3017–3026
Finkel RS, Mercuri E, Darras BT, et al. 2017 Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 377 1723–1732
Finkel RS, Chiriboga CA, Vajsar J, et al. 2021 Treatment of infantile-onset spinal muscular atrophy with nusinersen: final report of a phase 2, open-label, multicentre, dose-escalation study. Lancet Child Adolesc. Health 5 491–500
Finkel R, Farrar M, Vlodavets D, et al. 2022 FP.24 RAINBOWFISH: Preliminary efficacy and safety data in risdiplam-treated infants with presymptomatic spinal muscular atrophy (SMA). Neuromuscul. Disord. 32 S85–S86
Gambardella A, Mazzei R, Toscano A, et al. 1998 Spinal muscular atrophy due to an isolated deletion of exon 8 of the telomeric survival motor neuron gene. Ann. Neurol. 44 836–839
Gan LM, Lagerström-Fermér M, Carlsson LG, et al. 2019 Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat. Commun. 10 871
Gilliam TC, Brzustowicz LM, Castilla LH, et al. 1990 Genetic homogeneity between acute and chronic forms of spinal muscular atrophy. Nature 345 823–825
Hagenacker T, Wurster CD, Günther R, et al. 2020 Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol. 19 317–325
Hendrickson BC, Donohoe C, Akmaev VR, et al. 2009 Differences in SMN1 allele frequencies among ethnic groups within North America. J. Med. Genet. 46 641–644
Intellia and Regeneron 2022 Intellia and Regeneron announce updated Phase 1 data demonstrating a single dose of NTLA-2001, an investigational CRISPR therapy for transthyretin (ATTR) amyloidosis, resulted in rapid, deep and sustained reduction in disease-causing protein (https://ir.intelliatx.com/news-releases/news-release-details/intellia-and-regeneron-announce-updated-phase-1-data)
Jiang J, Huang J, Gu J, et al. 2019 Genomic analysis of a spinal muscular atrophy (SMA) discordant family identifies a novel mutation in TLL2, an activator of growth differentiation factor 8 (myostatin): a case report. BMC Med. Genet. 20 204
Kashima T and Manley JL 2003 A negative element in SMN2 exon 7 inhibits splicing in spinal muscular atrophy. Nat. Genet. 34 460–463
Kashima T, Rao N, David CJ, et al. 2007 hnRNP A1 functions with specificity in repression of SMN2 exon 7 splicing. Hum. Mol. Genet. 16 3149–3159
Kaur A, Kumari A, Kaur A, et al. 2022 Carrier frequency of Spino-muscular atrophy in individuals of a reproductive age group from North India. J. Family Med. Prim. Care 11 7870–7874
Keinath MC, Prior DE and Prior TW 2021 Spinal muscular atrophy: mutations, testing, and clinical relevance. Appl. Clin. Genet. 14 11–35
Kesari A, Rennert H, Leonard DG, et al. 2005 SMN1 dosage analysis in spinal muscular atrophy from India. BMC Med. Genet. 6 22
Kubo Y, Nishio H and Saito K 2015 A new method for SMN1 and hybrid SMN gene analysis in spinal muscular atrophy using long-range PCR followed by sequencing. J. Hum. Genet. 60 233–239
Le TT, Pham LT, Butchbach MER, et al. 2005 SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum. Mol. Genet. 14 845–857
le Hao T, Burghes AH and Beattie CE 2011 Generation and characterization of a genetic zebrafish model of SMA carrying the human SMN2 gene. Mol. Neurodegener. 6 24
Lefebvre S, Bürglen L, Reboullet S, et al. 1995 Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80 155–165
Lefebvre S, Burlet P, Liu Q, et al. 1997 Correlation between severity and SMN protein level in spinal muscular atrophy. Nat. Genet. 16 265–269
Liu Q, Fischer U, Wang F, et al. 1997 The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 90 1013–1021
Lorson CL and Androphy EJ 2000 An exonic enhancer is required for inclusion of an essential exon in the SMA-determining gene SMN. Hum. Mol. Genet. 9 259–265
Lorson CL, Strasswimmer J, Yao JM, et al. 1998 SMN oligomerization defect correlates with spinal muscular atrophy severity. Nat. Genet. 19 63–66
Lorson CL, Hahnen E, Androphy EJ, et al. 1999 A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 96 6307–6311
Lunn MR and Wang CH 2008 Spinal muscular atrophy. Lancet 371 2120–2133
Luo M, Liu L, Peter I, et al. 2014 An Ashkenazi Jewish SMN1 haplotype specific to duplication alleles improves pan-ethnic carrier screening for spinal muscular atrophy. Genet. Med. 16 149–156
Mailman MD, Hemingway T, Darsey RL, et al. 2001 Hybrids monosomal for human chromosome 5 reveal the presence of a spinal muscular atrophy (SMA) carrier with two SMN1 copies on one chromosome. Hum. Genet. 108 109–115
Masson R, Mazurkiewicz-Bełdzińska M, Rose K, et al. 2022 Safety and efficacy of risdiplam in patients with type 1 spinal muscular atrophy (FIREFISH part 2): secondary analyses from an open-label trial. Lancet Neurol. 21 1110–1119
McAndrew PE, Parsons DW, Simard LR, et al. 1997 Identification of proximal spinal muscular atrophy carriers and patients by analysis of SMNT and SMNC gene copy number. Am. J. Hum. Genet. 60 1411–1422
Melki J, Abdelhak S, Sheth P, et al. 1990 Gene for chronic proximal spinal muscular atrophies maps to chromosome 5q. Nature 344 767–768
Mendell JR, Al-Zaidy S, Shell R, et al. 2017 Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377 1713–1722
Mendell JR, Al-Zaidy SA, Lehman KJ, et al. 2021 Five-year extension results of the phase 1 START Trial of onasemnogene abeparvovec in spinal muscular atrophy. JAMA Neurol. 78 834–841
Mendell J, Wigderson M, Alecu I, et al. 2023 Long-term follow-up of onasemnogene abeparvovec gene therapy in patients with spinal muscular atrophy type 1 (P7–3.006). Neurology 100 2432
Mercuri E, Darras BT, Chiriboga CA, et al. 2018 Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med. 378 625–635
Mercuri E, Baranello G, Day JW, et al. 2019a Onasemnogene abeparvovec gene-replacement therapy (GRT) for spinal muscular atrophy type 1 (SMA1): Global pivotal phase 3 study program (STR1VE-US, STR1VE-EU, STR1VE-AP). J. Neurol. Sci. 405 277–278
Mercuri E, Baranello G, Kirschner J, et al. 2019b Update from SUNFISH Part 1: Safety, tolerability and PK/PD from the dose-finding study, including exploratory efficacy data in patients with type 2 or 3 spinal muscular atrophy (SMA) treated with risdiplam (RG7916) (S25.007). Neurology 92 S25.007
Mercuri E, Muntoni F, Baranello G, et al. 2021 Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 20 832–841
Mercuri E, Deconinck N, Mazzone ES, et al. 2022 Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 21 42–52
Michaud M, Arnoux T, Bielli S, et al. 2010 Neuromuscular defects and breathing disorders in a new mouse model of spinal muscular atrophy. Neurobiol. Dis. 38 125–135
Monani UR, Lorson CL, Parsons DW, et al. 1999 A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 8 1177–1183
Monani UR, Sendtner M, Coovert DD, et al. 2000 The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn(-/-) mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 9 333–339
Morrison KE 1996 Advances in SMA research: review of gene deletions. Neuromuscul. Disord. 6 397–408
Naryshkin NA, Weetall M, Dakka A, et al. 2014 SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science 345 688–693
Nilay M, Moirangthem A, Saxena D, et al. 2021 Carrier frequency of SMN1-related spinal muscular atrophy in north Indian population: The need for population based screening program. Am. J. Med. Genet. A. 185 274–277
Oskoui M, Day JW, Deconinck N, et al. 2023 Two-year efficacy and safety of risdiplam in patients with type 2 or non-ambulant type 3 spinal muscular atrophy (SMA). J. Neurol. 270 2531–2546
Parsons DW, McAndrew PE, Iannaccone ST, et al. 1998 Intragenic telSMN mutations: frequency, distribution, evidence of a founder effect, and modification of the spinal muscular atrophy phenotype by cenSMN copy number. Am. J. Hum. Genet. 63 1712–1723
Pellizzoni L 2007 Chaperoning ribonucleoprotein biogenesis in health and disease. EMBO Rep. 8 340–345
Poirier A, Weetall M, Heinig K, et al. 2018 Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol. Res. Perspect. 6 e00447
Prior TW, Leach ME and Finanger E 1993 Spinal muscular atrophy; in GeneReviews (University of Washington)
Prior TW, Krainer AR, Hua Y, et al. 2009 A positive modifier of spinal muscular atrophy in the SMN2 gene. Am. J. Hum. Genet. 85 408–413
Qiu J, Wu L, Qu R, et al. 2022 History of development of the life-saving drug “Nusinersen” in spinal muscular atrophy. Front. Cell. Neurosci. 16 942976
Ramdas S and Servais L 2020 New treatments in spinal muscular atrophy: an overview of currently available data. Expert Opin. Pharmacother. 21 307–315
Rochette CF, Gilbert N and Simard LR 2001 SMN gene duplication and the emergence of the SMN2 gene occurred in distinct hominids: SMN2 is unique to Homo sapiens. Hum. Genet. 108 255–266
Rodrigues NR, Owen N, Talbot K, et al. 1996 Gene deletions in spinal muscular atrophy. J. Med. Genet. 33 93–96
Rohner E, Yang R, Foo KS, et al. 2022 Unlocking the promise of mRNA therapeutics. Nat. Biotechnol. 40 1586–1600
Roy N, Mahadevan MS, McLean M, et al. 1995 The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell 80 167–178
Ruhno C, McGovern VL, Avenarius MR, et al. 2019 Complete sequencing of the SMN2 gene in SMA patients detects SMN gene deletion junctions and variants in SMN2 that modify the SMA phenotype. Hum. Genet. 138 241–256
Salort-Campana E and Quijano-Roy S 2020 Clinical features of spinal muscular atrophy (SMA) type 3 (Kugelberg-Welander disease). Arch. Pediatr. 27 7S23-7S28
Sangaré M, Hendrickson B, Sango HA, et al. 2014 Genetics of low spinal muscular atrophy carrier frequency in sub-Saharan Africa. Ann. Neurol. 75 525–532
Schorling DC, Pechmann A and Kirschner J 2020 Advances in treatment of spinal muscular atrophy - new phenotypes, new challenges, new implications for care. J. Neuromuscul. Dis. 7 1–13
Schultz M, Swoboda K, Farrar M, et al. 2019 AVXS-101 Gene-replacement therapy (GRT) in presymptomatic spinal muscular atrophy (SMA): Study update (P1.6–357). Neurology 92 P1.6–357
Sheth J, Patel H, Mehta S, et al. 2013 Clinical and molecular characterization of patients with gross hypotonia and impaired lower motor neuron function. Indian Pediatr. 50 591–593
Singh RN and Singh NN 2018 Mechanism of splicing regulation of spinal muscular atrophy genes. Adv. Neurobiol. 20 31–61
Soares VM, Brzustowicz LM, Kleyn PW, et al. 1993 Refinement of the spinal muscular atrophy locus to the interval between D5S435 and MAP1B. Genomics 15 365–371
Souza PVS, Pinto W, Ricarte A, et al. 2021 Clinical and radiological profile of patients with spinal muscular atrophy type 4. Eur. J. Neurol. 28 609–619
Stabley DL, Holbrook J, Scavina M, et al. 2021 Detection of SMN1 to SMN2 gene conversion events and partial SMN1 gene deletions using array digital PCR. Neurogenetics 22 53–64
Stolte B, Schreiber-Katz O, Günther R, et al. 2022 Prevalence of anti-adeno-associated virus serotype 9 antibodies in adult patients with spinal muscular atrophy. Hum. Gene. Ther. 33 968–976
Strauss KA, Farrar MA, Muntoni F, et al. 2022 Onasemnogene abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: the Phase III SPR1NT trial. Nat. Med. 28 1381–1389
Sugarman EA, Nagan N, Zhu H, et al. 2012 Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur. J. Hum. Genet. 20 27–32
Sun Y, Kong X, Zhao Z, et al. 2020 Mutation analysis of 419 family and prenatal diagnosis of 339 cases of spinal muscular atrophy in China. BMC Med. Genet. 21 133
Suthar R and Patil AN 2021 Spinal muscular atrophy therapeutics in India: Parental hopes and despair! Ann. Neurosci. 28 112–113
Tan CA, Westbrook MJ, Truty R, et al. 2020 Incorporating spinal muscular atrophy analysis by next-generation sequencing into a comprehensive multigene panel for neuromuscular disorders. Genet. Test. Mol. Biomarkers 24 616–624
Thompson TG, Morrison KE, Kleyn P, et al. 1993 High resolution physical map of the region surrounding the spinal muscular atrophy gene. Hum. Mol. Genet. 2 1169–1176
Verhaart IEC, Robertson A, Wilson IJ, et al. 2017 Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy - a literature review. Orphanet J. Rare Dis. 12 124
Verma IC, Bijarnia-Mahay S, Jhingan G, et al. 2015 Newborn screening: need of the hour in India. Indian J. Pediatr. 82 61–70
Verma IC, Kohli S, Shenbagam S, et al. 2020 Carrier screening of spinal muscular atrophy in North Indian population and its public health implications. Clin. Genet. 98 198–199
Vezain M, Saugier-Veber P, Goina E, et al. 2010 A rare SMN2 variant in a previously unrecognized composite splicing regulatory element induces exon 7 inclusion and reduces the clinical severity of spinal muscular atrophy. Hum. Mutat. 31 E1110–E1125
Vidal-Folch N, Gavrilov D, Raymond K, et al. 2018 Multiplex droplet digital PCR method applicable to newborn screening, carrier status, and assessment of spinal muscular atrophy. Clin. Chem. 64 1753–1761
Vidovic M, Freigang M, Aust E, et al. 2023 Cognitive performance of adult patients with SMA before and after treatment initiation with nusinersen. BMC Neurol. 23 216
Vijzelaar R, Snetselaar R, Clausen M, et al. 2019 The frequency of SMN gene variants lacking exon 7 and 8 is highly population dependent. PLoS One 14 e0220211
Ware G, Miller C, Jones D, et al. 2022 The clinical utility of a risk-modifying SNP to detect carriers for spinal muscular atrophy with increased sensitivity. Mol. Genet. Genomic Med. 10 e1897
Wijaya YOS, Nishio H, Niba ETE, et al. 2021 Detection of spinal muscular atrophy patients using dried saliva spots. Genes 12 1621
Wirth B, Hahnen E, Morgan K, et al. 1995 Allelic association and deletions in autosomal recessive proximal spinal muscular atrophy: association of marker genotype with disease severity and candidate cDNAs. Hum. Mol. Genet. 4 1273–1284
Wirth B, Schmidt T, Hahnen E, et al. 1997 De novo rearrangements found in 2% of index patients with spinal muscular atrophy: mutational mechanisms, parental origin, mutation rate, and implications for genetic counseling. Am. J. Hum. Genet. 61 1102–1111
Wirth B, Karakaya M, Kye MJ, et al. 2020 Twenty-five years of spinal muscular atrophy research: from phenotype to genotype to therapy, and what comes next. Annu. Rev. Genomics Hum. Genet. 21 231–261
Wu X, Wang SH, Sun J, et al. 2017 A-44G transition in SMN2 intron 6 protects patients with spinal muscular atrophy. Hum. Mol. Genet. 26 2768–2780
Zhang Y, Huang JJ, Wang ZQ, et al. 2012 Value of muscle enzyme measurement in evaluating different neuromuscular diseases. Clin. Chim. Acta. 413 520–524
Zhang Y, He J, Zhang Y, et al. 2020 The analysis of the association between the copy numbers of survival motor neuron gene 2 and neuronal apoptosis inhibitory protein genes and the clinical phenotypes in 40 patients with spinal muscular atrophy: Observational study. Medicine 99 e18809
Zhang C, Li Z, Chen M, et al. 2021 Cas12a and lateral flow strip-based test for rapid and ultrasensitive detection of spinal muscular atrophy. Biosensors 11 154
Zhong Q, Bhattacharya S, Kotsopoulos S, et al. 2011 Multiplex digital PCR: breaking the one target per color barrier of quantitative PCR. Lab Chip 11 2167–2174
Zhong ZJ, Zheng PM, Dou HH, et al. 2023 Adverse events in the treatment of spinal muscular atrophy in children and adolescents with nusinersen: A systematic review and meta-analysis. Front. Pediatr. 11 1152318
Acknowledgements
The authors thank Dr. Rakesh K Mishra and Dr. Alok Bhattacharya for their directions and insightful inputs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Alok Bhattacharya
This article is part of the Topical Collection: The Rare Genetic Disease Research Landscape in India.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aasdev, A., Sreelekshmi, R.S., Iyer, V.R. et al. Spinal muscular atrophy: Molecular mechanism of pathogenesis, diagnosis, therapeutics, and clinical trials in the Indian context. J Biosci 49, 36 (2024). https://doi.org/10.1007/s12038-023-00412-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-023-00412-9