Abstract
The aim of the present study was the direct comparison of two popular minerals, bauxite and palygorskite, as adsorbents for the removal of cephalexin (CPX) from aqueous solutions and the regeneration of the spent adsorbents through cold atmospheric plasma. Batch kinetics and isotherm studies were carried out to evaluate the effect of contact time, initial CPX concentration, adsorbent dosage, pH and temperature. The adsorbents were characterized by ATR-FTIR, N2 sorption, SEM and XRD, while several isotherm, kinetic and thermodynamic models were evaluated attempting to shed light on the adsorption mechanisms. CPX adsorption on both adsorbents was better described by Langmuir model, with an adsorption capacity of 112.36 mg/g for palygorskite and 11.79 mg/g for bauxite. Thermodynamics revealed the endothermic and the spontaneous character of the process, indicating chemisorption as the main adsorption mechanism for both adsorbents. The pseudo-second-order and the Elovich models fitted satisfactorily the adsorption onto bauxite, while adsorption onto palygorskite was well presented by Weber–Morris model, indicating that pore diffusion is also involved in the process. The adsorption capacity of both minerals decreased significantly after being used for several adsorption cycles and then almost completely recovered (regeneration efficiency was 99.6% and 98% for palygorskite and bauxite, respectively) inside a novel cold plasma microbubble reactor energized by high-voltage nanopulses, revealing the potential of these adsorbents to be reused. In addition to the regeneration of the adsorbents, the cold plasma completely eliminated the CPX transferred from the solid to the aqueous phase during the regeneration process.
Similar content being viewed by others
Introduction
In spite of the fact that numerous treatment methods have been examined over the years, the challenge in remediating wastewater is still much greater than it seems (Aggelopoulos 2022; Dotto and McKay 2020). Adsorption is among the most widely adopted, reliable and effective treatment processes for the removal of organic or inorganic contaminants from industrial wastewater (Sukmana et al. 2021; Makrygianni et al. 2019; Wang et al. 2022). Especially when natural products or agricultural wastes, being abundant in nature, are used, adsorption is fairly considered an eco-friendly approach. The thermodynamic and molecular properties of adsorption process such as affinity, capacity, shape of isotherm and the enthalpy change are the basic parameters to understand the binding processes and to provide information for the selection of adsorbents capable of preventing toxicity to the environment, animals and human (Kaur and Datta 2014; Stavrinou et al. 2018; Aggelopoulos et al. 2017; Tang et al. 2016).
The environmental pollution caused by antibiotics has become one of the most critical concerns today (Ukaogo et al. 2020). The ever-increasing presence of antibiotics in environment is mainly attributed to the necessity of their widespread use which is then disposed of as wastewater from domestic, hospital, veterinary and pharmaceutical industries in the aquatic environment. In addition, their degradation-resistant properties can cause a serious ecological and environmental problem and potential risks to human and animals (Azanu et al. 2018; Serwecińska 2020). Cephalexin (CPX) is a cephalosporin antibiotic being extensively used for the human and animals’ infections (Harrison and Bratcher 2008). Unfortunately, due to its widespread use, accumulated levels of cephalexin in drinking water have been implicated in causing mutagenic and carcinogenic effects in the human body, leading to a great need to develop appropriate treatment methods (Miao et al. 2016; Samarghandi et al. 2015; He et al. 2019). A recent study on the occurrence and fate of antibiotics present in river water in Iran confirmed that the most frequently found antibiotics were ciprofloxacin and cephalexin (Mirzaei et al. 2018).
Minerals, organic and inorganic materials are most commonly used as adsorbents (e.g., clay minerals, carbon, zeolites, polymers, etc.) having different adsorption capacities (Yousef et al. 2020; Singh et al. 2018). Clay minerals have an important role in wastewater treatment due to their unique reconstructed structure and good adsorption based on their ability to electrostatically interact with molecules or ions through ion exchange (Barakan and Aghazadeh 2021). Besides, advantages of huge reserves in nature, cheap and environmentally friendly character categorize clays as promising high-quality economical adsorbents. Among them, the palygorskite and bauxite have been considered as adsorbents for the removal of different types of pollutants, including antibiotics. In particular, ciprofloxacin and tetracycline have been examined with an adsorption capacity of 10–65 mg/g for palygorskite and ~ 6 mg/g for bauxite (Berhane et al. 2016; Chang et al. 2016; Habibi et al. 2018), while CPX adsorption on these minerals has not yet been tested. The effectiveness of the adsorption process for the removal of CPX from aqueous solutions has been reported previously, using other materials such as zeolite and activated carbon that present advantages such as simplicity and low cost (Samarghandi et al. 2015; Mohseni-Bandpi et al. 2016; Al-Khalisy et al. 2010; Acelas et al. 2021), however, with a relatively low adsorption capacity (~ 7 mg/g).
Furthermore, the adsorbents once exhausted may become hazardous for the environment resulting in secondary pollution (Younas et al. 2021). Therefore, their regeneration has been considered, mainly through chemical and thermal regeneration techniques. The two main drawbacks of chemical treatment are that the regeneration efficiency is determined by the solubility of the adsorbates and, secondly, the requirement to use additional chemicals in the process. For thermal treatment, high temperatures are mandatory for the effective oxidation of the adsorbates. In addition, the complex adsorbate–adsorbent interactions may result in an ineffective regeneration.
Cold atmospheric plasma (CAP) is a novel process which has proved its effectiveness in the fields of materials processing (Daletou and Aggelopoulos 2021) and wastewater treatment (Meropoulis and Aggelopoulos 2023; Meropoulis et al. 2021; Tang et al. 2018), among others. Plasma has emerged as an interesting alternative to conventional regeneration methods due to its high regeneration efficiency and low energy consumption (Zhou et al. 2016; Chen et al. 2013). Recently, very high regeneration efficiencies of 90% (Kalebić et al. 2022) and ~ 100% with simultaneous activation of the adsorbent during the regeneration process (Giannoulia et al. 2023) have been reported. The efficiency of plasma is based on the cooperative action of the produced reactive oxygen and nitrogen species (RONS) such as 1O2, ·OH, O, O2·−, O3, NO2–, NO3–, ONOO– and H2O2 which have high oxidation potentials (Aggelopoulos 2022). Therefore, cold plasma could be considered an alluring alternative for the effective, rapid, green and highly energy efficient regeneration of adsorbents.
In the present study, for the first time, the suitability of palygorskite and bauxite as adsorbents for the removal of CPX was investigated, and at the same time, a direct comparison was made on their effectiveness. In addition, to effectively mitigate the secondary pollution problem, both adsorbents were regenerated in a novel cold plasma reactor, operating at atmospheric air pressure, able to produce directly plasma bubbles in water. In this context, batch kinetics and isotherm studies were carried out to evaluate the impact of adsorbent dosage, contact time, initial CPX concentration, pH and temperature on the removal efficiency, while the adsorption isotherm, kinetic and thermodynamic parameters were determined. The specific surface area of each adsorbent was determined through N2 sorption, while for a better understanding of the adsorption mechanism a detailed physicochemical characterization by ATR-FTIR, SEM and XRD was also performed. In addition, the ability of the adsorbents to be reused for several cycles was investigated. Toward the sustainability of the process, the spent adsorbents were regenerated by underwater plasma microbubbles without the use of any chemical reagents and re-tested for their adsorption capacity.
Materials and methods
Materials and adsorbent preparation procedure
The raw material of palygorskite was purchased by Geohellas, while activated bauxite was kindly provided by a waste management industry (PELCO Achaean lubricants). The antibiotic cephalexin (CPX, C16H17N3O4S, M = 347.39 g mol−1) was of analytical grade and purchased from Sigma-Aldrich.
Preparation of the adsorbents
Palygorskite and bauxite were washed with triple-distilled water and dried in an oven overnight at 110 °C. The resulted solids remained in airtight containers and were subsequently used for the experiments.
Characterization of the adsorbents
N2 adsorption was used to determine the surface area of the samples, scanning electron microscopy (SEM) for their morphological characterization, powder X-ray diffraction (p-XRD) for determining the composition of the materials and attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) for examining the sorption mechanism. The BET surface area (SBET) of the two adsorbents was calculated by employing the Brunauer–Emmett–Teller (BET) model. Scanning electron microscopy was performed using a FE-SEM (Zeiss SUPRA 35VP-FEG) operating at 5 keV. The XRD analysis of the samples was performed by a Bruker D8 Advanced instrument, Ni-filtered with a CuKα1 radiation (λ = 0.154059 nm). The ATR/FTIR spectra were recorded by using a Bruker Optics ATR spectrometer (Alpha-P Diamond/Bruker Optics GmbH).
For the calculation of the point of zero charge (pHPZC), the solid addition method was used (Balistrieri and Murray 1981). To adjust the initial pH (pHi) of the solutions in the range of 1.0–12.0, aqueous solutions of 0.1 M HNO3 or NaOH were used, and then, 45 mg of bauxite and 30 mg of palygorskite were added in 15 mL of each aqueous solution. The vials were then placed in an incubator chamber with a constant temperature (28 °C) and a shaking speed of 18 rpm, until equilibrium was reached. The final step included the filtration of the suspensions and the determination of the supernatant’s final pH value (pHf). The cross point of the curve of ΔpH = pHi–pHf vs pHi determines the pHPZC value (Mall et al. 2006a).
Adsorption kinetic and equilibrium experiments
Artificially polluted water was generated by dissolving CPX into distilled water to achieve various initial concentrations (5–300 mg L−1). The adsorption experiments were performed in tightly sealed glass bottles on an overhead shaker at a speed of 18 rpm at constant temperature. A detailed parametric analysis was performed by examining the effect of several key-role parameters such as the initial pH (2.0–9.0), adsorbent dose (0.5–5 g/L), contact time and antibiotic concentration (5–300 mg L−1). To that end, tests were performed by adding the adsorbent in 15 mL of CPX solution. The impact of temperature was also investigated; the adsorption capacity was determined for both adsorbents at three different temperatures, i.e., 28, 40 and 50 °C. After each experiment, the supernatant was isolated by filtration of the suspension with a 0.22 μm pore size filter (Whatman, syringe filters) and analyzed by a UV–Vis spectrophotometer (Shimadzu, UV-1900). In order to quantify the adsorbed CPX concentration, the characteristic absorption band of CPX at 262 nm and the corresponding calibration curve were used. The CPX removal efficiency, R(%), was calculated by the following equation:
where C0 and Ce (mg/L) are the concentrations initially and at equilibrium.
The adsorption capacity at equilibrium, qe (mg/g) and the amount of the antibiotic adsorbed at a specific time t, qt (mg/g), were calculated as follows:
Ct (mg/L) is the cephalexin’s concentration at time t, while V (L) is the volume of the aqueous solution and m (g) is the adsorbent’s mass. All experiments were carried out in duplicate or triplicate.
Adsorbent regeneration through cold atmospheric plasma
Both palygorskite and bauxite were reused for four (4) adsorption cycles. Briefly, batch adsorption experiments were performed using the determined optimum values of the parameters tested. Specifically, the CPX solution (40 mg/L) was decanted from the sealed vials containing the proper adsorbent dosage, i.e., 2 g/L for palygorskite and 3 g/L for bauxite, one hour past sedimentation. Adsorbents were dried at room temperature overnight, and the next day 15 mL of fresh CPX solution (40 mg/L) was added in the sealed vials for a new adsorption cycle. Samples were collected at every cycle and subjected to analysis as described above. The process was repeated until the removal efficiency of the adsorbents dropped up to 50% of their initial removal efficiency and then subjected to regeneration.
The regeneration of the two adsorbents after their use for four cycles was examined using the cold atmospheric plasma (CAP) process. The detailed experimental setup has been previously presented (Hatzisymeon et al. 2021). Briefly, it consisted of a specially designed microbubble reactor, a nanosecond pulse generator (NPG-18/3500) producing positive high-voltage pulses of rising time of about 4 ns, a feeding gas system controlled by a flow meter and electrical characterization arrangement consisting of a digital oscilloscope (Rigol MSO2302A) in connection with voltage and current probes (Tektronix P6015A and Pearson electronic 2877, respectively).
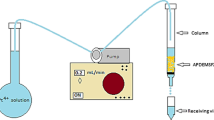
The schematic diagram of the plasma microbubble reactor is shown in Fig. 1. The reactor was comprised from a rodlike high-voltage stainless steel electrode placed in an inner quartz tube (5.0 mm internal diameter and 8.0 mm external diameter) which forms a coaxial dielectric barrier discharge (DBD) reactor with an outer quartz tube (12.0 mm and 15.0 mm internal and external diameter, respectively). The plasma feeding was injected between the two dielectrics and passed through the microholes located at the base of the outer dielectric tube resulting in the production of underwater plasma bubbles (Zhang et al. 2021; Meropoulis and Aggelopoulos 2023). The air flow rate was controlled by a mass flow controller (Aalborg GFC17) and was kept constant during the adsorbent regeneration experiments (i.e., 3 L/min). Before plasma ignition, 50 mL of distilled water containing the corresponding amount of adsorbent (30 mg for palygorskite and 45 mg for bauxite) was introduced inside the microbubble reactor. The plasma treatment time was 20 min, whereas the peak pulse voltage and pulse frequency were equal to 28 kV and 200 Hz, respectively. Cold plasma regeneration experiments were performed in duplicates.
For the calculation of the instantaneous power P(t), the waveforms of voltage V(t) and current I(t) were multiplied as previously described (Meropoulis et al. 2022). The pulse energy (Ep) dissipated in the reactor derived as the average of five successive measurements and through the integration of the instantaneous power P(t) over the pulse duration (t), according to Eq. (4):
In order to calculate the mean power P dissipated in the plasma microbubble reactor, the pulse frequency \(f\) was multiplied by the average pulse energy according to the following equation:
Energy consumption (\({E}_{cons}\); Wh/g) was calculated according to the following equation:
where \(m\) is the mass of the adsorbent treated for treatment time \(t\).
The regenerated adsorbents were tested for their removal efficiency as described above. Briefly, 2 g/L regenerated palygorskite and 3 g/L regenerated bauxite were placed in sealed vials with 15 mL of CPX solution (40 g/L), at 28 °C. After equilibrium has been established, samples were collected and subjected to analysis as described above.
Results and discussion
Characterization of palygorskite and bauxite
Point of zero charge
According to Fig. 2, the pHPZC was found ~ 6.4 and ~ 8.2 for bauxite and palygorskite, respectively. By principle, when the pH of any substance is less than the pHPZC (pH ≤ pHPZC), the sample is positively charged, leading to the preferable binding/interaction with anionic molecules, while on the contrary if the pH is greater than pHPZC (pH ≥ pHPZC), the samples develop a negative charge preferably interacting with cationic molecules.
BET analysis
The specific surface area of bauxite and palygorskite (Table 1) was determined by fitting the low-relative vapor pressure N2 adsorption isotherm to the BET equation. The presence of micropores and channels in bauxite’s and palygorskite’s structure combined with their fine particle size and rough structure are responsible for their relatively high specific surface area, 133.7 and 220.0 m2/g for bauxite and palygorskite, respectively. The particles size was ranged from 500 to 1000 μm for bauxite and 250 to 500 μm for palygorskite.
FTIR analysis
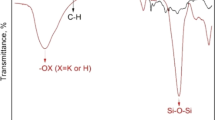
ATR-FTIR spectroscopy was performed to detect any potential changes on the surface chemistry of the materials after the adsorption process. In the ATR-FTIR spectra of both palygorskite and bauxite, several characteristic peaks were observed (Fig. 3 for palygorskite and Fig. S1 for bauxite) (Bourliva et al. 2018; Suárez and García-Romero 2006; Alhassan et al. 2022). For bauxite, the presence of hydroxyl groups (~ 3300 cm−1), Si–O–Al moieties of kaolinite Al2Si2O5(OH)4 (~ 1100–1000 cm−1) and water molecules occluded inside the aluminosilicate structure (~ 1630 cm−1) were observed. A small enhancement of the peak at 1038 cm−1 attributed to Si–O–Al moiety was noticed after the CPX adsorption (S1, Fig. S1) indicating indirectly that electrostatic interactions expressed as H bonds between kaolinite and CPX are most probably taking place. Many characteristic peaks were observed in the ATR-FTIR of palygorskite before and after CPX adsorption (Table 2). In particular, the broad peak at ~ 3550 cm−1 has been previously attributed to Al–Fe3+–OH, Al–Mg–OH or Al–Fe–OH stretching. The asymmetric band, centered at 1650 cm−1 corresponds to bending modes of absorbed and zeolitic water. Between 1200 and 400 cm−1 characteristic bands of silicate can be observed, mainly those corresponding to Si–O bonds in the tetrahedral sheet, and also to Mg-O stretching vibrations. The most intense peaks at 980 and 1020 cm−1 are those corresponding to stretching of the Si–O bond. The small shoulder at 887 cm−1 has been ascribed to the bending vibration mode of Al–Fe–OH bond, while the broad peak at 651 cm−1 is probably due to the Si–O–Si vibrations. After the adsorption small alterations on the relative intensities were observed in the region 880–1050 cm−1. This indicates that most probably H bonds are formed between the palygorskite and CPX. These H bonds may take place between the oxygen of the Si–O group of palygorskite (existing in the outer sphere of the crystal structure of palygorskite) and the NH2 group of the CPX. It should be also noted that as indicated from the kinetic/isotherm/thermodynamic study (discussed below), chemisorption is important on the adsorption process explaining hence these alterations observed in the FTIR spectra before and after CPX adsorption.
FE-SEM analysis
Representative SEM images of both adsorbents before and after CPX adsorption are shown in Fig. 4. Bauxite surface consists of fine particles and larger aggregations of different spherical sizes. It has been reported that the particles of fresh bauxite exhibit poorly crystallization in comparison with aged bauxite residue which has a stronger crystalline structures (Abd and Abbas 2019). After adsorption, the number of particles aggregations was increased probably due to bauxite-CPX interactions. Palygorskite displayed fibrous or rod-shaped formations, which is consistent with literature data (Zhang et al. 2015). Bundles of nanowires (diameter ~ 50 nm) and length up to several micrometers present straw-like aggregation morphology (Chen et al. 2012). After the adsorption, the surface becomes rough due to the CPX-palygorskite interactions.
XRD analysis
Palygorskite’s structure consists of modulated 2:1 layers, with a variable dioctahedral to trioctahedral geometry and with a general chemical formula (MgAl)2Si4O10(OH).4H2O. On the other hand bauxite is an aluminumore which mainly consists of gibbsite [Al(OH)3], bayerite [α-Al(OH)3], boehmite [γ-AlO(OH)], diaspore [α-AlO(OH)] and minor phases of kaolinite (Al2Si2O5(OH)4) and iron oxides. The XRD patterns for bauxite and palygorskite with the corresponding peak assignment are shown in Fig. 5. In the XRD pattern of palygorskite its different crystal phases were present (Bourliva et al. 2018; Habibi et al. 2018). On the other hand, in the XRD pattern of bauxite, peaks of the different minerals existing in its structure were observed (Alhassan et al. 2022).
XRD pattern of palygorskite and bauxite. The A, B, C, D, E, F and G symbols correspond to the crystal phases (A: palygorskite—(Mg,Al)2Si4O10(OH)·4(H2O), B: lizardite—Mg3(Si2O5)(OH)4, C: silicon oxide—SiO2, D: sillimanite—Al2(SiO4)O, E: aluminum oxide—δAl2O3, F: aluminum iron oxide—AlFeO3 and G: aluminum oxide—Al2O3)
Effect of experimental parameters
Effect of initial pH
The efficiency of the sorption process is pH dependent given that at certain pH values the adsorbent (palygorskite or bauxite) and the adsorbate (CPX) could exist in a cationic or ionic form. In particular, CPX has an isoelectric point at 4.5 and exists in a zwitterionic form; thus, both negatively and positively charged groups of CPX are present in the pH range 2.6 to 6.9 (Fig. 6). On the other hand, the piezoelectric charge, namely the pH value below/above in which the adsorbent’s surface is positively/negatively charged, was approximately at 6.4 and 8.2 for bauxite and palygorskite, respectively. For palygorskite, when the pH increased from 2 to 4, the removal of CPX increased significantly from 50 to 100%, while any further increase in pH value did not seem to affect the CPX removal (Fig. 6). For bauxite, in very acidic conditions (pH = 2), no CPX removal was observed, while as the pH increased up to pH = 6, an increase in removal efficiency was recorded. Notably, further increase in pH resulted in a decrease in removal efficiency. The highest removal efficiency of CPX was observed in both cases at pH around 6 (100 and 69% for palygorskite and bauxite, respectively) which could be attributed to the fact that CPX exists in its zwitterionic form (both negatively and positively charged groups of CPX) providing the possibility of the adsorbents to interact with CPX more intensely. On the other hand, in low pH values, the functional groups of the adsorbents are protonated competing the existing H3O+ in the low-pH solution to bind with the few negative sites of CPX explaining the dramatic decrease of removal efficiency in both palygorskite and bauxite. These results are in accordance with previous efforts where reduced removal efficiency of CPX was recorded (Khanday et al. 2019; Acelas et al. 2021; Samarghandi et al. 2015). At high pH values, where pH ≥ pHPZC (e.g., pH > 7 for bauxite and pH > 9 for palygorskite), an electrostatic repulsion between the anionic form of CPX and the negatively charged adsorbent is anticipated which subsequently will result in the decrease the removal rate. The latter is less profound in the case of palygorskite most likely due to the fact that the PZC of palygorskite is relatively high, i.e., ~ 8.2 (higher than the one of bauxite). From all the above, it is assumed that these adsorbents could favor the adsorption of charged pollutants.
Effect of palygorskite and bauxite adsorbent dosage
The effect of adsorbent dosage on CPX adsorption on both palygorskite and bauxite was investigated (Fig. 7). CPX removal efficiency was an increasing function of the adsorbent dosage. For palygorskite, the CPX removal efficiency increased drastically (from 10 to 95%) with increasing dosage from 0.5 to 2 g/L due to the increase in available adsorption sites (Mall et al. 2006b; Gusain et al. 2016). Further increase in adsorbent dosage slightly increased the CPX removal efficiency resulting in complete removal of CPX at dosage > 3 g/L. For bauxite, a gradual increase in removal efficiency was observed as its dosage increased from 0.5 to 5 g/L with the maximum removal efficiency being ~ 75%. In both cases, 0.5–1 g/L is not considered ideal for achieving respectable CPX removal, regardless of the high \({q}_{e}\). Balancing the optimal removal efficiency and as high as possible \({q}_{e}\), 2 g/L and 3 g/L were selected as the optimum adsorbent dosage for palygorskite and bauxite, respectively.
Effect of contact time
The effect of contact time on the adsorption capacity of palygorskite and bauxite is presented in Fig. 8. The adsorption capacity of palygorskite increased significantly within the first 30 h and then remained constant. For bauxite, equilibrium was reached after 24 h, and therefore, a more rapid adsorption kinetic compared to palygorskite was observed.
Effect of initial cephalexin concentration
Another critical parameter is the initial concentration of pollutant affecting decisively its removal efficiency. For palygorskite, almost complete CPX removal was achieved for the initial concentration range of 5–50 mg/L, while further increasing the initial concentration resulted in a gradual decrease in CPX removal (Fig. 9). However, the removal efficiency remained high (75%) even when the initial CPX concentration was 300 mg/L. For bauxite, the removal efficiency of CPX was a decreasing function of its initial concentration in aqueous solution. For an initial CPX concentration of 5 and 100 mg/L, the removal efficiency was ~ 60% and ~ 25%, respectively. In general, at higher initial pollutant concentration, more CPX molecules were not adsorbed due to saturation of binding sites (Samarghandi et al. 2015). Comparing the two adsorbents, palygorskite showed superior performance compared to bauxite as it was much more effective for a very wide range of initial CPX concentrations in water.
Isotherms study
CPX adsorption isotherms were fitted to the Langmuir and Freundlich models to describe the interaction between the adsorbates and adsorbents (Langmuir 1918; Freundlich 1907). The adsorption capacity, \({q}_{e}\) (mg/g), was an increasing function of the equilibrium CPX concentration in solution (Fig. 10) in agreement with previously published results (Song et al. 2016). Apparently, \({q}_{e}\) gradually increased reaching its maximum at the higher concentration with the corresponding \({q}_{e}\) values being 111.66 and 8.46 mg/g for palygorskite and bauxite, respectively.
The Langmuir model assumes that a monolayer adsorption occurs on the surface of a structurally homogeneous adsorbent, considering that all active sites are identical and energetically equivalent, whereas no interaction between adsorbed molecules occurs (Foo and Hameed 2010). The linearized Langmuir equation is described by the following equation (Aggelopoulos et al. 2017):
where \({q}_{e}\) (mg/g) is the amount of CPX adsorbed per unit mass of adsorbent at equilibrium, \({C}_{e}\) (mg/L) is the equilibrium concentration of the CPX in solution, \(Q\) (mg/g) is the maximum adsorption capacity of the adsorbent corresponding to monolayer coverage and \(b\) (L/mg) is the Langmuir adsorption constant describing the affinity of binding sites and the free energy of sorption. The dimensionless constant separation factor,\({R}_{L}\), is an indicator of the adsorption capacity:
where \({C}_{0}\) (mg/L) is the initial CPX concentration in aqueous solution. The adsorption process is considered favorable when \(0<{R}_{L}<1\) and unfavorable when \({R}_{L}>1\).
On the contrary, Freundlich isotherm model describes a multilayer adsorption where a non-uniform distribution of adsorption heat and affinities occur over the heterogeneous surface taking into account that interactions are taking place between adsorbed molecules (Foo and Hameed 2012). The linearized form of Freundlich equation is expressed as follows (Aggelopoulos et al. 2017):
where \(K\) ((mg/g)(L/mg)1/n) is the Freundlich adsorption constant related to the maximum adsorption capacity of the adsorbent and \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 n}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{$n$}}\) is a constant related to the adsorption intensity varying with the heterogeneity of the adsorbent (Pathania et al. 2017). When the \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 n}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{$n$}}\) values are in the 0.1–1.0 range, the adsorption process could be considered favorable (Özcan et al. 2004).
In Table 3, the calculated Langmuir and Freundlich isotherm constants, along with the correlation coefficients (\({R}^{2}\)), are presented. Langmuir model fitted the experimental data of palygorskite almost perfectly and very effectively the corresponding ones of bauxite (based on the \({R}^{2}\) values). The maximum monolayer adsorption capacities for palygorskite and bauxite were found to be 112.36 and 11.79, respectively, being very close to the experimental values. The \({R}_{L}\) and \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 n}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{$n$}}\) values revealed the favorable character of the adsorption in both cases (\(0<{R}_{L}<1\) and \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 n}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{$n$}} <1\)), indicating that the adsorption bond/interaction between the adsorbent and the adsorbate is strong. For both adsorbents, Langmuir model described clearly better the experimental data compared to the Freundlich model, indicating that a monolayer adsorption is taking place.
Adsorption kinetics
The adsorption kinetic study is considered a valuable tool by means of practical application, the appropriate design of the process and the operation control. In addition, the kinetic studies could be considered significant since provide valuable insights into the adsorption mechanism. In this study, four different adsorption kinetic models were investigated: the pseudo-first-order (PFO), the pseudo-second-order (PSO), the Elovich and the Weber–Morris models.
The pseudo-first-order kinetic equation is described by the following equation (Lagergren 1898):
where \({k}_{1}\) (1/min) is the rate constant. The PFO model fitted satisfactorily the data of palygorskite. In contrast, the PFO model did not satisfactorily fit bauxite adsorption over the entire time range (Fig. S2, Table 4). The correlation coefficients (\({R}^{2})\) were found to be 0.922 and 0.686 for palygorskite and bauxite, respectively, while the predicted adsorption capacity, \({q}_{e, cal}\), was relatively close to the \({q}_{e, exp}\) for palygorskite and significantly different for bauxite (Table 4). It is noteworthy that the initial bauxite adsorption data (up to 1 h) fitted almost perfectly to PFO model (R2 > 0.999) and the \({q}_{e, cal}\) coincided with the experimental one. In addition, the kinetic constant \({k}_{1}\) of bauxite for the initial stages was high enough, indicating that adsorption was much more rapid for bauxite compared to palygorskite.
The following pseudo-second-order kinetic model was also tested (Ho and McKay 1998):
where \({k}_{2}\) (g/mg min) is the rate constant. In contrast to the pseudo-first-order model, the pseudo-second-order kinetic model was found to fit very well (\({R}^{2}\) ≥ 0.992) the experimental data of bauxite (Fig. S3), indicating that chemisorption plays an important role in the process. On the other hand, the experimental data of palygorskite were better fitted through the PFO than PSO.
The Elovich kinetic model is regarded as one of the most useful models for describing chemical adsorption and it was also tested for fitting the experimental data. The Elovich model is expressed by the following equation (Aharoni and Tompkins 1970):
where a (mg/g min) is the initial adsorption rate and 1/b (mg/g) indicates the available sites for adsorption. The Elovich model fitted well enough the experimental data of bauxite and palygorskite (Fig. S4). The high 1/b values are indicative for the numerous available sites on the adsorbent for chemisorption (Table 2). The 1/b value of palygorskite is much higher than the one of bauxite (11.59 and 0.281 for palygorskite and bauxite, respectively), indicating that a great number of active sites are available for interactions with CPX; this is also supported by the high adsorption capacity achieved for palygorskite.
Finally, in order to evaluate the contribution of CPX diffusion within adsorbent pores, the data were fitted to the Weber–Morris intraparticle diffusion model (Acelas et al. 2021):
The parameters of the intraparticle diffusion model Kp and C were calculated (Table 4). The experimental data of palygorskite were effectively fitted, and the plot \({q}_{t}\) versus \({t}^{1/2}\) was linear (Fig. S5), indicating that pore diffusion is one of the adsorption processes. The high Kp value indicates the high rate that the CPX molecules enter inside the pores of the palygorskite through intraparticle diffusion. The high BET surface area of palygorskite (Table 1) promotes the diffusion of the CPX inside the pores of the adsorbent. For bauxite, the curve was not linear and could be divided into two linear regions (Fig. S5), meaning that the adsorption process is not governed by intraparticle diffusion (Dawood and Sen 2012). In the first region (5 min to 1 h), CPX molecules are rapidly transferred from the solution to the bauxite’s external surface through film diffusion. In the second region (2 h to 24 h), the Kp is significantly reduced, meaning that the diffusion inside the pores of bauxite is low (Stavrinou et al. 2018).
In general, minerals are considered an advantageous option for adsorption, considering that they are abundant natural materials. Palygorskite and bauxite have been previously examined as adsorbents for the removal of toxic organic compounds from water, while studies referring to antibiotics (cephalexin is a widely used antibiotic) are still very limited (Bourliva et al. 2018; Habibi et al. 2018; Chang et al. 2016; Wang et al. 2020; Alhassan et al. 2022). For instance, palygorskite has been investigated for the adsorption of tetracycline, ciprofloxacin, sulfamethoxazole, metronidazole and spiramycin (Habibi et al. 2018; Alhassan et al. 2022; Rivera et al. 2022; Chang et al. 2016, 2009). Comparing the adsorption capacity of this study with literature data, a higher adsorption was achieved herein (Qthis study = 112.36 mg/g for CPX) compared to the maximum adsorption ever reported (Q = 101 mg/g for tetracycline). On the other hand, studies on the adsorption of antibiotics on bauxite are even fewer, with similar low adsorption capacities as the present study. In terms of comparison, palygorskite’s adsorption capacity was extremely higher compared to bauxite, even if bauxite interacted and adsorbed more rapidly the pollutant. Hence, palygorskite could be considered an advantageous solution for the removal of CPX from wastewater. However, the ability of bauxite to adsorb rapidly CPX, could be an important asset and therefore its activation/modification should be considered to improve its adsorption capacity.
Thermodynamic behavior
In order to evaluate the thermodynamic parameters οφ CPX adsorption on the two adsorbents, experiments on three different temperatures, namely 28, 40 and 50 °C, were performed (Fig. 11). The thermodynamic parameters of the standard free energy (\(\Delta G\)), enthalpy (\(\Delta H\)) and entropy (\(\Delta S\)) were determined (Table 5) by the following equations (Roy et al. 2013):
where \(R\) (8.314 J/mol K) is the ideal gas constant, \(T\) (K) is the temperature and \({k}_{c}\) (L/g) is the standard thermodynamic equilibrium constant defined by \({\text{qe}}/{\text{Ce}}\). For both adsorbents, \(\Delta G\) was negative for all temperatures and decreased with increasing temperature, indicating that CPX adsorption is a spontaneous process. Moreover, the decrease in \(\Delta G\) as temperature increases, suggests that the adsorption process becomes more favorable at higher temperatures. This phenomenon can be attributed to the enhanced mobility of adsorbate ions/molecules in the solution as temperature rises, coupled with increased adsorbate/adsorbent affinity at higher temperatures. The positive \(\Delta S\) for both adsorbents confirmed the increased randomness at the solid-solution interface during the removal process and the affinity of the adsorbate/adsorbent molecules. In addition, positive \(\Delta S\) is indicative of increased degree of freedom for the adsorbed molecules (Saha and Chowdhury 2011). The positive \(\Delta H\) for both adsorbents, indicates that the adsorption is an endothermic process. \(\Delta H\) was determined as 71.02 and 44.47 kJ/mol for palygorskite and bauxite, respectively, which are above 40 kJ/mol, indicating that the adsorption mechanism is mainly controlled by chemisorption (Cantu et al. 2014).
Analysis of the adsorption mechanism
The adsorption mechanism is the key-role player affecting the overall adsorption performance. There are two types of adsorption that may occur, the chemical or/and the physical adsorption of chemical compounds onto substances. When a material possesses a large specific surface area (SBET), the enclosement of a molecule inside the adsorbent pore/cavity is feasible through physisorption. In the case of chemisorption, electrostatic interactions, complexation, ion exchange and hydrogen bonds between the adsorbent and the adsorbate may take place. In the present study, considering that (i) a significant surface area was measured and (ii) palygorskite, bauxite and CPX possess positively and negatively charged active sites, both physical and chemical sorption are possible to occur. The elucidation of the adsorption mechanism was based on all data derived from adsorption studies and minerals characterization. In brief, the three main processes taking place during the adsorption are: (i) CPX molecules diffuse from the solution to the external surface (macropores) of the adsorbent, (ii) subsequent diffusion within the micropores of the adsorbents (intraparticle diffusion) and (iii) electrostatic interactions and hydrogen bonding between the active sites of adsorbent and CPX. The extent of each of the three processes indicates the significance of either physisorption or chemisorption; this extent seemed to be slightly different between bauxite and palygorskite. For bauxite, kinetic, isotherm and thermodynamic analysis revealed that chemisorption was the main process of CPX adsorption onto its surface, while as revealed from Weber–Morris model the intraparticle diffusion was not important. Similarly, chemisorption was revealed the main adsorption mechanism for palygorskite, but the CPX diffusion inside its pores is indicative of the supportive role of CPX physisorption onto palygorskite pore structure.
In addition, when CPX existed in zwitterionic form (both negatively and positively charged groups of CPX) a maximum removal efficiency was observed (Fig. 6), indicating that the adsorbents interact with both positive and negative sites of CPX. The existence of electrostatic interactions was further supported by ATR spectroscopy; characteristic vibrations were affected after CPX adsorption. The presence of hydroxyl groups in the adsorbents suggests possible hydrogen bonding with the amino, thiol and carboxylate groups of CPX. Electrostatic interactions may also be generated between the unpaired electrons of oxygen atoms connected with Si, Al, etc. and the protonated amines on CPX. This mechanism has been also previously reported for the adsorption of CPX and other antibiotics (Noman et al. 2021). Figure 12 describes schematically the possible occurring interactions due to chemisorption between the antibiotic and the adsorbents.
Toward the sustainability of the process: the regeneration of adsorbents through underwater plasma bubbling
Regeneration efficiency of adsorbent by plasma
One of the major issues related with the adsorption process is the recovery and the sustainable management of spent adsorbents (Baskar et al. 2022). Various technologies have been examined for their regeneration including desorption, photodegradation and biodegradation. The highly reactive species generated through cold plasma (e.g., 1O2, ·OH, O3, NO2–, NO3–, ONOO–, H2O2) are able to remove and degrade antibiotics in soil and wastewater (Aggelopoulos et al. 2020a, 2020b) and therefore might be efficient for the adsorbed CPX molecules onto the minerals surface restoring their initial capacity. Very recently, the effectiveness of cold plasma to regenerate natural clinoptilolite, which has been used for the adsorption of the antibiotic ciprofloxacin, has been reported; the spent adsorbent was regenerated by ~ 90% (Kalebić et al. 2022).
In the present study, a plasma microbubble reactor, energized by high-voltage (HV) nanosecond pulses, was employed for the first time for the regeneration of spent bauxite and palygorskite (after their reuse for four cycles). Even if both bauxite and palygorskite were able to be reused for several cycles (Fig. 13), their effectiveness was gradually decreased with their adsorption capacity being ~ 40–50% lower after 4 cycles of reuse. Interestingly, when spent adsorbents were treated with HV nanopulses inside the plasma microbubble reactor, their effectiveness was almost completely recovered. After the regeneration experiments, both adsorbents exhibited almost the same adsorption capacity to that of their first use with the regeneration efficiency being 99.6% and 98% for palygorskite and bauxite, respectively, revealing the potential of these adsorbents to be reused. According to the results, it is obvious that underwater plasma bubbling achieved almost complete restoration of the saturated palygorskite and bauxite. It is important to note that plasma bubbles not only regenerated the adsorbents, but also effectively eliminated the concentration of CPX that had been transferred from the solid phase to the aqueous solution during the regeneration experiments. This was verified through the use of UV–Vis measurements on the aqueous solution following the regeneration experiments. In contrast to chemical regeneration methods, which involve the transfer of pollution from the adsorbent to the liquid phase, the present study proposes a green process that eliminates the generation of secondary pollution. Therefore, this work presents an environmentally friendly, sustainable and highly effective approach for water treatment.
CPX removal efficiency during several adsorption cycles (reuse) and after adsorbent regeneration by cold atmospheric plasma (CAP) for (a) palygorskite (contact time: 48 h, C0 = 100 mg/L, pH: 6.2, adsorbent dosage 2 g/L, T = 28 °C) and (b) bauxite (contact time: 24 h, C0 = 10 mg/L, pH: 6.2, adsorbent dosage 3 g/L, T = 28 °C)
Energy requirements of adsorbent regeneration by plasma
The recorded single-pulse voltage and current waveforms during adsorbent regeneration through underwater cold plasma bubbles and the resulting instantaneous power are shown in Fig. 14. At a pulse voltage equal to 28 kV, the measured peak pulse current was ~ 40 A. Despite the fact that the applied voltage was a unipolar pulse, the current was bipolar with positive (primary discharge) and negative pulses (secondary discharge) as it consists of conduction and displacement current. The positive current pulse increased with the rise of the pulse voltage and the negative pulse started at the falling edge of the pulse voltage. The peak of instantaneous power corresponding to the primary discharge was very high (~ 1.0 MW), while the power of the secondary discharge is provided by the dielectric layer and is deposited during the primary discharge. However, the mean power dissipated in the plasma bubble reactor was very low (~ 0.9 W) due to the quite low duty cycle involved. Based on the plasma regeneration time (20 min) and the mass of adsorbents regenerated at each experiment (45 mg for bauxite and 30 mg for palygorskite), the energy consumption (see Eq. (6)) was calculated ~ 10 Wh/g and ~ 6.7 Wh/g, for palygorskite and bauxite, respectively. These values are among the lowest in the literature (Table 6), possibly due to the combination of energy efficient HV nanopulses and enhanced plasma species diffusion and mass transfer from the gas to liquid phase in the current cold plasma bubble reactor. Therefore, underwater plasma microbubbles energized by HV nanopulses provided a unique cost-effective and green solution, simultaneously exhibiting two different important functionalities: the regeneration of the adsorbent and the elimination of the pollutant transferred from the adsorbent to the aqueous phase used for its regeneration.
Conclusions
The efficiency of two popular minerals, palygorskite and bauxite, was thoroughly investigated and compared for the removal of the antibiotic cephalexin (CPX) from synthetically polluted water. Several operating parameters were examined, such as contact time, adsorbent dosage, pH, pollutant concentration and temperature. Given that CPX exists in its zwitterionic form at pH = 3–7, an effective interaction between CPX and the active sites of the adsorbents took place, supported by ATR/FTIR spectroscopy where characteristic vibrations were affected after CPX adsorption. Palygorskite showed superior performance compared to bauxite as it was much more effective for a very wide range of initial CPX concentrations in water. CPX adsorption onto both minerals was better described by the Langmuir isotherm model; the maximum adsorption capacity was found to be 112.36 and 11.79 mg/g for palygorskite and bauxite, respectively. The thermodynamics study revealed that adsorption occurs spontaneously for both adsorbents (\(\Delta G\)<0), and is an endothermic process (\(\Delta H\)>0). Moreover, since the value of \(\Delta H\) was higher than 40 kJ/mol for both adsorbents, the adsorption mechanism was mainly controlled by chemisorption. The PSO kinetic model fitted in the most appropriate way the experimental data of bauxite, indicating the dominant character of chemisorption in the process, while as revealed from Weber–Morris model intraparticle diffusion was limited. In the case of palygorskite, the CPX diffusion inside its pores was indicative of the supportive role of CPX physisorption into palygorskite pore structure. The presence of hydroxyl groups in both adsorbents suggests possible hydrogen bonding with the amino, thiol and carboxylate groups of CPX. After 4 reuse cycles, the adsorption capacity of both minerals decreased by ~ 50%, while they were almost completely regenerated (regeneration efficiency was 99.6% and 98% for palygorskite and bauxite, respectively) when exposed to plasma treatment in a novel plasma microbubble reactor energized by high-voltage nanosecond pulses. The energy consumption of the current nanopulsed-plasma bubble regeneration process was very low and was estimated to be ~ 10 Wh/g and ~ 6.7 Wh/g, for palygorskite and bauxite, respectively. Finally, the introduction of spent adsorbents into the plasma reactor also resulted in the complete elimination of CPX transferred from solid phase to aqueous solution during the regeneration experiments. Therefore, a green, rapid, cost-effective and sustainable method for the regeneration of adsorbents without the use of chemical agents was achieved. Our next steps include the testing of these adsorbents (supported by this cold plasma-based regeneration process) for peculiar classes of pollutants with unique chemical structures and properties (e.g., perfluoroalkyl substances, PFAS).
References
Abd SS, Abbas AM (2019) Preparation, characterization and adsorption capacity of bauxite-carbon nanotube composite. Nat Environ Pollut Technol 18(3):863–869
Acelas N, Lopera SM, Porras J, Torres-Palma RA (2021) Evaluating the removal of the antibiotic cephalexin from aqueous solutions using an adsorbent obtained from palm oil fiber. Molecules 26(11):3340. https://doi.org/10.3390/Molecules26113340
Aggelopoulos CA (2022) Recent advances of cold plasma technology for water and soil remediation: a critical review. Chem Eng J 428:131657. https://doi.org/10.1016/J.Cej.2021.131657
Aggelopoulos CA, Moschopoulou E, Klepetsanis PG, Tsakiroglou CD (2017) Valorization of fruit wastes (Pistachio Shells) as adsorbent for the removal of Zn from aqueous solutions under adverse acidic conditions. Desalin Water Treat 74:174–183. https://doi.org/10.5004/Dwt.2017.20738
Aggelopoulos CA, Hatzisymeon M, Tataraki D et al (2020a) Remediation of ciprofloxacin-contaminated soil by nanosecond pulsed dielectric barrier discharge plasma: influencing factors and degradation mechanisms. Chem Eng J 393:124768. https://doi.org/10.1016/J.Cej.2020.124768
Aggelopoulos CA, Meropoulis S, Hatzisymeon M et al (2020b) Degradation of antibiotic enrofloxacin in water by gas-liquid Nsp-Dbd plasma: parametric analysis, effect of H2O2 and CaO2 additives and exploration of degradation mechanisms. Chem Eng J 398:125622. https://doi.org/10.1016/J.Cej.2020.125622
Aharoni C, Tompkins FC (1970) Advances In Catalysis, Edited By D. D. Eley, H. Pines & P. B. Weisz, p. 1–49: Academic Press.
Alhassan SI, Wang H, He Y et al (2022) Fluoride remediation from on-site wastewater using optimized bauxite nanocomposite (Bx-Ce-La@500): synthesis maximization, and mechanism of F─removal. J Hazard Mater 430:128401. https://doi.org/10.1016/J.Jhazmat.2022.128401
Al-Khalisy RS, Ama A-H, Al-Dujaili AH (2010) Aqueous phase adsorption of cephalexin onto bentonite and activated carbon. Sep Sci Technol 45:1286–1294. https://doi.org/10.1080/01496391003689017
Azanu D, Styrishave B, Darko G et al (2018) Occurrence and risk assessment of antibiotics in water and lettuce in ghana. Sci Total Environ 622–623:293–305. https://doi.org/10.1016/J.Scitotenv.2017.11.287
Balistrieri LS, Murray JW (1981) The surface chemistry of goethite (Alpha Feooh) in major ion seawater. Am J Sci 281:788–806. https://doi.org/10.2475/Ajs.281.6.788
Barakan S, Aghazadeh V (2021) The advantages of clay mineral modification methods for enhancing adsorption efficiency in wastewater treatment: a review. Environ Sci Pollut Res 28:2572–2599. https://doi.org/10.1007/S11356-020-10985-9
Baskar AV, Bolan N, Hoang SA et al (2022) Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: a review. Sci Total Environ 822:153555. https://doi.org/10.1016/J.Scitotenv.2022.153555
Berhane TM, Levy J, Krekeler MPS et al (2016) Adsorption of bisphenol a and ciprofloxacin by palygorskite-montmorillonite: effect of granule size, solution chemistry and temperature. Appl Clay Sci 132–133:518–527. https://doi.org/10.1016/J.Clay.2016.07.023
Bourliva A, Sikalidis A, Papadopoulou L et al (2018) Elimination of Cu2+ and Ni2+ ions from aqueous solutions by adsorption onto natural palygorskite and vermiculite. Clay Miner 53:1–15. https://doi.org/10.1180/Clm.2017.1
Cantu Y, Remes A, Reyna A et al (2014) Thermodynamics, kinetics, and activation energy studies of the sorption of chromium (iii) and chromium (Vi) to A Mn3O4 nanomaterial. Chem Eng J 254:374–383. https://doi.org/10.1016/J.Cej.2014.05.110
Chang P-H, Li Z, Yu T-L et al (2009) Sorptive removal of tetracycline from water by palygorskite. J Hazard Mater 165:148–155. https://doi.org/10.1016/J.Jhazmat.2008.09.113
Chang P-H, Jiang W-T, Li Z et al (2016) Interaction of ciprofloxacin and probe compounds with palygorskite Pfl-1. J Hazard Mater 303:55–63. https://doi.org/10.1016/J.Jhazmat.2015.10.012
Chen H, Zhong A, Wu J et al (2012) Adsorption behaviors and mechanisms of methyl orange on heat-treated palygorskite clays. Ind Eng Chem Res 51:14026–14036. https://doi.org/10.1021/Ie300702j
Chen J, Pan X, Chen J (2013) Regeneration of activated carbon saturated with odors by non-thermal plasma. Chemosphere 92:725–730. https://doi.org/10.1016/J.Chemosphere.2013.04.014
Daletou MK, Aggelopoulos CA (2021) Highly-energy efficient oxidation of mwcnt with nanosecond pulsed dielectric barrier discharge plasma. Appl Surf Sci 563:150139. https://doi.org/10.1016/J.Apsusc.2021.150139
Dawood S, Sen TK (2012) Removal of anionic dye congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res 46:1933–1946. https://doi.org/10.1016/J.Watres.2012.01.009
Dotto GL, Mckay G (2020) Current scenario and challenges in adsorption for water treatment. J Environ Chem Eng 8:103988. https://doi.org/10.1016/J.Jece.2020.103988
Dutta T, Kim T, Vellingiri K et al (2019) Recycling and regeneration of carbonaceous and porous materials through thermal or solvent treatment, chemical engineering journal, volume 364, 2019. Issn 514–529:1385–8947
Fallah Z, Roberts EP (2019) Combined adsorption/regeneration process for the removal of trace emulsified hydrocarbon contaminants, chemosphere. ISSN 230:596–605
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10. https://doi.org/10.1016/J.Cej.2009.09.013
Foo KY, Hameed BH (2012) Preparation, characterization and evaluation of adsorptive properties of orange peel based activated carbon via microwave induced K2CO3 activation. Biores Technol 104:679–686. https://doi.org/10.1016/J.Biortech.2011.10.005
Freundlich H (1907) Über Die Adsorption In Lösungen. Zeitschrift Für Physikalische Chemie 57u: 385. https://doi.org/10.1515/Zpch-1907-5723
Giannoulia S, Triantaphyllidou I-E, Tekerlekopoulou AG, Aggelopoulos CA (2023) Mechanisms of individual and simultaneous adsorption of antibiotics and dyes onto halloysite nanoclay and regeneration of saturated adsorbent via cold plasma bubbling. Nanomaterials 13:341. https://doi.org/10.3390/Nano13020341
Gusain D, Dubey S, Upadhyay SN et al (2016) Studies on optimization of removal of orange G from aqueous solutions by a novel Nano adsorbent, Nano zirconia. J Ind Eng Chem 33:42–50. https://doi.org/10.1016/J.Jiec.2015.08.028
Habibi A, Belaroui LS, Bengueddach A et al (2018) Adsorption of metronidazole and Spiramycin by an Algerian palygorskite. Effect of modification with tin. Microporous Mesoporous Mater 268:293–302. https://doi.org/10.1016/J.Micromeso.2018.04.020
Harrison CJ, Bratcher D (2008) Cephalosporins: a review. pediatrics in review 29: 264–267; Quiz 273. https://doi.org/10.1542/Pir.29-8-264
Hatzisymeon M, Tataraki D, Tsakiroglou C et al (2021) Highly energy-efficient degradation of antibiotics in soil: extensive cold plasma discharges generation in soil pores driven by high voltage nanopulses. Sci Total Environ 786:147420. https://doi.org/10.1016/J.Scitotenv.2021.147420
He J, Zhang Y, Guo Y et al (2019) Photocatalytic degradation of cephalexin by Zno nanowires under simulated sunlight: kinetics, influencing factors and mechanisms. Environ Int 132:105105. https://doi.org/10.1016/J.Envint.2019.105105
Ho YS, Mckay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124. https://doi.org/10.1016/S0923-0467(98)00076-1
Kalebić B, Škoro N, Kovač J et al. (2022) Regeneration of the ciprofloxacin-loaded clinoptilolite by non-thermal atmospheric plasma. Appl Surf Sci 153379. https://doi.org/10.1016/J.Apsusc.2022.153379
Kaur M, Datta M (2014) Diclofenac sodium adsorption onto montmorillonite: adsorption equilibrium studies and drug release kinetics. Adsorpt Sci Technol 32:365–387. https://doi.org/10.1260/0263-6174.32.5.365
Khanday WA, Ahmed MJ, Okoye PU et al (2019) Single-step pyrolysis of phosphoric acid-activated chitin for efficient adsorption of cephalexin antibiotic. Biores Technol 280:255–259. https://doi.org/10.1016/J.Biortech.2019.02.003
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar 24(4):1–39. https://doi.org/10.1021/Ic102040y
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/Ja02242a004
Makrygianni M, Lada ZG, Manousou A et al (2019) Removal of anionic dyes from aqueous solution by novel pyrrolidinium-based polymeric ionic liquid (Pil) as adsorbent: investigation of the adsorption kinetics, equilibrium isotherms and the adsorption mechanisms involved. J Environ Chem Eng 7:103163. https://doi.org/10.1016/J.Jece.2019.103163
Mall ID, Srivastava VC, Agarwal NK (2006a) Removal of orange-G and methyl violet dyes by adsorption onto bagasse fly ash—kinetic study and equilibrium isotherm analyses. Dyes Pigments 69:210–223. https://doi.org/10.1016/J.Dyepig.2005.03.013
Mall ID, Srivastava VC, Kumar GVA et al (2006b) Characterization and utilization of mesoporous fertilizer plant waste carbon for adsorptive removal of dyes from aqueous solution. Colloids Surf a: Physicochem Eng Asp 278:175–187. https://doi.org/10.1016/J.Colsurfa.2005.12.017
Meropoulis S, Aggelopoulos CA (2023) Plasma microbubbles vs gas-liquid dbd energized by low-frequency high voltage nanopulses for pollutants degradation in water: destruction mechanisms, composition of plasma-activated water and energy assessment. J Environ Chem Eng 11(3):109855. https://doi.org/10.1016/J.Jece.2023.109855
Meropoulis S, Rassias G, Bekiari V et al (2021) Structure-degradation efficiency studies in the remediation of aqueous solutions of dyes using nanosecond-pulsed Dbd plasma. Sep Purif Technol 274:119031. https://doi.org/10.1016/J.Seppur.2021.119031
Meropoulis S, Giannoulia S, Skandalis, et al (2022) Key-study on plasma-induced degradation of cephalosporins in water: process optimization, assessment of degradation mechanisms and residual toxicity. Sep Purif Technol 298:121639. https://doi.org/10.1016/J.Seppur.2022.121639
Miao M-S, Liu Q, Shu L et al (2016) Removal of cephalexin from effluent by activated carbon prepared from alligator weed: kinetics, isotherms, and thermodynamic analyses. Process Saf Environ Protect 104:481–489. https://doi.org/10.1016/J.Psep.2016.03.017
Mirzaei R, Yunesian M, Nasseri S et al (2018) Occurrence and fate of most prescribed antibiotics in different water environments of Tehran, Iran. Sci Total Environ 619–620:446–459. https://doi.org/10.1016/J.Scitotenv.2017.07.272
Mohseni-Bandpi A, Al-Musawi TJ, Ghahramani E et al (2016) Improvement of zeolite adsorption capacity for cephalexin by coating with magnetic Fe3O4 nanoparticles. J Mol Liq 218:615–624. https://doi.org/10.1016/J.Molliq.2016.02.092
Noman EA, Al-Gheethi A, Mohamed RMSR et al (2021) Sustainable approaches for removal of cephalexin antibiotic from non-clinical environments: a critical review. J Hazard Mater 417:126040. https://doi.org/10.1016/J.Jhazmat.2021.126040
Özcan AS, Erdem B, Özcan A (2004) Adsorption of acid blue 193 from aqueous solutions onto Na–bentonite and Dtma-bentonite. J Colloid Interface Sci 280:44–54. https://doi.org/10.1016/J.Jcis.2004.07.035
Pathania D, Sharma S, Singh P (2017) Removal Of Methylene Blue By Adsorption Onto Activated Carbon Developed From Ficus Carica Bast. Arab J Chem 10:S1445–S1451. https://doi.org/10.1016/J.Arabjc.2013.04.021
Rivera A, Hernadez D, Quiñones L et al (2022) Cuban natural palygorskite nanoclays for the removal of sulfamethoxazole from aqueous solutions. Chemrxiv Cambridge: Cambridge Open Engage. https://doi.org/10.1016/J.Envpol.2020.113944
Roy A, Adhikari B, Majumder SB (2013) Equilibrium, kinetic, and thermodynamic studies of Azo dye adsorption from aqueous solution by chemically modified lignocellulosic jute fiber. Ind Eng Chem Res 52:6502–6512. https://doi.org/10.1021/Ie400236s
Saha P, Chowdhury S (2011) Insight into adsorption thermodynamics. Thermodynamics 16:349–364
Samarghandi MR, Al-Musawi TJ, Mohseni-Bandpi A et al (2015) Adsorption of cephalexin from aqueous solution using natural zeolite and zeolite coated with manganese oxide nanoparticles. J Mol Liq 211:431–441. https://doi.org/10.1016/J.Molliq.2015.06.067
Sepehr MN, Kazemian H, Ghahramani E et al (2014) Defluoridation of water via light weight expanded clay aggregate (Leca): adsorbent characterization competing ions, chemical regeneration, equilibrium and kinetic modeling. J Taiwan Inst Chem Eng 45(4):1821–1834
Serwecińska L (2020) Antimicrobials and antibiotic-resistant bacteria: a risk to the environment and to public health. Water 12:3313
Singh NB, Nagpal G, Agrawal S et al (2018) Water purification by using adsorbents: a review. Environ Technol Innov 11:187–240. https://doi.org/10.1016/J.Eti.2018.05.006
Song Y, Xu H, Ren J (2016) Adsorption study for removal of sunset yellow by ethylenediamine-modified peanut husk. Desalin Water Treat 57:17585–17592. https://doi.org/10.1080/19443994.2015.1086897
Stavrinou A, Aggelopoulos CA, Tsakiroglou CD (2018) Exploring the adsorption mechanisms of cationic and anionic dyes onto agricultural waste peels of banana, cucumber and potato: adsorption kinetics and equilibrium isotherms as a tool. J Environ Chem Eng 6:6958–6970. https://doi.org/10.1016/J.Jece.2018.10.063
Suárez M, García-Romero E (2006) Ftir spectroscopic study of palygorskite: influence of the composition of the octahedral sheet. Appl Clay Sci 31:154–163. https://doi.org/10.1016/J.Clay.2005.10.005
Sukmana H, Bellahsen N, Pantoja F et al (2021) Adsorption and coagulation in wastewater treatment—review. Prog Agric Eng Sci 17:49–68. https://doi.org/10.1556/446.2021.00029
Tang S, Yuan D, Zhang Q et al (2016) Fe-Mn Bi-metallic oxides loaded on granular activated carbon to enhance dye removal by catalytic ozonation. Environ Sci Pollut Res 23:18800–18808. https://doi.org/10.1007/S11356-016-7030-5
Tang S, Yuan D, Rao Y, et al. (2018) Evaluation of antibiotic oxytetracycline removal in water using a gas phase dielectric barrier discharge plasma. J Environ Manage. Nov 15;226:22–29
Ukaogo PO, Ewuzie U, Onwuka CV (2020) Microorganisms For Sustainable Environment And Health, Edited By P. Chowdhary, A. Raj, D. Verma & Y. Akhter, Pp. 419–429: Elsevier.
Wang Y, Nie Q, Huang B et al (2020) Removal of ciprofloxacin as an emerging pollutant: a novel application for bauxite residue reuse. J Clean Prod 253:120049. https://doi.org/10.1016/J.Jclepro.2020.120049
Wang T, He J, Lu J et al (2022) Adsorptive removal of Ppcps from aqueous solution using carbon-based composites: a review. Chin Chem Lett. https://doi.org/10.1016/J.Cclet.2021.09.029
Younas F, Mustafa A, Farooqi ZUR et al (2021) Current and emerging adsorbent technologies for wastewater treatment: trends, limitations and environmental implications. Water 13:215
Yousef R, Qiblawey H, El-Naas MH (2020) Adsorption as a process for produced water treatment: a review. Processes 8:1657
Zhang XN, Mao GY, Jiao YB et al (2014) Adsorption of anionic dye on magnesium hydroxide-coated pyrolytic bio-char and reuse by microwave irradiation. Int J Environ Sci Technol 11:1439–1448. https://doi.org/10.1007/S13762-013-0338-5
Zhang Y, Wang W, Zhang J et al (2015) A Comparative study about adsorption of natural palygorskite for methylene blue. Chem Eng J 262:390–398. https://doi.org/10.1016/J.Cej.2014.10.009
Zhang T, Zhou R, Wang P et al (2021) Degradation of cefixime antibiotic in water by atmospheric plasma bubbles: performance, degradation pathways and toxicity evaluation. Chem Eng J 421:127730. https://doi.org/10.1016/J.Cej.2020.127730
Zhou R, Zhou R, Zhang X et al (2016) An efficient bio-adsorbent for the removal of dye: adsorption studies and cold atmospheric plasma regeneration. J Taiwan Inst Chem Eng 68:372–378. https://doi.org/10.1016/J.Jtice.2016.09.030
Acknowledgements
The authors thank the Laboratory of Applied Molecular Spectroscopy at ICE-HT/FORTH (Head: Dr. G.A. Voyiatzis) and Dr. Z. Lada for obtaining the ATR-FTIR spectra, and Dr. V. Drakopoulos for the SEM images.
Funding
This work was funded by the Horizon 2020 Framework Programme (Grant No.: 101037509).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. The authors did not receive support from any organization for the submitted work and have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giannoulia, S., Tekerlekopoulou, A.G. & Aggelopoulos, C.A. Exploration of cephalexin adsorption mechanisms onto bauxite and palygorskite and regeneration of spent adsorbents with cold plasma bubbling. Appl Water Sci 14, 51 (2024). https://doi.org/10.1007/s13201-024-02101-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02101-w