Abstract
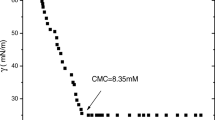
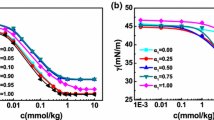
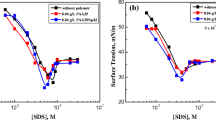
New biamphiphilic surfactants (BSs) have been synthesized based on alkylmethylmorpholinium cation and dodecyl sulfate anion (Mor-n(DS), n = 4, 6, 8, 10). The structure of the biamphiphiles has been characterized by IR spectroscopy, 1H NMR spectroscopy, mass spectrometry, and elemental analysis. The aggregation behavior of the biamphiphiles in aqueous solutions has been assessed by tensiometry, conductometry, fluorescence spectroscopy (using a pyrene probe) and dynamic and electrophoretic light scattering. It has been shown that an increase in the hydrophobic tail length by two carbon atoms in an amphiphilic cation leads to an increase in the surface activity of the surfactant by ~5 units and a decrease in the aggregation threshold of the systems by 1.5–2 times. It has been established that the formation of aggregates with hydrodynamic diameters of 20–120 nm depending on the alkyl chain length of the alkylmethylmorpholinium cation and BS concentration. The zeta potential of the systems ranges from –25 to –100 mV and decreases with increasing biamphiphile concentration. Spectrophotometry has been employed to show a significant solubilization capacity of the biamphiphiles with respect to a hydrophobic dye Orange OT. The compounds obtained may be of interest for biomedical applications and other high-tech areas.







Similar content being viewed by others
REFERENCES
Kotenko, A.A. and Khil’ko, S.L., The surface properties of solutions of dicationic imidazolium surfactants with short bridge fragments, Colloid J., 2021, vol. 83, no. 2, pp. 211–218. https://doi.org/10.1134/S1061933X21020058
Dement’eva, O.V., Mesoporous silica container particles: New approaches and new opportunities, Colloid J., 2020, vol. 82, no. 5, pp. 479–501. https://doi.org/10.1134/S1061933X20050038
Massarweh, O. and Abushaikha, Ah. S., The use of surfactants in enhanced oil recovery: A review of recent advances, Energy Reports, 2020, vol. 6, pp. 3150–3178. https://doi.org/10.1016/j.egyr.2020.11.009
Johnson, P., Trybala, A., Starov, V., and Pinfield, V. J., Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants, Adv. Colloid Interface Sci., 2021, vol. 288, p. 102340. https://doi.org/10.1016/j.cis.2020.102340
Kuznetsova, D.A., Kuznetsov, D.M., Vasileva, L.A., Amerhanova, S.K., Valeeva, D.N., Salakhieva, D.V., Nikolaeva, V.A., Nizameev, I.R., Islamov, D.R., Usachev, K.S., Voloshina, A.D., and Zakharova, L.Ya., Complexation of oligo- and polynucleotides with methoxyphenyl-functionalized imidazolium surfactants, Pharmaceutics, 2022, vol. 14, no. 12, p. 2685. https://doi.org/10.3390/pharmaceutics14122685
Dement’eva, O.V., Naumova, K.A., Shishmakova, E.M., Senchikhin, I.N., Zhigletsova, S.K., Klykova, M.V., Dunaitsev, I.A., Kozlov, D.A., Rudoy, V.M., Synthesis of bifunctional silica container particles on antiseptic micelles with solubilized curcumin and assessment of their biological activity, Colloid J., 2021, vol. 83, no. 6, pp. 651–661. https://doi.org/10.1134/S1061933X21060028
Zhil’tsova, E.P., Islamov, D.R. and Zakharova, L.Y., Estimation of the shape factor of aggregates in self-associating systems based on metallosurfactants, Colloid J, 2023, vol. 85, no. 3, pp. 358–365. https://doi.org/10.1134/S1061933X23600252
Kashapov, R.R., Mirgorodskaya, A.B., Kuznetsov, D.M., Razuvaeva, Yu.S., and Zakharova, L.Ya., Nanosized supramolecular systems: From colloidal surfactants to amphiphilic macrocycles and superamphiphiles, Colloid J., 2022, vol. 84, no. 5, pp. 502–517. https://doi.org/10.1134/S1061933X22700016
Pavlov, R.V., Gaynanova, G.A., Kuznetsova, D.A., Vasileva, L.A., Zueva, I.V., Sapunova, A.S., Buzyurova, D.N., Babaev, V.M., Voloshina, A.D., Lukashenko, S.S., Rizvanov, I.Kh., Petrov, K.A., Zakharova, L.Ya., and Sinyashin, O.G., Biomedical potentialities of cationic geminis as modulating agents of liposome in drug delivery across biological barriers and cellular uptake, Int. J. Pharm., 2020, vol. 587, p. 119640. https://doi.org/10.1016/j.ijpharm.2020.119640
Chowdhury, S., Rakshit, At., Acharjee, An., and Saha, B., Biodegradability and biocompatibility: Advancements in synthetic surfactants, J. Mol. Liq., 2021, vol. 324, p. 115105. https://doi.org/10.1016/j.molliq.2020.115105
Ghosh, S., Ray, A., Pramanik, N., and Ambade, B., Can a catanionic surfactant mixture act as a drug delivery vehicle?, C. R. Chim., 2016, vol. 19, no. 8, pp. 951–954. https://doi.org/10.1016/j.crci.2016.03.020
Ghosh, S., Ray, A., and Pramanik, N., Self-assembly of surfactants: An overview on general aspects of amphiphiles, Biophys. Chem., 2020, vol. 265, p. 106429. https://doi.org/10.1016/j.bpc.2020.106429
El Seoud, O.A., Keppeler, N., Malek, N.I., and Galgano, P.D., Ionic liquid-based surfactants: Recent advances in their syntheses, solution properties, and applications, Polymers, 2021, vol. 13, no. 7, p. 1100. https://doi.org/10.3390/polym13071100
Wasserscheid, P., van Hal, R., and Bösmann, A., 1-n-Butyl-3-methylimidazolium ([bmim]) octylsulfate—an even ‘greener’ ionic liquid, Green Chem., 2002, vol. 4, no. 4, pp. 400–404. https://doi.org/10.1039/B205425F
Rao, K.S., Trivedi, T.J., and Kumar, A., Aqueous-biamphiphilic ionic liquid systems: Self-assembly and synthesis of gold nanocrystals/microplates, J. Phys. Chem. B, 2012, vol. 116, no. 49, pp. 14363–14374. https://doi.org/10.1021/jp309717n
Bharmoria, P., Mehta, M.J., Pancha, I., and Kumar, A., Structural and functional stability of cellulase in aqueous-biamphiphilic ionic liquid surfactant solution, J. Phys. Chem. B, 2014, vol. 118, no. 33, pp. 9890–9899. https://doi.org/10.1021/jp506211b
Pal, A., Punia, R., and Dubey, G.P., Formation of mixed micelles in an aqueous mixture of a biamphiphilic surface active ionic liquid and an anionic surfactant: Experimental and theoretical study, J. Mol. Liq., 2021, vol. 337, p. 116355. https://doi.org/10.1016/j.molliq.2021.116355
Pal, A. and Punia, R., Self-aggregation behaviour of cationic surfactant tetradecyltrimethylammonium bromide and bi-amphiphilic surface active ionic liquid 3‑methyl-1-pentylimidazolium dodecylsulfate in aqueous solution, J. Mol. Liq., 2020, vol. 304, p. 112803. https://doi.org/10.1016/j.molliq.2020.112803
Shi, J. and Shen, X., Construction of supramolecular self-assemblies based on the biamphiphilic ionic liquid−β-cyclodextrin system, J. Phys. Chem. B, 2014, vol. 118, no. 6, pp. 1685–1695. https://doi.org/10.1021/jp4113188
Singh, G., Singh, G., and Kang, T.S., Micellization behavior of surface active ionic liquids having aromatic counterions in aqueous media, J. Phys. Chem. B, 2016, vol. 120, no. 6, pp. 1092–1105. https://doi.org/10.1021/acs.jpcb.5b09688
Singh, G., Komal, Singh, M., Singh, O., and Kang, T.S., Hydrophobically driven morphologically diverse self-assembled architectures of deoxycholate and imidazolium-based biamphiphilic ionic liquids in aqueous medium, J. Phys. Chem. B, 2018, vol. 122, no. 50, pp. 12227–12239. https://doi.org/10.1021/acs.jpcb.8b10161
Kaur, M., Kaur, H., Singh, M., Singh, G., and Kang, T.S., Biamphiphilic ionic liquid based aqueous microemulsions as an efficient catalytic medium for cytochrome c, Phys. Chem. Chem. Phys., 2021, vol. 23, no. 1, pp. 320–328. https://doi.org/10.1039/D0CP04513F
Pavlov, R., Valeeva, F., Gaynanova, G., Kuznetsov, D., and Zakharova, L., Aggregation of morpholinium surfactants with amino alcohols as additives: A close look, Surf. Innovations, 2023, vol. 11, nos. 1–3, pp. 169–177. https://doi.org/10.1680/jsuin.22.00006
Hong, J.Y., Kim, J-K., Song, Y.K., Park, J.S., and Kim, C.K., A new self-emulsifying formulation of itraconazole with improved dissolution and oral absorption, Journal of Controlled Release, 2006, vol. 110, no. 2, pp. 332–338. https://doi.org/10.1016/j.jconrel.2005.10.002
Mirgorodskaya, A.B., Lukashenko, S.S., Yatskevich, E.I., Kulik, N.V., Voloshina, A.D., Zobov, V.V., Zakharova, L.Y., Konovalov, A.I., Kudryavtsev, D.B., and Panteleeva, A.R., Aggregation behavior, anticorrosion effect, and antimicrobial activity of alkylmethylmorpholinium bromides, Prot. Met. Phys. Chem. Surf., 2014, vol. 50, pp. 538–542. https://doi.org/10.1134/S207020511404011X
Chiappe, C., Pomelli, C.S., and Rajamani, S., Influence of structural variations in cationic and anionic moieties on the polarity of ionic liquids, J. Phys. Chem. B, 2011, vol. 115, no. 31, pp. 9653–9661. https://doi.org/10.1021/jp2045788
Obliosca, J.M., Arco, S.D., and Huang, M.H., Synthesis and optical properties of 1-alkyl-3-methylimidazolium lauryl sulfate ionic liquids, J. Fluoresc., 2007, vol. 17, pp. 613–618. https://doi.org/10.1007/s10895-007-0236-7
Kuznetsova, D.A., Kuznetsov, D.M., Vasileva, L.A., Toropchina, A.V., Belova, D.K., Amerhanova, S.K., Lyubina, A.P., Voloshina, A.D., and Zakharova, L.Ya., Pyrrolidinium surfactants with a biodegradable carbamate fragment: Self-assembling and biomedical application, J. Mol. Liq., 2021, vol. 340, p. 117229. https://doi.org/10.1016/j.molliq.2021.117229
Kuznetsova, D.A., Kuznetsov, D.M., Amerhanova, S.K., Buzmakova, E.V., Lyubina, A.P., Syakaev, V.V., Nizameev, I.R., Kadirov, M.K., Voloshina, A.D., and Zakharova, L.Ya., Cationic imidazolium amphiphiles bearing a methoxyphenyl fragment: Synthesis, self-assembly behavior, and antimicrobial activity, Langmuir, 2022, vol. 38, no. 16, pp. 4921–4934. https://doi.org/10.1021/acs.langmuir.2c00299
Kuznetsov, D.M., Kuznetsova, D.A., Gabdrakhmanov, D.R., Lukashenko, S.S., Nikitin, Y.N., and Zakharova, L.Ya., Triallyl ammonium amphiphiles: Self-assembly and complexation with bovine serum albumin, Surf. Innovations, 2022, vol. 10, nos. 4–5, pp. 298–311. https://doi.org/10.1680/jsuin.21.00044
Kuznetsov, D.M., Kuznetsova, D.A., and Zakharova, L.Ya., Liposomes modified with borneol-containing surfactants for transdermal delivery of hydrophilic substrates, Russ. Chem. Bull., 2022, vol. 71, no. 9, pp. 1887–1896. https://doi.org/10.1007/s11172-022-3606-z
Samarkina, D.A., Gabdrakhmanov, D.R., Lukashenko, S.S., Khamatgalimov, A.R., Kovalenko, V.I., and Zakharova, L.Y., Cationic amphiphiles bearing imidazole fragment: From aggregation properties to potential in biotechnologies, Colloids Surf., A, 2017, vol. 529, pp. 990–997. https://doi.org/10.1016/j.colsurfa.2017.07.018
Perinelli, D.R., Cespi, M., Lorusso, N., Palmieri, G.F., Bonacucina, G., and Blasi, P., Surfactant self-assembling and critical micelle concentration: One approach fits all?, Langmuir, 2020, vol. 36, no. 21, pp. 5745–5753. https://doi.org/10.1021/acs.langmuir.0c00420
Vasileva, L.A., Kuznetsova, D.A., Valeeva, F.G., Vasileva, E.A., Lukashenko, S.S., Gaynanova, G.A., and Zakharova, L.Ya., Micellar nanocontainers based on cationic surfactants with a pyrrolidinium head group for increasing drug bioavailability, Russ. Chem. Bull., 2021, vol. 70, pp. 1341–1348. https://doi.org/10.1007/s11172-021-3221-4
Perinelli, D.R., Cespi, M., Casettari, L., Vllasaliu, D., Cangiotti, M., Ottaviani, M.F., Giorgioni, G., Bonacucina, G., and Palmieri, G.F., Correlation among chemical structure, surface properties and cytotoxicity of N-acyl alanine and serine surfactants, Eur. J. Pharm. Biopharm., 2016, vol. 109, pp. 93–102. https://doi.org/10.1016/j.ejpb.2016.09.015
Shaban, S.M., Kang, J., and Kim, D.–H., Surfactants: Recent advances and their applications, Composites Communications, 2020, vol. 22, p. 100537. https://doi.org/10.1016/j.coco.2020.100537
Kolesnikova, E.N. and Glukhareva, N.A., Micellization in solutions of anionic surfactants with two ionogenic groups, Colloid J., 2008, vol. 70, no. 2, pp. 184–188. https://doi.org/10.1134/s1061933x08020105
Mabrouk, M.M., Hamed, N.A., and Mansour, F.R., Spectroscopic methods for determination of critical micelle concentrations of surfactants; A comprehensive review, Appl. Spectrosc. Rev., 2023, vol. 58, no. 3, pp. 206–234. https://doi.org/10.1080/05704928.2021.1955702
Chatterjee, A., Moulik, S.P., Sanyal, S.K., Mishra, B.K., and Puri, P.M., Thermodynamics of micelle formation of ionic surfactants: A critical assessment for sodium dodecyl sulfate, cetyl pyridinium chloride and dioctyl sulfosuccinate (Na salt) by microcalorimetric, conductometric, and tensiometric measurements, J. Phys. Chem. B, 2001, vol. 105, no. 51, pp. 12823–12831. https://doi.org/10.1021/jp0123029
Piñeiro, L., Novo, M., and Al–Soufi, W., Fluorescence emission of pyrene in surfactant solutions, Adv. Colloid Interface Sci., 2015, vol. 215, pp. 1–12. https://doi.org/10.1016/j.cis.2014.10.010
Aguiar, J., Carpena, P., Molina–Bolívar, J.A., and Carnero Ruiz, C., On the determination of the critical micelle concentration by the pyrene 1 : 3 ratio method, J. Colloid Interface Sci., 2003, vol. 258, no. 1, pp. 116–122. https://doi.org/10.1016/S0021-9797(02)00082-6
Pisárčik, M., Devínsky, F., and Pupák, M., Determination of micelle aggregation numbers of alkyltrimethylammonium bromide and sodium dodecyl sulfate surfactants using time-resolved fluorescence quenching, Open Chem., 2015, vol. 13, no. 1, pp. 922–931. https://doi.org/10.1515/chem-2015-0103
Israelachvili, J.N., Mitchell, D.J., and Ninham, B.W., Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers, J. Chem. Soc., Faraday Trans. 2, 1976, vol. 72, pp. 1525–1568. https://doi.org/10.1039/F29767201525
Vashishat, R., Sanan, R., Ray, D., Aswal, V.K., and Mahajan, R.K., Biamphiphilic ionic liquids-drug mixtures: Interactional and morphological aspects, ChemistrySelect, 2018, vol. 3, no. 25, pp. 7089–7099. https://doi.org/10.1002/slct.201801296
Zakharova, L.Ya., Vasilieva, E.A., Mirgorodskaya, A.B., Zakharov, S.V., Pavlov, R.V., Kashapova, N.E., and Gaynanova, G.A., Hydrotropes: Solubilization of nonpolar compounds and modification of surfactant solutions, J. Mol. Liq., 2023, vol. 370, p. 120923. https://doi.org/10.1016/j.molliq.2022.120923
Saha, U., De, R., and Das, B., Interactions between loaded drugs and surfactant molecules in micellar drug delivery systems: A critical review, J. Mol. Liq., 2023, vol. 382, p. 121906. https://doi.org/10.1016/j.molliq.2023.121906
Tehrani-Bagha, A. and Holmberg, K., Solubilization of hydrophobic dyes in surfactant solutions, Materials, 2013, vol. 6, no. 2, pp. 580–608. https://doi.org/10.3390/ma6020580
Vasilieva, E.A., Kuznetsova, D.A., Valeeva, F.G., Kuznetsov, D.M., and Zakharova, L.Ya., Role of polyanions and surfactant head group in the formation of polymer–colloid nanocontainers, Nanomaterials, 2023, vol. 13, no. 6, p. 1072. https://doi.org/10.3390/nano13061072
Gabdrakhmanov, D.R., Samarkina, D.A., Krylova, E.S., Kapitanov, I.V., Karpichev, Y., Latypov, Sh.K., Semenov, V.E., Nizameev, I.R., Kadirov, M.K., and Zakharova, L.Ya., Supramolecular systems based on novel amphiphiles and a polymer: aggregation and selective solubilization, J. Surfactants Deterg., 2019, vol. 22, no. 4, pp. 865–874. https://doi.org/10.1002/jsde.12257
Tehrani–Bagha, A. and Holmberg, K., Solubilization of hydrophobic dyes in surfactant solutions, Materials, 2013, vol. 6, no. 2, pp. 580–608. https://doi.org/10.3390/ma6020580
Mirgorodskaya, A.B., Yackevich, E.I., Gabdrakhmanov, D.R., Lukashenko, S.S., Zuev, Yu.F., and Zakharova, L.Ya., Self-organization and lipoplex formation of cationic surfactants with morpholinium head group, J. Mol. Liq., 2016, vol. 220, pp. 992–998. https://doi.org/10.1016/j.molliq.2016.05.010
Mata, J., Varade, D., Ghosh, G., and Bahadur, P., Effect of tetrabutylammonium bromide on the micelles of sodium dodecyl sulfate, Colloids Surf., A, 2004, vol. 245, nos. 1–3, pp. 69–73. https://doi.org/10.1016/j.colsurfa.2004.07.009
Funding
The work was supported by the Russian Science Foundation (project no. 23-73-01035).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of int-erest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
APPENDIX
APPENDIX
4-Butyl-4-methylmorpholinium dodecyl sulfate. Yield, 1.3 g (80.6%); M.p., 62–65°C. IR spectrum (KBr), (ν, cm–1): 3460, 2959, 2920, 2852, 1648, 1469, 1381, 1223, 1131, 1113, 1075, 1019, 992, 912, 896, 827, 724, 631, 586. 1H NMR spectrum (400 MHz, CDCl3, δ ppm, J Hz): 0.87 t (OSO\(_{3}^{ - }\)–(CH2)11–CH3, 3H, 3JHH 6.8); 1.00 t (N+–(CH2)3–CH3, 3H, 3JHH 7.3); 1.24–1.34 m (N+–(CH2)2–CH2–CH3, OSO\(_{3}^{ - }\)–(CH2)3(CH2)8– CH3, 18H); 1.42–1.48 m (OSO\(_{3}^{ - }\)–(CH2)2–CH2–(CH2)8–CH3, 2H); 1.60–1.67 m (OSO\(_{3}^{ - }\)–CH2-CH2–(CH2)9–CH3, 2H); 1.76 m (N+–CH2–CH2–CH2–CH3, 2H); 3.38 s (N+–CH3, 3H); 3.51 m (N+–CH2–(CH2)2–CH3, 2H); 3.59 – 3.69 two m (N+–CH2–CH2–O–, 4H); 3.96 – 4.09 two m (OSO\(_{3}^{ - }\)–CH2–(CH2)10–CH3, N+–CH2–CH2–O–, 6H). Found, %: C, 59.47; H, 10.74; N, 3.22; S, 7.48. Calculated for C21H45NO5S%: C, 59.54; H, 10.71; N, 3.31; S, 7.57. ESI mass spectrum, m/z: [M–C12H25OSO3]+ 158.05; [C12H25OSO3]– 265.14 (calc. m/z for C21H45NO5S 423.30).
4-Hexyl-4-methylmorpholinium dodecyl sulfate. Yield 1.1 g (71.4%). M.p. 87−92°C. IR spectrum (KBr), ν, cm–1: 3456, 2957, 2923, 2854, 1638, 1468, 1379, 1248, 1222, 1132, 1117, 1062, 999, 919, 900, 790, 724, 623, 580. NMR spectrum 1H (600 MHz, D2O, δ ppm, J Hz): 0.95 t (OSO\(_{3}^{ - }\)–(CH2)11–CH3, 3H, 3JHH 6.9); 1.00 t (N+–(CH2)5–CH3, 3H, 3JHH 6.9); 1.37–1.46 m (N+–(CH2)2–(CH2)3–CH3, OSO\(_{3}^{ - }\)–(CH2)2–(CH2)9–CH3, 24H); 1.74 m (OSO\(_{3}^{ - }\)–CH2–CH2–(CH2)9–CH3, 2H); 1.89 m (N+–CH2–CH2–(CH2)3–CH3, 2H); 3.28 s (N+–CH3, 3H); 3.53–3.63 m (N+–CH2–CH2–O–, N+–CH2–(CH2)4–CH3, 6H); 4.05 m (OSO\(_{3}^{ - }\)–CH2–(CH2)10–CH3, 2H); 4.12 m (N+–CH2–CH2–O–, 4H). Found, %: C, 61.08; H, 10.99; N, 3.10; S, 7.01. Calculated for C23H49NO5S%: C, 61.16; H, 10.93; N, 3.11; S, 7.10. ESI mass spectrum, m/z: [MC12H25OSO3]+ 186.15; [C12H25OSO3]– 265.12 (calc. m/z for C23H49NO5S 451.33).
4-Methyl-4-octylmorpholinium dodecyl sulfate. Yield 1.1 g (74.3%). M.p. 100–102°C. IR spectrum (KBr), ν, cm–1: 3447, 2957, 2924, 2853, 1637, 1468, 1439, 1379, 1251, 1226, 1120, 1096, 1061, 1016, 995, 913, 900, 857, 784, 723, 644, 62 2, 581, 536. NMR spectrum 1H (600 MHz, CDCl3, δ ppm, J Hz): 0.87 t (OSO\(_{3}^{ - }\)–(CH2)11–CH3, 3H, 3JHH 7.1); 0.88 t (N+–(CH2)7–CH3, 3H, 3JHH 7.1); 1.24–1.39 two m (N+–(CH2)2–(CH2)5–CH3, OSO\(_{3}^{ - }\)–(CH2)2–(CH2)9–CH3, 28Н); 1.64 m (OSO\(_{3}^{ - }\)–CH2–CH2–(CH2)9–CH3, 2H); 1.76 m (N+–CH2–CH2–(CH2)5–CH3, 2H); 3.38 s (N+–CH3, 3H); 3.48 m (N+–CH2–(CH2)6–CH3, 2H); 3.54–3.57, 3.66–3.68 two m (N+–CH2–CH2–O–, 4H); 3.95–3.99 m (N+–CH2–CH2–O–, 4H); 4.04–4.08 m (OSO\(_{3}^{ - }\)–CH2–(CH2)10–CH3, 2H). Found, %: C, 62.66; H, 11.15; N, 2.84; S, 6.60. Calculated for C25H53NO5S: C, 62.59; H, 11.13; N, 2.92; S, 6.68. ESI mass spectrum, m/z: [MC12H25OSO3]+ 214.03; [C12H25OSO3]– 265.14 (calc. m/z for C25H53NO5S 479.36).
4-Decyl-4-methylmorpholinium dodecyl sulfate. Yield 1.1 g (78%). M.p. 98–100°C. IR spectrum (KBr), ν, cm–1: 3489, 2957, 2922, 2853, 1640, 1469, 1381, 1248, 1227, 1120, 1061, 1015, 992, 904, 855, 786, 723, 623, 581. NMR spectrum 1H (400 MHz, CDCl3, δ ppm, J Hz): 0.86–0.89 t (OSO\(_{3}^{ - }\)–(CH2)11–CH3, N+–(CH2)9–CH3, 6H); 1.25–1.37 two m (N+–(CH2)2–(CH2)7–CH3, OSO3¯–(CH2)2–(CH2)9–CH3, 32Н); 1.65 m (OSO\(_{3}^{ - }\)–CH2–CH2–(CH2)9–CH3, 2H); 1.77 m (N+–CH2–CH2–(CH2)7–CH3, 2H); 3.38 s (N+–CH3, 3H); 3.48 m (N+–CH2–(CH2)8–CH3, 2H); 3.54–3.58, 3.65–3.69 two m (N+–CH2–CH2–O–, 4H); 3.95–4.01 m (N+–CH2–CH2–O–, 4H); 4.04–4.10 m (OSO\(_{3}^{ - }\)–CH2–(CH2)10–CH3, 2H). Found, %: C, 63.79; H, 11.28; N, 2.82; S, 6.24. Calculated for C27H57NO5S%: C, 63.86; H, 11.31; N, 2.76; S, 6.31. ESI mass spectrum, m/z: [MC12H25OSO3]+ 242.07; [C12H25OSO3]– 265.15 (calc. m/z for C27H57NO5S 507.40).
Rights and permissions
About this article
Cite this article
Kuznetsov, D.M., Kuznetsova, D.A., Valeeva, F.G. et al. New Polyfunctional Biamphiphilic Surfactants Based on Alkylmethylmorpholinium Cation and Dodecyl Sulfate Anion. Colloid J 86, 64–85 (2024). https://doi.org/10.1134/S1061933X23601051
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X23601051