Abstract
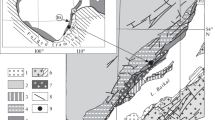
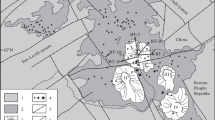
Oxygen crystallising was obtained from olivine, chromite separates from host rock samples, and gabbro from ultramafic cumulates, harzburgite, and dunite samples collected from tectonites of the Guleman ophiolites. The study includes the oxygen isotope compositions from the Guleman ophiolite and the evolutionary scenarios of geochemical and isotopic signatures. The olivine and chromitite δ18Oolivine/chromite and δ18Omelt values point out these minerals and melt isotopic compositions similar to mantle values and rich, heavy stable isotopes. The highest δ18Ochromite isotopic composition values indicate that as the heavy isotope in the melt increases, the concentration in the mineral increases. Excluding a dunite sample, the values of the δ18Owhole rock of the dunite and pyroxenite samples are similar to mantle values. The gabbro samples have higher δ18Owhole rock values than the two harzburgites. Hereby, the chromite formations are the later fractional crystallisation products than olivine and dunites due to the lowering of δ18O. The lower oxygen isotopic composition in studied samples than the normal mantle values, and these values point out subducted hydrothermally alteration. The higher δ18O isotopic compositions could be explained by serpentinization on the ocean floor at low temperatures.




Similar content being viewed by others
REFERENCES
G. Aktaş and H. F. Robertson, in The Geological Evolution of the Eastern Mediterranean, Ed. by J. E. Dixon and A. H. F. Robertson, Spec. Publ.—Geol. Soc. London (Blackwell Sci. Publ., Oxford, London, 1984), vol. 17, pp. 375–401.
I. Bindeman, A. Gurenko, O. Sigmarsson, and M. Chaussidon, Geochim. Cosmochim. Acta 72, 4397–4420 (2008). https://doi.org/10.1016/j.gca.2008.06.010
C. E. Bucholz, O. Jagoutz, J. A. VanTongeren, J. Setera, and Z. Wang, Geochim. Cosmochim. Acta 207, 154–184 (2017). https://doi.org/10.1016/j.gca.2017.03.027
J. Chaumba, Can. Mineral. 52 (3), 473–486 (2014). https://doi.org/10.3749/canmin.52.3.473
C. Chen, B.-X. Su, Y. Xiao, P. A. Sakyi, X.-Q. He, K.-N. Pang, İ. Uysal, A. Avcı, and L.-P. Qin, Lithos 342–343, 361–369 (2019).
J. M. Eiler, K. A. Farley, J. W. Valley, E. Hauri, H. Craig, S. Hart, and E. M. Stolper, Geochim. Cosmochim. Acta 61, 2281–2293 (1996). https://doi.org/10.1016/s0016-7037(97)00075-6
J. M. Eiler, Rev. Mineral. Geochem. 43, 319–364 (2001). https://doi.org/10.1515/9781501508745-008
J. Eiler, M. Edward, E. M. Stolper, and M. C. McCanta, J. Petrol. 52 (8), 1393–1413 (2011). https://doi.org/10.1093/petrology/egr006
T. Engin, M. Balcı, Y. Sümer, and Y. Z. Özkan, Bull. Miner. Res. Explor. 95, 34–56 (1981).
M. A. Ertür, M. Beyarslan, S. L. Chung, and T. H. Lin, Geosci. Front. 9 (6), 1829–1847 (2018). https://doi.org/10.1016/j.gsf.,2017.09.008
R. T. Gregory and H. P. Taylor, J. Geophys. Res. 86, 2737–2755 (1981). https://doi.org/10.1029/jb086ib04p0273
C. Höfer, S. Kraus, H. Miller, et al., J. S. Am. Earth Sci. 14, 113–126 (2001). https://doi.org/10.1016/s0895-9811(01)00011-6
T. K. Kyser, Rev. Mineral. Geochem. 16, 141–164 (1986). https://doi.org/10.1515/9781501508936-010
D. Lowry, W. U. Peter, P. W. U. Appel, and H. Rollinson, Precambrian Res. 126 (3), 272–288 (2003). https://doi.org/10.1016/S0301-9268(03)00099-8
D. Mattey, D. Lowry, and C. Macpherson, Earth Planet. Sci. Lett. 128, 231–241 (1994). https://doi.org/10.1016/0012-821x(94)90147-3
M. T. McCulloch, R. T. Gregory, G. J. Wasserburg, and H. P. Taylor, J. Geophys. Res. 86, 2721–2735 (1981). https://doi.org/10.1029/jb086ib04p02721
K. Muehlenbachs, Chem. Geol. 145 (3-4), 263–273 (1998). https://doi.org/10.1016/S0009-2541(97)00147-2
Y. Z. Özkan and O. Öztunalı, in Proc. Int. Symp. on the Geology of the Taurus Belt, Sept. 26–29, 1984, Ed. by O. Tekeli and M. C. Göncüoğlu (Ankara, 1984), pp. 285–294.
G. Özek, M. Akgül, N. Nurlu, and N. Yapici, Konjes 20 (2), 29–44 (2017). https://doi.org/10.17780/ksujes.298746
O. Parlak, T. Rızaoğlu, U. Bağcı, et al., Tectonophysics 473 (2), 285–294 (2009).
M. E. Rizeli, A. F. Bingöl, K. L. Wang, and H. Y. Lee, Lithos 436, 106958 (2023).
A. H. F. Robertson, Lithos 65 (1–2), 1–67 (2002). https://doi.org/10.1016/S0024-4937(02)00160-3
N. G. Rudraswami, Y. Marrocchi, M. S. Prasad, D. Fernandes, J. Villeneuve, and S. Taylor, Meteorit. Planet. Sci. 54/6, 1347–1361 (2019). https://doi.org/10.1111/maps.13281
S. Rouméjon, M. J. Williams, and G. L. Früh-Green, Lithos 323, 156–173 (2018).
R. O. Sack and M. S. Ghiorso, Am. Min. 76 (5–6), 827–847 (1991).
S. Villiger, P. Ulmer, O. Müntener, and A. B. Thompson, J. Petrol. 45, 2369–2388 (2004). https://doi.org/10.1093/petrology/egh042
A. Sar, M. A. Ertürk, and M. Rizeli, Lithos 350–351 (105263) (2019). https://doi.org/10.1016/j.lithos.2019.105263
A. M. C. Şengör and Y. Yılmaz, Tectonophysics 75, 181–241 (1981). https://doi.org/10.1016/0040-1951(81)90275-4
H. P. Taylor and M. F. Sheppard, Rev. Mineral. Geochem. 16, 227–271 (1986).
I. Uysal, A. Kapsiotis, R. M. Akmazc, et al., Ore Geol. Rev. 93, 98–113 (2018). https://doi.org/10.1016/j.oregeorev.2017.12.017
C. G. Wang, W. L. Xu, D. B. Yang, Y. S. Liu, F. P. Pei, Q. L. Li, and Q. J. Zhou, Geochem. Geophys. Geosyst. 19, 1913–1924 (2018).
H. Yu, H. F. Zhang, H. Zou, and J. F. Xu, Am. Min. 107 (5), 904–913 (2022). https://doi.org/10.2138/am-2022-7990
F. Z. Zhao and Y. F. Zheng, Chem. Geol. 193, 59–80 (2003).
Y. F. Zheng, Geochim. Cosmochim. Acta 55, 2299–2307 (1991).
Y. F. Zheng, Geochim. Cosmochim. Acta 57, 1079–1091 (1993).
ACKNOWLEDGMENTS
The authors thank Prof. Dr. Torsten Vennemann for oxygen isotope analysis at Laussane University Labs. We thank the anonymous reviewers for their helpful criticism and insightful remarks, which significantly enhanced our manuscript.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ertürk, M.A., Kara, H. & Kalender, L. Oxygen Isotope Composition of the Silicate Minerals and Chrome Ores in the Guleman Ophiolite in Southeastern Türkiye. Dokl. Earth Sc. (2024). https://doi.org/10.1134/S1028334X23602651
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1028334X23602651