Abstract
Knowledge of the large carnivore guild is important in view of a possible lion reintroduction into the Comoé National Park (CNP), northern Côte d’Ivoire. We used camera trapping to assess activity patterns, habitat selection, and ecological factors influencing the occupancy of the spotted hyena Crocuta crocuta. Our results showed that the presence of leopard Panthera pardus, the only current other large carnivore and thus potential competitor, did not influence hyena occupancy. Hyena occupancy was only significantly related to Euclidian distance to nearest water point. Manly’s alpha habitat selection index values show a preference of spotted hyena for shrub savannah (αSa = 0.71), and Pianka’s overlap index showed low spatial competition with leopard (OIJ(PNC) = 0.12). Our findings showed that spotted hyena were mostly nocturnal, and generally active at the same time slots with leopard, leading to a high index of temporal overlap (OIJ(time) = 0.78). The kernel density estimation confirmed that spotted hyenas and leopard shared almost half of their activity patterns (Δ1 = 0.49 and 95% CI = 0.26–0.71). Prey diversity is high in CNP but anthropogenic threats appear to suppress population growth of prey and predators. Conservation efforts should continue to reduce pressures before contemplating a potential lion Panthera leo reintroduction.
Similar content being viewed by others
Introduction
The last century has seen an upsurge in anthropogenic disturbance, with important consequences for ecosystems on a planetary scale (Mallon et al. 2015; Sarrazin and Barbault 1996). Among these consequences are declining biodiversity and species extinction (Edmond 2010; Ceballos et al. 2017). According to the World Wildlife Fund, populations of endangered species declined by 60% between 1970 and 2014, and 22 to 36% of known mammal species are currently threatened with extinction worldwide (Bauer et al. 2015; Craigie et al. 2010; Hours 2018). This worrying situation also extends to birds with 14%, and amphibians with 31 to 53% in danger of extinction (IUCN SSC Cat Specialist Group 2018).
In West African savannah regions, where wildlife is mainly confined to protected areas (Brashares et al. 2001), anthropogenic pressures are intensifying the degree of decline of many species. The situation is extremely worrying as there is accelerating population decline in many species due to hunting, habitat loss, and degradation (IUCN SSC Cat Specialist Group 2018). Several species of large carnivores are thus threatened and in danger of extinction (Bauer et al. 2015; IUCN SSC Cat Specialist Group 2018; Nowell and Jackson 1996; Woodroffe 2000).
Predators play an essential role in maintaining ecosystems and their removal can have negative cascading effects (Krebs et al. 1995; Miller et al. 2001). Predators are also important in traditional and modern cultures, both socio-culturally and economically (Simberloff 1998). Their value in sport hunting, tourism, and traditional and spiritual-medicinal uses gives them considerable economic and socio-cultural worth (Maddox 2003; Sogbohossou et al. 2017).
As one of the largest protected areas in West Africa, the Comoé National Park (CNP) is representative of the savannah biomes of Côte d’Ivoire (WCF 2008, 2010). This conservation area is a vast reservoir of diverse flora and fauna, maintaining the ecological processes by which these species interact (Lauginie 2007). The CNP has been a UNESCO World Heritage site since 1983 (Kouadio et al. 2006). However, over time, human activities such as poaching, gold mining, and transhumance have had a negative impact on the quality of the natural habitats present in the park (Atta et al. 2021; OIPR 2015). The PNC has the largest viable population of spotted hyena Crocuta crocuta and leopard Panthera pardus in Côte d’Ivoire (OIPR 2015), although leopards are also found in Tai National Park (Jenny 1996).
CNP was home to populations of large carnivores including the lion, which disappeared in the civil war a decade ago (Aglissi et al. 2023a) as have cheetahs Acinonyx jubatus and African wild dogs Lycaon pictus. The anthropogenic pressures that led to the extinction of the lion persist in the CNP, and predators such as spotted hyenas (hyenas hereafter) and leopards are under constant threat from intensive poaching and habitat destruction in this ecosystem (Atta et al. 2020). Research and conservation actions focused on these predators are needed for a sound conservation strategy. The study of diversity and spatio-temporal habitat use is of particular importance. However, scientific research on these predators in the wild is limited to work by Kouakou (2018) and the Office Ivoirien des Parcs et Réserves (OIPR) in 2015, 2018, and 2021, providing an overview of the threats and an inventory of the groups present in the CNP. This lack of research is especially problematic as management has formulated the reintroduction of lions to the park as a key objective for the next decade (Aglissi et al. 2023b).
As the hyena is the most common large carnivore currently present in the protected area, our research questions were (1) how does it select habitat; (2) what is the spatial and temporal overlap with leopards, the only potential competitor in the area; and (3) which anthropogenic and ecological factors influence its occupancy? A better understanding of these dynamics will shed light on ecological processes and inform appropriate conservation measures; it will also establish baselines for the monitoring of lion reintroduction if and when that happens.
Methods
Study area
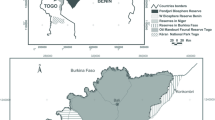
Comoé National Park is located in the northeast of the Republic of Côte d’Ivoire, between latitudes 8°30′–9°36′ North and longitudes 3°6′–4°25′ West, covering an area of approximately 11,500 km2 (Fig. 1).The major watercourse is the Comoé River, which crosses the park in its western part from North to South. The climate is tropical with a rainy and a dry season; the latter can last up to 8 months and covers the period from October to May. This is the period for development and management activities in CNP. The rainy season runs from June to September, and the park is mostly inaccessible during this period. March is the hottest month, with average temperatures around 37 °C, while January is the coolest with an average temperature of around 15 °C. Annual precipitation ranges from 900 to 1200 mm with an average of 1084 mm per year (Fischer et al. 2002; OIPR 2015). There are four main vegetation types in CNP: gallery forests, forest patches, tree savannah, and grassy savannah. Savanna formations occupy more than 80% of the total area of the park (Poilecot 1990; Schweter 2016). CNP is not fenced but “hard edged,” i.e., with communities living all around it (Aglissi et al. 2023a, b).
As CNP is located in the savannah-forest transition zone, it hosts both savannah and forest species, including the chimpanzee Pan troglodytes and forest elephant Loxodonta cyclotis (Lauginie 2007; OIPR 2015). Despite the fact that the secondary streams and most pools dry up for several months of the year, the Comoé, Iringou, and Kongo Rivers, along with some other tributaries, provide wildlife with ample access to water. Several saltpans allow the animals to meet their mineral requirements. Recent work in CNP, including the last aerial census, identified 19 mammal species, with Western hartebeest Acelaphus buselaphus major, African buffalo Syncerus caffer, kob Kobus kob, and roan antelope Hippotragus equinus the most common (Atta et al. 2020; Bouché, 2016; Linchant et al. 2022; Viljoen 2019). In unpublished camera trapping sessions in previous years, hyenas were regularly detected but leopards were rare; no density estimates are available.
Data collection and analysis
We deployed cameras traps in 125 cells of a grid of 5 km × 5 km from February to June 2022; we excluded grid cells in marginal or encroached areas of CNP (Fig. 1). Camera traps were rotating between stations and mean camera days in stations were 33.82 ± 6.11. We used Browning Strike Force Pro XD, Browning Strike Force Pro HD, and Victure Trail Camera HC300 passive infrared cameras. We placed one camera trap per station, at a convenient location within a 500-m radius centroid of the grid cell. Others have placed cameras along roads inside their grid cells to maximize detection, as roads are often intensively used by wildlife. However, there are very few roads in CNP so cameras were instead placed near to other cues in the centroid, such as evidence of wildlife presence (footprints, droppings, carcasses), or proximity of a waterhole or saline. Cameras were attached to trees or poles at a height between 40 and 50 cm above the ground and set to operate continuously 24 h a day with three images per trigger and a 30-s interval after each trigger (Sogbohossou and Aglissi 2017). We carried out a regular check every 10 to 15 days to verify camera functioning, download pictures, and proceed to battery and/or device changes as needed.
We recorded the GPS coordinates for all camera traps installed at stations as well as habitat characteristics (Table 1). Habitat classification was done by visual inspection at the camera station; we discerned the following vegetation types: tree savannah (consisting of trees over 5 to 8 m tall), shrub savannah (consisting of shrubs over 5 m tall), wooded savannah, and open forest (Formation of closed, multi-layered forests with a fairly closed canopy and including both gallery and island forests that are mapped separately in Fig. 1) (Schweter 2016). Soil types were classified as sandy, clayey, silty, hydromorphic, or lateritic, also by visual inspection at the camera station. Other variables were measured by gathering evidence within the 500-m centroid (signs of poaching such as remains of a campfire, of transhumance such as footprints of herds of cattle, of gold mining such as holes in the ground, and occurrence of fires in stations), or by calculation (Euclidean distance of the camera to nearest village, road, or waterpoint). The presence of wildlife was determined from the camera’s images and divided into leopard, small carnivores (average adult body mass < 50 kg), large prey (> 200 kg), or small prey (< 200 kg).
To avoid pseudo-replication, when investigating the spatio-temporal activity pattern of hyena, we considered only independent captures that were taken from different stations or at least 30 min apart from the same station, or depicted unambiguously different individuals in the same station (Ancrenaz et al. 2012; Farris et al. 2015). For each detected species, we calculated the capture rate (Ancrenaz et al. 2012; Burton et al. 2015; MacKenzie et al. 2003) and the frequency of independent capture events following Hausser et al. (2017) (Fiske and Chandler 2011; Kellner et al. 2023). We assessed the overlap between the hyena and leopard with Pianka’s index (Farris et al. 2015; Pianka 1973; Sogbohossou et al. 2018):
where Pij and Pik are the proportions of site i used by species j and k respectively. This index has a value from 0 (no overlap) to 1 (complete overlap).
The temporal activity pattern was estimated using the number of independent captures over the time of 24-day hours. Times of observations were converted to radians for the overlap estimation through the probability density function using the kernel density estimate (Foster et al. 2013; Ridout and Linkie 2009). We estimated the coefficient of overlap (Δ), which varies from 0 (no overlap) to 1 (complete overlap). Based on our sample size, we estimated Δ1, which is more suitable for small samples. We calculated the 95% confidence interval (95% CI) of Δ using 10,000 bootstrap samples (Sogbohossou et al. 2018).
The occupancy rate of each species was then calculated (Schuette et al. 2013), and preferential habitats of hyena and leopard were determined by calculating the Manly alpha habitat selection index for the different habitat types surveyed. The calculation was done following the method presented by Manly et al. (1972):
For each habitat type, when the value of Manly’s alpha index is:
-
✔ \({\varvec{\upalpha}}\mathbf{i}=\boldsymbol{ }\frac{1}{{\varvec{m}}}\), there is no preference
-
✔ \({\varvec{\upalpha}}\mathbf{i}>\boldsymbol{ }\frac{1}{{\varvec{m}}}\), then habitat i is preferred
-
✔ αi <\(\frac{1}{{\varvec{m}}}\) reflects avoidance of habitat i
In these equations, αi is the Manly alpha selection index for habitat i, ri is the proportion of sites used in the habitat type by a given species (i = 1, 2, 3 …m), ni is the proportion of habitat i available in the study environment, and m is the maximum number of habitat types in the environment. In our case, the number of habitats surveyed was four (m = 4) (Schweter 2016).
To determine factors influencing large carnivores’ occupancy, we added variables linked to wildlife presence to the anthropogenic and environmental variables after image processing. These were as follows: presence of small prey (bush duiker Sylvicapra grimmia, red-flanked duiker Cephalophus rufilatus, Maxwell’s duiker Philantomba maxwelli, yellow-backed duiker Cephalophus silvicultor and oribi Ourebia ourebi) and presence of large prey (buffalo, roan antelope, hartebeest, kob antelope, waterbuck Kobus ellipsiprymnus defassa, bushbuck Tragelaphus scriptus and common warthog Phacochoerus africanus). The presence of small carnivores (African civet Civettictis civetta, serval Leptailurus serval, striped jackal Canis adustus, common genet Genetta genetta, honey badger Mellivora capensis, marsh mongoose Atilax paludinosus, slender mongoose Herpestes sanguineus and white-tailed mongoose Ichneumia albicauda) was also included. The presence of leopard also as potential competitor was also included.
We performed occupancy analysis on hyena detections, investigating the influence of various factors on their presence and detectability. The factors include habitat types (tree savannah, shrub savannah, wooded savannah, and open forest), soil types (sandy, clayey, silty, hydromorphic, or lateritic), human disturbance (signs of poaching, of transhumance, of gold mining, and occurrence of fires), environmental covariates (altitude, presence of waterpoint, Euclidean distance of the camera to nearest waterpoint, village, or road), and presence of wildlife (leopard, small carnivores, or large and small prey). We organized our data for the single season occupancy models using the unmarked package in R (Fiske and Chandler 2011; Kellner et al. 2023). Next, multiple MacKenzie et al. (2002) occupancy models were constructed, each exploring separate covariates and different combinations of covariates additively. The models were based on zero-inflated binomial models (Doherty 2006; Royle and Dorazio 2008). The occupancy state (Eq. 1) and observation (Eq. 2) process are modeled respectively:
\({{\text{Z}}}_{{\text{i}}}\) is the true occupancy state \(\uppsi\) parameter describing the true state, drawn from a Bernoulli distribution. \({p}_{{\text{ij}}}\) is the detection probability.
Using model selection criteria based on AIC values, we identified the model with the best fit and parsimony. Finally, we focused on this selected model, transforming parameter estimates back to original scales for easier interpretation.
We did not perform occupancy modeling for leopard as the number of detections was too low for this species.
Results
For a total trapping effort of 3205 days, we obtained 3057 independent captures for 32 mammal species (Table 2). We gathered 53 independent large carnivore detections (n = 42 for the spotted hyena and n = 11 for the leopard). Herbivores, all potential prey except the elephant Loxodonta africana, scored the highest number of independent captures with 69% (n = 2101 for 11 species), followed by small carnivores (13%; n = 399 for eight species), and primates (10%; n = 310 for five species). Further details are presented in Table 2.
Distribution and habitat selection of spotted hyena
Of 125 stations, 10% (n = 13) detected hyena or leopard, with only 2% (n = 3) detecting both. Hyena detection did was not different between stations that had or had not detected leopard (χ2 = 1.496; p = 0.221). The Pianka’s spatial overlap index was low (OIJ (PNC) = 0.12), suggesting a low spatial competition between hyena and leopard. The Manly’s alpha habitat selection index showed a preference of hyenas for shrub savannah (αSa = 0.71; Fig. 2). Leopard also showed a preference for shrub savannah (αSa = 0.64) and a slight preference for wooded savannah (αSb = 0.27). Thus, although the two species were rarely detected in the same station, they prefer the same habitat types.
Temporal overlap
Both predator species were nocturnal, except one leopard detection during the day. Hyena activity peaked between 2 and 6 h while leopard activity peaked between 18 and 22 h. The Pianka’s overlap index was high, Oij (time) = 0.78 (n = 41 captures). In addition, the kernel density estimation revealed that the significant temporal overlap between spotted hyenas and leopard was important as Δ1 = 0.49 (95% CI = 0.26–0.71) (Fig. 3).
Determinants of spotted hyena occupancy
A total of 30 models were tested (Table 3); model 17 was the best, indicating that the only significant covariate was EDNWP (Euclidian Distance to Nearest Water Point). This covariate exhibits an estimate of 0.811 on the logit scale, yet a standard error of 14.1 and non-significant z-value (0.0576, p-value = 0.954) indicate that this variable is not statistically influential in explaining occupancy. In terms of detection, this covariate that is related to detection shows marginal significance (− 0.0228 on the logit scale, z = − 2.05, p-value = 0.04) (Table 4), suggesting a weak influence on detection probability (0.217 \(\pm\) 0.0193, Table 5). The model’s AIC value of 767.9654 indicates its goodness of fit, while the successful convergence after 21 iterations denotes a stable optimization process across 125 sites.
Discussion
Our study confirmed the absence from the CNP of three of the five large carnivores found in West Africa: the lion, cheetah, and wild dog. The number of spotted hyena and leopard detections was low, despite the availability of a diversity of prey. Large predator numbers are not determined by prey abundance alone (Kolowski and Holekamp 2009). Many anthropogenic drivers such as illegal activities and edge effects affect large carnivores (Balme et al. 2010; Duangchatrasiri et al. 2019; Milda et al. 2023; Sogbohossou et al. 2018). It should be noted that our study area corresponds to the core of CNP, where anthropogenic pressures are more controlled and prey abundance is higher; detections would probably have been even lower outside this core (Henschel and Ray 2003; Sogbohossou et al. 2018).
As observed elsewhere in Africa, hyenas in CNP were strictly nocturnal whereas leopards were largely nocturnal (Hayward and Hayward 2007; Hayward and Slotow 2009; Schuette et al. 2013; Sogbohossou et al. 2018). Their activity peaks were at different times (2:00 to 4:00 for hyena, 18:00 to 20:00 for leopard). Similar findings were found for hyenas in Pendjari National Park, Benin (Sogbohossou et al. 2018). The CNP is always prone to numerous anthropogenic disturbances such as poaching and illegal gold mining, which can further explain strictly nocturnal behavior of both species (Barrueto et al. 2014; Sogbohossou et al. 2018). In addition, the CNP belong to sub-Saharan regions where daytime temperature is often high. This could also explain the restriction of spotted hyena activities to hours of darkness in this protected area where humans are frequently abroad daylight hours (Kolowski et al. 2007).
Spotted hyena and leopard had low spatial but high temporal overlap. Our results revealed that spotted hyena and leopard mainly preferred shrub savannahs, but leopard was also detected in wooded savannahs. The main use of shrub savannas by spotted hyena could be explained by putatively higher prey density and proximity to seasonal streams, as in (Kolowski and Holekamp 2009). In addition, as species’ detection occurs during their movements, for these two predators, their observations in shrub savannahs would also be justified by the search for food and water resources. The leopard is known to prefer to keep its prey in trees, which could explain its detection in wooded savannahs, unlike the spotted hyena (Bohm and Honer 2015; Ouchaou et al. 2015; Stein et al. 2020). The Pianka’s overlap index indicates a high overlap in habitat selection between the two species. So, even though the two species avoid crossing each other, they tend to use the same habitat types in CNP.
For the hyena, as for most wildlife, distance to water is an important parameter. Indeed, waterholes are poles of attraction for prey, and opportunistic predators like the hyena prefer these areas (Kolowski and Holekamp 2009). Even though they drink infrequently, spotted hyenas are relatively dependent on water (Bohm and Honer 2015). Our occupancy analysis did not find other significant covariates, but this may in part be due to the low number of detections.
Our study covered the core area with higher prey densities; we do not expect higher detection rates in the rest of CNP. Our low number of detections suggest low abundance despite the diversity of prey species, and despite CNP being one of the largest national parks in the region. This suggests that anthropogenic disturbance is limiting numbers of both prey and large carnivores. The low diversity and abundance of predators currently present in CNP may be an asset for a possible future lion reintroduction. However, before contemplating a lion reintroduction program, management needs to improve in order to reduce disturbance and increase prey availability in CNP.
Availability of data and materials
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
References
Aglissi J, Sogbohossou EA, Bolam J, Bauer H (2023) Community knowledge on factors behind extirpation of lion Panthera leo in Comoé National Park, Côte d’Ivoire (West Africa). Afr J Ecol 00:1–11. https://doi.org/10.1111/aje.13214
Aglissi J, Sogbohossou EA, Bauer H (2023) Community perspectives on the prospect of lion (Panthera leo) reintroduction to Comoé National Park, Côte d’Ivoire (West Africa). Wildlife Biology e01116. https://doi.org/10.1002/wlb3.01116
Ancrenaz M, Hearn AJ, Ross J, Sollman R, Wilting A (2012) 2012. In BBEC Publication (Issue January, Handbook for wildlife monitoring using camera-traps
Atta CJA, Blaise K, Ouattara S, Yao Roger K (2020) Abondance et distribution des buffles Syncerus caffer (Sparrman, 1779) dans le Parc National de la Comoé, Nord-Est de la Côte d’Ivoire. J Ani Plant Sci 45(3):8024–8037. https://doi.org/10.35759/janmplsci.v45-3.6
Atta CJA, Ouattara S, Blaise K, Yao Roger K (2021) Some uses of the African buffalo Syncerus caffer (sparrman, 1779) by the populations living around the Comoé National Park (North-East Ivory Coast). J Anim Plant Sci 47(2):8484–8496. https://doi.org/10.35759/JAnmPlSci.v47-2.6
Balme GA, Slotow R, Hunter LTB (2010) Edge effects and the impact of non-protected areas in carnivore conservation: leopards in the Phinda-Mkhuze Complex. South Africa Animal Conservation 13(3):315–323. https://doi.org/10.1111/j.1469-1795.2009.00342.x
Barrueto M, Ford AT, Clevenger AP (2014) Anthropogenic effects on activity patterns of wildlife at crossing structures. Ecosphere 5(3). https://doi.org/10.1890/ES13-00382.1
Bauer H, Chapron G, Nowell K, Henschel P, Funston P, Hunter LTB, Macdonald DW, Packer C (2015) Lion (Panthera leo) populations are declining rapidly across Africa, except in intensively managed areas. Proc Natl Acad Sci USA 112(48):14894–14899. https://doi.org/10.1073/pnas.1500664112
Bohm T, Honer OR (2015) Spotted hyaena (Crocuta crocuta). The IUCN Red List of Threatened Species 2015, III Part A e.T5674A45194782
Bouché P (2016) Comptage aérien de la faune du Parc national de la Comoé. Site du Patrimoine mondial et d’une de Réserve de Biosphère - Côte d’Ivoire et des deux zones de biodiversité. p 64
Brashares JS, Arcese P, Sam MK (2001) Human demography and reserve size predict wildlife extinction in West Africa. Proceedings of the Royal Society b: Biological Sciences 268(1484):2473–2478. https://doi.org/10.1098/rspb.2001.1815
Burton AC, Neilson E, Moreira D, Ladle A, Steenweg R, Fisher JT, Bayne E, Boutin S (2015) Wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. J Appl Ecol 52(3):675–685. https://doi.org/10.1111/1365-2664.12432
Ceballos G, Ehrlich PR, Dirzo R (2017) Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc Natl Acad Sci USA 114(30):E6089–E6096. https://doi.org/10.1073/pnas.1704949114
Craigie ID, Baillie JEM, Balmford A, Carbone C, Collen B, Green RE, Hutton JM (2010) Large mammal population declines in Africa’s protected areas. Biol Cons 143(9):2221–2228. https://doi.org/10.1016/J.BIOCON.2010.06.007
Duangchatrasiri S, Jornburom P, Jinamoy S, Pattanvibool A, Hines JE, Arnold TW, Fieberg J, Smith JLD (2019) Impact of prey occupancy and other ecological and anthropogenic factors on tiger distribution in Thailand’s western forest complex. Ecol Evol 9(5):2449–2458. https://doi.org/10.1002/ece3.4845
Edmond EP (2010) Convention sur la diversité biologique — Il faudra établir un protocole lors de la rencontre de Nagoya. Le Devoir. Repéré à. Accessed 17 Apr 2010 https://www.ledevoir.com/societe/environnement/287103/convention-sur-la-diversite-biologique-il-faudra-etablir-un-protocole-lors-de-la-rencontre-de-nagoya
Farris ZJ, Gerber BD, Karpanty S, Murphy A, Andrianjakarivelo V, Ratelolahy F, Kelly MJ (2015) When carnivores roam: temporal patterns and overlap among Madagascar’s native and exotic carnivores. J Zool 296(1):45–57. https://doi.org/10.1111/jzo.12216
Fischer F, Gross M, Linsenmair KE (2002) Updated list of the larger mammals of the Comoé National Park. Ivory Coast Mammalia 66(1):83–92. https://doi.org/10.1515/mamm.2002.66.1.83
Fiske IJ, Chandler RB (2011) Unmarked: An R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Soft 43(10):1–23. https://doi.org/10.18637/jss.v043.i10
Foster VC, Sarmento P, Sollmann R, Tôrres N, Jácomo ATA, Negrões N, Fonseca C, Silveira L (2013) Jaguar and Puma activity patterns and predator-prey interactions in four brazilian biomes. Biotropica 45(3):373–379. https://doi.org/10.1111/btp.12021
Hausser Y, Tagand R, Vimercati E, Mermod S, Fischer C (2017) Comparing survey methods to assess the conservation value of a community-managed protected area in western Tanzania. Afr J Ecol 55(1):1–11. https://doi.org/10.1111/aje.12301
Hayward MW, Hayward GJ (2007) Activity patterns of reintroduced lion Panthera leo and spotted hyaena Crocuta crocuta in the Addo Elephant National Park. South Africa African Journal of Ecology 45(2):135–141. https://doi.org/10.1111/j.1365-2028.2006.00686.x
Hayward MW, Slotow R (2009) Temporal partitioning of activity in large african carnivores: tests of multiple hypotheses. African Journal of Wildlife Research 39(2):109–125. https://doi.org/10.3957/056.039.0207
Henschel P, Ray J (2003) Léopards dans les forêts pluviales d’Afrique: méthodes de relevé et de surveillance. Wild Conserv Soc 60
Hours C (2018) La Terre a perdu 60 % de ses animaux sauvages en 44 ans. Le Devoir. Repéré à. Accessed 30 Oct 2018 https://www.ledevoir.com/societe/environnement/540185/la-terre-a-perdu-60-de-ses-animaux-sauvages-en-44-ans
IUCN SSC Cat Specialist Group (2018) Directives pour la Conservation du Lion en Afrique 158
Jenny D (1996) Spatial organization of leopards Panthera pardus in Taï National Park, Ivory Coast: is rainforest habitat a “tropical haven”? J Zool 240(3):427–440. https://doi.org/10.1111/j.1469-7998.1996.tb05296.x
Kellner KF, Smith AD, Royle JA, Kéry M, Belant JL, Chandler RB (2023) The unmarked R package: twelve years of advances in occurrence and abundance modelling in ecology. Methods Ecol Evol 14(6):1408–1415. https://doi.org/10.1111/2041-210X.14123
Kolowski JM, Holekamp KE (2009) Ecological and anthropogenic influences on space use by spotted hyaenas. J Zool 277(1):23–36. https://doi.org/10.1111/j.1469-7998.2008.00505.x
Kolowski JM, Katan D, Theis KR, Holekamp KE (2007) Daily patterns of activity in the spotted hyena. J Mammal 88(4):1017–1028. https://doi.org/10.1644/06-MAMM-A-143R.1
Kouadio BH, Tahoux MT, Biemi J (2006) Apport de la Télédétection et d'un système d'information Géographique (SIG) à la Gestion Intégrée et participative des aires protégées en Côte D'Ivoire: application au Parc national de la Comoé (Reserve de Biosphère). Unesco /Mab, p 82
Kouakou JP (2018) Données préliminaires sur la présence des grands carnivores dans le Parc National de la Comoé (Cote d’Ivoire). Mémoire à l’Université Felix Houphouët Boigny Abidjan, p 46
Krebs CJ, Boutin S, Boonstra R, Sinclair ARE, Smith JNM, Dale MRT, Martin K, Turkington R (1995) Impact of food and predation on the snowshoe hare cycle. Science 269(5227):1112–1115. https://doi.org/10.1126/science.269.5227.1112
Lauginie F (2007) Conservation de la nature et aires protégées en Côte d’Ivoire. Edition CEDA- NEI. p 688
Linchant J, Sabdano N, Loïc E, Vincke X (2022) Rapport d’inventaire aérien du Parc National de la Comoé 2022, version révisée du 14.11.22
MacKenzie DI, Nichols JD, Hines JE, Knutson MG, Franklin AB (2003) Estimating site occupancy, colonization, and local extinction when a species is detected imperfectly. Ecology 84(8):2200–2207. https://doi.org/10.1890/02-3090
Mackenzie DI, Nichols JD, Lachman GB, Droege S, Andrew J, Langtimm CA, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83(8):2248–2255
Doherty F Jr (2006) Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence, by Darryl I. MacKenzie, James D. Nichols, J. Andrew Royle, Kenneth H. Pollock, Larissa L. Bailey, James E. Hines. Wild Soc Bull 34:1243–1244. https://doi.org/10.2193/0091-7648(2006)34[1243:BREAMI]2.0.CO;2
Maddox TM (2003) The ecology of cheetahs and other large carnivores in a pastoralist-dominated buffer zone. Doctoral thesis (Ph.D), UCL (University College London)
Mallon DP, Hoffman M, McGowan PJK (2015) An IUCN situation analysis of terrestrial and freshwater fauna in West and Central Africa. International Union for Conservation of Nature. https://doi.org/10.2305/iucn.ch.2015.ssc-op.54.en
Manly BFJ, Miller P, Cook LM (1972) Analysis of a selective predation experiment. Am Nat 106(952):719–736. https://doi.org/10.1086/282808
Milda D, Ashish K, Ramesh T, Kalle R, Thanikodi M (2023) Evaluation of anthropogenic pressure on the occupancy patterns of large mammals in the Western and Eastern Ghats. Landscape Ecol 38(2):409–422. https://doi.org/10.1007/s10980-022-01592-9
Miller B, Dugelby B, Foreman D, del Rio CM, Noss R, Phillips M, Reading R, Soule ME, Terborgh J, Willcox L (2001) TThe importance of large carnivores to healthy ecosystems. Endangered Species Update 18:202–210. Retrieved from http://scholar.googleusercontent.com/scholar?
Nowell K Jackson P (1996) Wild cats: Status Survey and Conservation Action Plan. IUCN, Gland, Switzerland. Ouchaou B, Merzoug S, Roubet C (2015) – P10. Panthère (léopard): Paléontologie. Encyclopédie berbère, Peeters Publishers (Louvain/Paris), XXXVII, p 6092–6097
OIPR (2015) OFFICE IVOIRIEN DES PARCS ET RESERVES Site du Patrimoine mondial et d’une Réserve de biosphère
Ouchaou B, Merzoug S, Roubet C (2015) – P10. Panthère (léopard): Paléontologie. Encyclopédie berbère, Peeters Publishers (Louvain/Paris), XXXVII, p 6092–6097
Pianka ER (1973) Lizard species diversity. Ecology 48:333–351
Poilecot P (1990) Les formations végétales du Parc national de la Comoé. Actes de séminaire de Korhogo sur l’aménagement des aires protégées. – UNESCO, pp 285–308
Ridout MS, Linkie M (2009) Estimating overlap of daily activity patterns from camera trap data. J Agric Biol Environ Stat 14(3):322–337. https://doi.org/10.1198/jabes.2009.08038
Royle JA, Dorazio RM (2008) Hierarchical modeling and inference in ecology: the analysis of data from populations, metapopulations and communities. Academic Press. https://doi.org/10.1016/B978-0-12-374097-7.50001-5
Sarrazin F, Barbault R (1996) Reintroduction: challenges and lessons for basic ecology. Trends Ecol Evol 11(11):474–478. https://doi.org/10.1016/0169-5347(96)20092-8
Schuette P, Wagner AP, Wagner ME, Creel S (2013) Occupancy patterns and niche partitioning within a diverse carnivore community exposed to anthropogenic pressures. Biol Cons 158(February):301–312. https://doi.org/10.1016/j.biocon.2012.08.008
Schweter (2016) Mission d'appui à l’interprétation des images satellites du Parc national de la Comoé et sa zone périphérique, rapport de mission. p 28
Simberloff, D. 1998. ScienceDirect_-_Biological_Conservation_Flagships_umbrellas_and_keystones_Is_single-species_man‐agement_passé_in_the_landscape_era_Simberloff_1998.pdf. – Biol Conserv 83:247–257
Sogbohossou EA, Ismaila TI, Marcel H, Sinsin B (2017) Diversite Et Importance Socio-Economique Des Services Ecosystemiques Dans La Reserve De Biosphere De La Pendjari Au Nord-Benin De La Pendjari Au Nord-Benin. J Rech Sci Univ Lomé 19(3):15–28
Sogbohossou EA, Aglissi J (2017) Diversity of small carnivores in Pendjari biosphere reserve, Benin. J Entomol Zool Stud 5:1429–1433
Sogbohossou EA, Kassa BD, Waltert M, Khorozyan I (2018) Spatio-temporal niche partitioning between the African lion (Panthera leo leo) and spotted hyena (Crocuta crocuta) in western African savannas. Euro J Wildl Res 64:1. https://doi.org/10.1007/s10344-017-1159-5
Stein AB, Athreya V, Gerngross P, Balme G, Henschel P, Karanth U, Miquelle D, Rostro-Garcia S, Kamler JF, Laguardia A, Khorozyan, I, Ghoddousi A (2020) Panthera pardus (amended version of 2019 assessment). The IUCN Red List of Threatened Species 2020, 8235, e.T15954A163991139
Viljoen P (2019) Parc national de la Comoé et les zones de biodiversité de Warigue et Mont Tingui. Enquête aérienne sur la faune. Résumé des résultats
WCF (2008) Rapport de l’inventaire faunique par survol du mois de mars 2008. OIPR, Abidjan
WCF (2010) Rapport de l’inventaire faunique par survol du mois de mars 2010. OIPR, Abidjan
Woodroffe R (2000) Predators and people: using human densities to interpret declines of large carnivores. Anim Conserv 3(2):165–173. https://doi.org/10.1017/S136794300000086X
Acknowledgements
We would like to thank our field team Mr. Xavier Kouame and Mr. Bakary Ouattara, the CNP Director Lieutenant-Colonel Roger Yao Koudio and Lieutenant-Colonel Amara Ouattara, responsible for wildlife monitoring, for research authorization and collaboration, and local people for their hospitality during the surveys. We also grateful to Mr. Stanislas M. Gandaho for his help with statistical analysis, the NGO SOS-Savane, and the University of Oxford’s WildCRU for supporting us through the provision of camera traps for the fieldwork.
Funding
The International Union for Conservation of Nature (IUCN) funded the present study through the PAPBio Program “Regional Governance of Protected Areas in West Africa” (NUMERO DE REFERENCE DU CONTRAT 2021/05/KL/100; AWARD NUMBER AVBF-00072), with co-financing from the German Technical Cooperation (GIZ of Côte d’Ivoire) through the Pro2GRN Program (CONTRAT VN 1508-290-22; PROJET PN 290-GIZ Côte d’Ivoire).
Author information
Authors and Affiliations
Contributions
AJ is the designer of the study. He was responsible of the data collection, data processing and data analysis, and the manuscript writing. EAS and HB contributed to the design of the study, data analysis and the manuscript writing. SF, OS, and BS contributed to data analysis and the manuscript writing. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Compliance with ethical standards as this research did not use biological material. Fieldwork was conducted with the relevant clearance from the General Management of the Office Ivoirien des Parcs et Réserves of Côte d’Ivoire (Authorization N°076/MINNED/OIPR/DR of January 28, 2022).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aglissi, J., Sogbohossou, E.A., Soro, F. et al. Ecological determinants of spotted hyena Crocuta crocuta occupancy in Comoé National Park, Côte d’Ivoire. Eur J Wildl Res 70, 21 (2024). https://doi.org/10.1007/s10344-024-01768-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-024-01768-5