Abstract
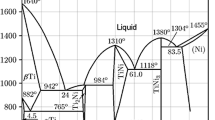
A novel synthesis method of Ti–Al powder was developed based on the shuttle of disproportionation and proportionation reactions of titanium ions in various molten salts. Fine Ti–Al intermetallic compound powder was successfully synthesized from bulk metals of titanium and aluminum as raw materials. Uniform Ti–Al intermetallic compound powder in the form of TiAl3 was obtained at temperatures lower than 600 °C, while in the form of mixture of TiAl2 and TiAl3 was obtained at temperatures higher than 650 °C. The obtained intermetallic powders in the size of micrometer scale are agglomerated by numerous primary particles with size of several tens of nanometers. The addition of AlCl3 into the reaction solvent does not change the compound phase of the Ti–Al powder but promotes the growth of the primary particles. The phase of the Ti–Al intermetallic compound powders only depends on the reaction temperature in various solvent salts with the different equilibrium constants of disproportionation reaction.
Graphical Abstract












Similar content being viewed by others
References
Fang ZZ, Paramore JD, Sun P et al (2018) Powder metallurgy of titanium—past, present, and future. Int Mater Rev 63:407–459. https://doi.org/10.1080/09506608.2017.1366003
Dimiduk DM (1999) Gamma titanium aluminide alloys—an assessment within the competition of aerospace structural materials. Mater Sci Eng A 263:281–288. https://doi.org/10.1016/S0921-5093(98)01158-7
Gupta K, Laubscher RF (2017) Sustainable machining of titanium alloys: a critical review. Proc Inst Mech Eng Part B J Eng Manuf 231:2543–2560. https://doi.org/10.1177/0954405416634278
Froes FH, Eylon D, Eichelman GE, Burte HM (1980) Developments in titanium powder metallurgy. JOM 32:47–54. https://doi.org/10.1007/BF03354547
Froes FH, Eylon D (1990) Powder metallurgy of titanium alloys. Int Mater Rev 35:162–184. https://doi.org/10.1179/095066090790323984
McCracken CG, Motchenbacher C, Barbis DP (2010) Review of titanium powder production methods. Int J Powder Metall 46:19–26
Martín A, Cepeda-Jiménez CM, Pérez-Prado MT (2020) Gas atomization of γ-TiAl alloy powder for additive manufacturing. Adv Eng Mater 22:1900594. https://doi.org/10.1002/adem.201900594
Barbis DP, Gasior RM, Walker GP et al (2015) 7-Titanium powders from the hydride–dehydride process. In: Qian M, Froes (Sam) FH (eds) Titanium powder metallurgy. Butterworth-Heinemann, Boston, pp 101–116
Sun P, Fang ZZ, Zhang Y, Xia Y (2017) Review of the methods for production of spherical Ti and Ti alloy powder. JOM 69:1853–1860. https://doi.org/10.1007/s11837-017-2513-5
Du C, Wang N, Hou J et al (2013) Facile synthesis of Nb–Al alloy powders via sodiothermic reduction in molten salts. J Alloys Compd 555:405–411. https://doi.org/10.1016/j.jallcom.2012.11.180
Wang Y, Ma J, Tao J et al (2006) Synthesis of CaWO4 nanoparticles by a molten salt method. Mater Lett 60:291–293. https://doi.org/10.1016/j.matlet.2005.08.037
Yang L, Wang Y, Liu R et al (2020) In-situ synthesis of nanocrystalline TiC powders, nanorods, and nanosheets in molten salt by disproportionation reaction of Ti(II) species. J Mater Sci Technol 37:173–180. https://doi.org/10.1016/j.jmst.2019.08.017
Du C, Xiao J, Zhang B, Zhu H (2021) Facile synthesis of fine Ti–Al intermetallic compound powders via sodiothermic reduction in molten CaCl2. Intermetallics 129:107038. https://doi.org/10.1016/j.intermet.2020.107038
Zhu F, Li L, Cheng X et al (2020) Direct electrochemical reduction of low titanium chlorides into titanium aluminide alloy powders from molten eutectic KCl–LiCl–MgCl2. Electrochim Acta 357:136867. https://doi.org/10.1016/j.electacta.2020.136867
Zhua F, Li K, Song W et al (2021) Composition and structure of Ti–Al alloy powders formed by electrochemical co-deposition in KCl–LiCl–MgCl2–TiCl3–AlCl3 molten salt. Intermetallics 139:107341. https://doi.org/10.1016/j.intermet.2021.107341
Song J, Mukherjee A (2016) Influence of F− on the electrochemical properties of titanium ions and Al–Ti alloy electrodeposition in molten AlCl3–NaCl. RSC Adv 6:82049–82056. https://doi.org/10.1039/C6RA18417K
Lu X, Ono T, Takeda O, Zhu H (2019) Production of fine titanium powder from titanium sponge by the shuttle of the disproportionation reaction in molten NaCl–KCl. Mater Trans 60:405–410. https://doi.org/10.2320/matertrans.MA201811
Zhu X, Wang Q, Song J et al (2014) The equilibrium between metallic titanium and titanium ions in LiCl–KCl melts. J Alloys Compd 587:349–353. https://doi.org/10.1016/j.jallcom.2013.09.151
Wang Q, Song J, Hu G et al (2013) The equilibrium between titanium ions and titanium metal in NaCl–KCl equimolar molten salt. Metall Mater Trans B 44:906–913. https://doi.org/10.1007/s11663-013-9853-5
Song J, Wang Q, Kang M et al (2014) The equilibrium between titanium ions and metallic titanium in the molten binary mixtures of LiCl. Electrochemistry 82:1047–1051. https://doi.org/10.5796/electrochemistry.82.1047
Wu J, Song J, Zhu H et al (2019) Equilibrium between metallic titanium and titanium ions in MgCl2–LiCl molten salt. Mater Trans 60:374–378. https://doi.org/10.2320/matertrans.MA201801
Wang S, Du C, Zhang B et al (2022) Direct synthesis of intermetallic compounds through thermo-reduction and electrochemical deposition. In: Ouchi T, Azimi G, Forsberg K et al (eds) Rare metal technology 2022. Springer International Publishing, Cham, pp 299–308
Ning X, Åsheim H, Ren H et al (2011) Preparation of titanium deposit in chloride melts. Metall Mater Trans B 42:1181–1187. https://doi.org/10.1007/s11663-011-9559-5
Schuster JC, Ipser H (1990) Phases and phase relations in the partial system TiAl3–TiAl/Phasen und phasenbeziehungen im teilbereich TiAl3–TiAl. Int J Mater Res 81:389–396. https://doi.org/10.1515/ijmr-1990-810601
Acknowledgements
We thank K. Kobayashi for his experimental assistance. Financial support was provided by the Grant-in-Aid for Scientific Research, JSPS KAKENHI grant nos. 20H02492. The authors gratefully acknowledge support from the International Joint Graduate Program in Material Science (GP-MS) at Tohoku University.
Funding
This work was funded by Japan Society for the Promotion of Science (Grant No. 20H02492).
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was Adam Clayton Powell.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Terigele, T., Wang, S., Xiao, J. et al. Synthesis of Ti–Al Intermetallic Compound Fine Powder Using Shuttle Reactions of Titanium Ions in the Molten Salt. J. Sustain. Metall. 10, 267–277 (2024). https://doi.org/10.1007/s40831-024-00791-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-024-00791-9