Abstract
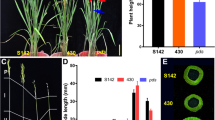
Cleistogamy or closed flowering is a widely used trait in barley (Hordeum vulgare) breeding because it reduces the risk of fungal infection in florets at anthesis. Cleistogamy in barley is caused by a point mutation within the microRNA172 (miR172) target site of the Cly1 gene, which encodes the Apetala2 (AP2) transcription factor. Because cleistogamy is not apparent in cultivars of hexaploid wheat (Triticum aestivum), a strategy to develop cleistogamous wheat was proposed by inducing point mutations in all three AP2 homoeologs, which are the wheat orthologs of barley Cly1. In this study, we investigated the effects of miR172 target site mutations on wheat cleistogamy using double mutants by combining three previously obtained mutant alleles (AP2-A1, D1 and D2) in a near-isogenic background. The AP2-D2 allele had the greatest effect on reducing the anther extrusion rate and lodicule size compared with the other two mutant alleles. The double mutant containing the AP2-A1 and AP2-D2 alleles had a much greater suppression of anther extrusion by reducing the lodicule size than the single AP2-D2 mutant, suggesting cumulative effects of the two mutant alleles. In addition, both single and double mutants exhibited compact spikes and shorter plant heights due to reduced rachis and culm internodes in the upper parts. The presence or absence of the wild-type AP2-B homoeolog had no significant effect on phenotype. This study provides insights into the cumulative effects of mutant AP2 alleles in suppressing open flowering and provides a basis for further research on the development of complete cleistogamy in hexaploid wheat.





Similar content being viewed by others
Data availability
The datasets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Anwar N, Ohta M, Yazawa T, Sato Y, Li C, Tagiri A, Sakuma M, Nussbaumer T, Bregitzer P, Pourkheirandish M, Wu J, Komatsuda T (2018) miR172 downregulates the translation of cleistogamy 1 in barley. Ann Bot 122:251–265. https://doi.org/10.1093/aob/mcy058
Cheignon M, Schaever J, Cornier N (1973) Elongation of stamen filament of graminaceae as growth phenomenon. Cr Acad Sci D Nat 276:319–322
Daniell H (2002) Molecular strategies for gene containment in transgenic crops. Nat Biotechnol 20:581–586. https://doi.org/10.1038/nbt0602-581
Debernardi JM, Lin H, Chuck G, Faris JD, Dubcovsky J (2017) microRNA172 plays a crucial role in wheat spike morphogenesis and grain threshability. Development 144:1966–1975. https://doi.org/10.1242/dev.146399
Debernardi JM, Greenwood JR, Jean Finnegan E, Jernstedt J, Dubcovsky J (2020) APETALA 2-like genes AP2L2 and Q specify lemma identity and axillary floral meristem development in wheat. Plant J 101:171–187. https://doi.org/10.1111/tpj.14528
Debernardi JM, Woods DP, Li K, Li C, Dubcovsky J (2022) MiR172-APETALA2-like genes integrate vernalization and plant age to control flowering time in wheat. PLoS Genet 18:e101057. https://doi.org/10.1371/journal.pgen.1010157
Graham S, Browne RA (2009) Anther extrusion and Fusarium head blight resistance in European wheat. J Phytopathol 157:580–582. https://doi.org/10.1111/j.1439-0434.2008.01524.x
Greenwood JR, Finnegan EJ, Watanabe N, Trevaskis B, Swain SM (2017) New alleles of the wheat domestication gene Q reveal multiple roles in growth and reproductive development. Development 144:1959–1965. https://doi.org/10.1242/dev.146407
He X, Lillemo M, Shi J, Wu J, Bjørnstad Å, Belova T, Dreisigacker S, Duveiller E, Singh P (2016) QTL characterization of Fusarium head blight resistance in CIMMYT bread wheat line Soru#1. PLoS ONE 11:e0158052. https://doi.org/10.1371/journal.pone.0158052
Heslop-Harrison Y, Heslop-Harrison JS (1996) Lodicule function and filament extension in the grasses: potassium ion movement and tissue specialization. Ann Bot 77:573–582. https://doi.org/10.1093/aob/77.6.573
Honda I, Turuspekov Y, Komatsuda T, Watanabe Y (2005) Morphological and physiological analysis of cleistogamy in barley (Hordeum vulgare). Physiol Plant 124:524–531. https://doi.org/10.1111/j.1399-3054.2005.00541.x
Houston K, McKim SM, Comadran J, Bonar N, Druka I, Uzrek N, Cirillo E, Guzy-Wrobelska J, Collins NC, Halpin C, Hansson M, Dockter C, Druka A, Waugh R (2013) Variation in the interaction between alleles of HvAPETALA2 and microRNA172 determines the density of grains on the barley inflorescence. Proc Natl Acad Sci U S A 110:16675–16680. https://doi.org/10.1073/pnas.1311681110
Kirby EJM, Appleyard M (1981) Cereal development guide. Cereal Unit, National Agricultural Centre, Stoneleigh, Kenilworth, Warwickshire CV8 2LZ, England
Kubo K, Kawada N, Masaya F, Koichi H, Shunsuke O, Nakajima T (2010) Effect of cleistogamy on Fusarium head blight resistance in wheat. Breed Sci 60:405–411. https://doi.org/10.1270/jsbbs.60.405
Kubo K, Fujita M, Kawada N, Nakajima T, Nakamura K, Maejima H, Ushiyama T, Hatta K, Matsunaka H (2013) Minor differences in anther extrusion affect resistance to Fusarium head blight in wheat. J Phytopathol 161:308–314. https://doi.org/10.1111/jph.12060
Lu Q, Lillemo M, Skinnes H, He X, Shi J, Ji F, Dong Y, Bjørnstad A (2013) Anther extrusion and plant height are associated with type I resistance to Fusarium head blight in bread wheat line “Shanghai-3/Catbird.” Theor Appl Genet 126:317–334. https://doi.org/10.1007/s00122-012-1981-9
Lombardo F, Kuroki M, Yao SG, Shimizu H, Ikegaya T, Kimizu M, Ohmori S, Akiyama T, Hayashi T, Yamaguchi T, Koike S, Yatou O, Yoshida H (2016) The superwoman1-cleistogamy2 mutant is a novel resource for gene containment in rice. Plant Biotechnol J 15:97–106. https://doi.org/10.1111/pbi.12594
Mesterhazy A (1995) Types and components of resistance to Fusarium head blight of wheat. Plant Breed 114:377–386. https://doi.org/10.1111/j.1439-0523.1995.tb00816
Mesterhazy A, Bartok T, Mirocha CG, Komoroczy R (1999) Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant Breed 118:97–110. https://doi.org/10.1046/j.1439-0523.1999.118002097
Nair SK, Wang N, Turuspekov Y, Pourkheirandish M, Sinsuwongwat S, Chen G, Sameri M, Tagiri A, Honda I, Watanabe Y, Kanamori H, Wicker T, Stein N, Nagamura Y, Matsumoto T, Komatsuda T (2010) Cleistogamous flowering in barley arises from the suppression of microRNA-guided HvAP2 mRNA cleavage. Proc Natl Acad Sci U S A 107:490–495. https://doi.org/10.1073/pnas.0909097107
Nanape AB, Haine HM, Sugimoto K, Kobayashi F, Oono Y, Handa H, Komatsuda T, Kakeda K (2023) Mutations within the miR172 target site of wheat AP2 homoeologs regulate lodicule size and rachis internode length. Breed Sci. https://doi.org/10.1270/jsbbs.23019. (in press)
Ni DH, Li J, Duan YB, Yang YC, Wei PC, Xu RF, Li CR, Liang DD, Li H, Song FS, Ni JL, Li L, Yang JB (2014) Identification and utilization of cleistogamy gene cl7(t) in rice (Oryza sativa L.). J Exp Bot 65:2107–2117. https://doi.org/10.1093/jxb/eru074
Ning S, Wang N, Sakuma S, Pourkheirandish M, Wu J, Matsumoto T, Koba T, Komatsuda T (2013a) Structure, transcription and post-transcriptional regulation of the bread wheat orthologs of the barley cleistogamy gene Cly1. Theor Appl Genet 126:1273–1283. https://doi.org/10.1007/s00122-013-2052-6
Ning S, Wang N, Sakuma S, Pourkheirandish M, Koba T, Komatsuda T (2013b) Variation in the wheat AP2 homoeologs, the genes underlying lodicule development. Breed Sci 63:255–266. https://doi.org/10.1270/jsbbs.63.255
Ohmori S, Tabuchi H, Yatou O, Yoshida H (2012) Agronomic traits and gene containment capability of cleistogamous rice lines with the superwoman1-cleistogamy mutation. Breed Sci 62:124–132. https://doi.org/10.1270/jsbbs.62.124
Patil V, McDermott HI, McAllister T, Cummins M, Silva JC, Mollison E, Meikle R, Morris J, Hedley PE, Waugh R, Dockter C, Hansson M, McKim SM (2019) APETALA2 control of barley internode elongation. Development 146:dev170373. https://doi.org/10.1242/dev.170373
Shoesmith JR, Solomon CU, Yang X, Wilkinson LG, Sheldrick S, van Eijden E, Couwenberg S, Pugh LM, Eskan M, Stephens J, Barakate A, Drea S, Houston K, Tucker MR, McKim SM (2021) APETALA2 functions as a temporal factor together with BLADE-ON-PETIOLE2 and MADS29 to control flower and grain development in barley. Development 148:dev194894. https://doi.org/10.1242/dev.194894
Simons KJ, Fellers JP, Trick HN, Zhang Z, Tai YS, Gill BS, Faris JD (2006) Molecular characterization of the major wheat domestication gene Q. Genetics 172:547–555. https://doi.org/10.1534/genetics.105.044727
Tang C, Zhang H, Zhang P, Ma Y, Cao M, Hu H, Shah FA, Zhao W, Li M, Wu L (2019) iTRAQ-based quantitative proteome analysis reveals metabolic changes between a cleistogamous wheat mutant and its wild-type wheat counterpart. PeerJ 7:e7104. https://doi.org/10.7717/peerj.7104
Turuspekov Y, Mano Y, Honda I, Kawada N, Watanabe Y, Komatsuda T (2004) Identification and mapping of cleistogamy genes in barley. Theor Appl Genet 109:480–487. https://doi.org/10.1007/s00122-004-1673-1
Turuspekov Y, Kawada N, Honda I, Watanabe Y, Komatsuda T (2005) Identification and mapping of a QTL for rachis internode length associated with cleistogamy in barley. Plant Breed 124:542–545. https://doi.org/10.1111/j.1439-0523.2005.01161.x
Ueno K, Itoh H (1997) Cleistogamy in wheat: genetic control and the effect of environmental conditions. Cereal Res Commun 25:185–189. https://doi.org/10.1007/BF03543455
van Doorn WG, van Meeteren U (2003) Flower opening and closure. A review. J Exp Bot 54:1801–1812. https://doi.org/10.1093/jxb/erg213
Wang N, Ning S, Pourkheirandish M, Honda I, Komatsuda T (2013) An alternative mechanism for cleistogamy in barley. Theor Appl Genet 126:2753–2762. https://doi.org/10.1007/s00122-013-2169-7
Wang N, Ning S, Wu J, Tagiri A, Komatsuda T (2015) An epiallele at cly1 affects the expression of floret closing (cleistogamy) in barley. Genetics 199:95–104. https://doi.org/10.1534/genetics.114.171652
Wang N, Kakeda K, Tomokazu M, Liu C, Yoshida M, Kawada N, Komatsuda T (2021) A novel mutant allele at the Cleistogamy 1 locus in barley. Theor Appl Genet 134:3183–3193. https://doi.org/10.1007/s00122-021-03884-1
Xu BJ, Chen Q, Zheng T, Jiang YF, Qiao YY, Guo ZR, Cao YL, Wang Y, Zhang YZ, Zong LJ, Zhu J, Liu CH, Jiang QT, Lan XJ, Ma J, Wang JR, Zheng YL, Wei YM, Qi PF (2018) An overexpressed Q allele leads to increased spike density and improved processing quality in common wheat (Triticum aestivum). G3 (Bethesda) 8:771–778. https://doi.org/10.1534/g3.117.300562
Yoshida M, Kawada N, Nakajima T (2007) Effect of infection timing on Fusarium head blight and mycotoxin accumulation in open- and closed-flowering barley. Phytopathology 97:1054–1062. https://doi.org/10.1094/PHYTO-97-9-1054
Yoshida M, Kawada N, Tohnooka T (2005) Effect of row type, flowering type and several other spike characters on resistance to Fusarium head blight in barley. Euphytica 141:217–227. https://doi.org/10.1007/s10681-005-7008-8
Zadoks JC, Changt TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Zajączkowska U, Denisow B, Łotocka B, Dołkin-Lewko A, Rakoczy-Trojanowska M (2021) Spikelet movements, anther extrusion and pollen production in wheat cultivars with contrasting tendencies to cleistogamy. BMC Plant Biol 21:136. https://doi.org/10.1186/s12870-021-02917-7
Zhang J, Xiong H, Guo H, Li Y, Xie X, Xie Y, Zhao L, Gu J, Zhao S, Ding Y, Liu L (2022) Identification of the Q gene playing a role in spike morphology variation in wheat mutants and its regulatory network. Front Plant Sci 12:807731. https://doi.org/10.3389/fpls.2021.807731
Zhang Z, Li A, Song G, Geng S, Gill BS, Faris JD, Mao L (2019) Comprehensive analysis of Q gene near-isogenic lines reveals key molecular pathways for wheat domestication and improvement. Plant J 102:299–310. https://doi.org/10.1111/tpj.14624
Zhao Y, Ma R, Xu D, Bi H, Xia Z, Peng H (2019) Genome-wide identification and analysis of the AP2 transcription factor gene family in wheat (Triticum aestivum L.). Front Plant Sci 10:1286. https://doi.org/10.3389/fpls.2019.01286
Acknowledgements
We are grateful to Prof. Chiharu Nakashima and Prof. Nobuhito Sekiya for their invaluable advice on this study. We thank Miyoko Nitta, Kiyomasa Watanabe and Ryotaro Ishigaki for their technical assistance.
Funding
This work was supported in part by the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics-based Technology for Agricultural Improvement, Grant Nos. IVG1003 and IVG3004) and the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Nos. 18H02176 and 18K19211). Agetha Bigie Nanape was supported by a Japanese Government (Monbukagakusho: MEXT) Scholarship for Research Students.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this study. Material preparation, data collection and analysis were performed by Agetha Bigie Nanape and Katsuyuki Kakeda. Agetha Bigie Nanape wrote the first draft of the manuscript, and all authors reviewed the previous versions of the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nanape, A.B., Komatsuda, T. & Kakeda, K. Accumulation of mutations in the AP2 homoeologs causes suppression of anther extrusion with altered spike and culm development in hexaploid wheat. Mol Breeding 44, 19 (2024). https://doi.org/10.1007/s11032-024-01458-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-024-01458-9