Abstract
Purpose
The study aimed to provide an overview of the behavior, fate and ecotoxicity of boron and sulfur in the sediments of Lake Burullus, Egypt, after the degradation of the lake area.
Materials and methods
Changes in the studied lake area due to human activities were estimated using Erdas Imagine v14 and ArcGIS. Sequential and non-sequential extraction techniques for boron and sulfur fractionation in sediments were evaluated.
Results and discussion
A remote-sensing tracking investigation revealed a decrease in area of about 19.4%. Boron geochemical fractions had the order of residual (BF5, 61.48 ± 3.84%) > bound oxide (BF3, 15.47 ± 9.64%) > specifically adsorbed (BF2, 13.34 ± 9.85%) > bound organic (BF4, 9.85 ± 12.40%) > water-soluble and exchangeable (BF1, 2.2 ± 2.66%). Sulfur gave the order of the fractions as residual (SF5, 70.52 ± 16.92%) > phosphate extractable (SF2, 15.47 ± 9.34%) > bound organic (SF4, 7.20 ± 5.35%) > CaCl2 -extractable (SF3, 9.85 ± 12.40%) > water-soluble sulfur (SF1, 1.34 ± 1.52%). A worst-case scenario involving boron and sulfur toxicity values was studied using mixture risk characterization ratios (RCRmix) based on the predicted no effect concentration (PNEC) and sum of toxic units (STU).
Conclusions
The RCRmix and STU of boron showed high long-term risks to aquatic organisms, while sulfur showed high short-term risks. Invertebrates were the most sensitive species to boron and sulfur. This study sheds light on the ecological impact of boron and sulfur in Lake Burullus sediments, highlighting potential risks to aquatic organisms. Further research could focus on developing mitigation strategies to address these concerns.
Graphical Abstract

Similar content being viewed by others
1 Introduction
Boron and sulfur are common elements that play important roles in environmental and biological processes. Their concentrations in aquatic ecosystems are influenced by both natural and anthropogenic factors. Elevated levels of boron and sulfate can impact organisms and water quality (Davies and Hall 2007). Lake Burullus, Egypt receives drainage from surrounding agricultural areas, and human activities have led to changes in the lake’s area over time. However, limited information exists on the geochemistry and fractionation of boron and sulfur in the lake sediments and limited information about the changes in the lake’s area over time.
Boron analysis provides insights into redox conditions and paleoenvironmental changes (Raitzsch et al. 2018). The sulfur cycle is controlled by redox potential, sulfate levels, organic matter availability, and dissolved metal ions (Liu and Han 2021; Chen et al. 2021; El Zokm et al. 2023). Sulfate enters water bodies from natural and human sources like wastewater, fertilizers and industrial effluent (Liu and Han 2021; Chen et al. 2021). Excess sulfate can induce eutrophication by remobilizing phosphates and ammonium (Chen et al. 2021). Studies on boron and sulfur fractionation and distribution aid in assessing contamination and toxicity risks (Arisekar et al. 2022; Casillas et al. 2022). There is also value in predicting sediment pollution using established risk assessment approaches (EC (European Commission) 2003; Finizio and Vighi 2014; El Zokm et al. 2022).
Activities around Lake Burullus, Egypt was responsible for significant changes in land use/land cover that have contributed to decrease the lake area between 1972 and 2020, primarily affecting eastern and southern regions. Such human-driven modifications likely influence sediment geochemistry through altering surface runoff and contaminant inputs from surrounding areas. The present study hypothesized that boron and sulfur fractionation patterns in lake sediments may differ spatially depending on proximity to locations where the most severe area loss has occurred, owing to potentially varying impacts over time from land-based pollution sources like boron/sulfur-laden wastewater and agricultural runoff that have been estimated at 4 billion m3 annually (Al-Afify et al. 2023).
This study aims to provide baseline data on boron and sulfur fractionation in Lake Burullus sediments while investigating potential linkages to spatial patterns of lake area change over the recent decades. By analyzing trace element concentrations and speciation at sites near zones of greatest and lesser area decline, insights may emerge regarding how geochemical fractions correlate with the magnitude of anthropic alterations. As the first investigation of boron and sulfur behavior in these sediments, coupled with high-resolution analysis of historical lake area reductions using remote sensing, this work establishes an improved understanding of factors controlling sediment contamination at this ecologically threatened coastal site. Sequential and non-sequential extraction techniques were used to identify the boron and sulfur fractions, respectively. Spatial trends and relationships with sediment properties were evaluated. Risk indices were determined to classify contamination levels. The ecological risk of boron and sulfur was also assessed using predicted no-effect concentrations and toxic units after the area change in the lake due to human activities using Erdas Imagine v14 and ArcGIS. This research contributes new insights into controlling factors and pollution implications, providing guidance for lake management.
2 Materials and methods
2.1 Area of study
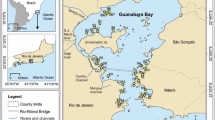
Lake Burullus is a shallow, brackish lake, located in the Kafr El-Sheikh Governorate (30° 22′–31° 35′N; 30° 33′–31° 08′E) on the eastern side of the Rosetta branch of the Nile River (Fig. 1). The lake connects to the sea at its northeast through the Burullus inlet. It receives 4 × 109 m3 y−1 drainage water from multiple drains, the main pollution (Al-Afify et al. 2023). The waters of the estuaries in the Rosetta estuary of the Nile River mix with the waters of the lake through the Brimbal Canal (El-Naggar and Rifaat 2022).
2.2 Region changes detection
Five Landsat images from 1972 to 2020 were analyzed using Erdas Imagine and ArcGIS to detect changes in the area of Lake Burullus (Fig. 2). For the 1972 image, the lake area was manually digitized due to low spectral resolution. For 1984–2020 images, the Normalized Difference Water Index (NDWI) and Normalized Difference Vegetation Index (NDVI) were applied to determine water bodies and wet vegetation, represented as raster images. These were merged and converted to a vector feature class to calculate total lake area, validated through visual interpretation. The analysis revealed a decrease in the lake area of approximately 147.4 km2 from 760 km2 in 1972 to 612.6 km2 in 2020, a reduction of around 19.4% (Table S1), mainly due to construction, cultivation and fish farming in eastern and southern regions. Since 2008, the area has increased slightly by 0.03% (Table S1), potentially from higher image resolution and dredging activities.
2.3 Sediment sampling and analysis
During November 2019, 16 sediment surface samples were collected from Lake Burullus using a Van Veen Grab sampler (Fig. 1) to apply the fractionation technique for both boron and sulfur. Field-wet samples were bagged and promptly sent to the lab. Sediment samples were air-dried, and particle sizes < 50 μm were achieved through mortar grinding (Abreu et al. 2016). The remaining sediment samples were stored at 4 °C. For grain size analysis, samples were dried, sectioned using the cone and quarter method, washed, and then dried at 105 °C for mechanical assessment (Folk 1974). Mechanical granulometric analysis was performed on 100 g of each sediment sample. Analysis involved dry sieving using a standard set of screens according to the Wentworth scale, with one Ø interval increments. The sieves separated particles into standard size fractions defined by Ø units. The mass of sediment retained on each sieve was accurately measured. Mean grain size, sorting, skewness, and kurtosis were computed from the size fraction data. The total organic carbon (TOC) content, total carbonate (TCO3) content and total phosphorus (TP) content were determined in the sediment samples (Molnia 1974; Aspila 1976; Loring and Rantala 1992).
In order to determine the concentrations of total boron and total sulfur, a precise amount of 0.2 g of finely powdered sediment sample was subjected to a digestion process. This was achieved by employing an acidic mixture composed of 3 mL of HNO3, 2 mL of HClO4, and 1 mL of HF, at 80 °C. The digested sample was filtered, and then the supernatant was transferred to a 25 mL volumetric flask containing 0.1 M HCl (Black 1965). The sulfur in solution or in an extractable form was analyzed using inductively coupled optical plasma emission spectroscopy (ICP-OES) (Mahanta et al. 2017).
2.4 Boron fractionation
The boron fractionation in sediment samples was accomplished using a sequential extraction technique proposed by Hou et al. (1994) and modified by Datta et al. (2002). Table S2 shows the various boron fractions identified in the sediment samples. For each step, the suspension was centrifuged, and the supernatant solution was analyzed for total boron, while all fractions in the extracts were analyzed using the colorimetric curcumin technique (APHA-AWWA-WPCF 1999).
2.5 Sulfur fractionation
Extraction was performed separately for each fraction, as specified in Table S3. Sequential extraction was not applied (Xiao-quan et al. 1992; Shan and Chen 1995; Likens et al. 2002; Morche 2008).
2.6 Pollution state
To study boron and sulfur contamination in lake sediment samples, enrichment factor (EF), contamination factor (CF), the individual contamination factors (ICF), global contamination factor (GCF), and risk assessment code (RAC) were determined for each site (Passos et al. 2011; Soliman et al. 2018; Liu et al. 2020; Sattarova et al. 2022; Table S4).
2.7 Ecological risk assessment
The ecological hazard assessment of boron and sulfur in Lake Burullus sediments was carried out following the European Commission Technical Guidance Document (EC 2003) and other documented literature studies (Finizio et al. 2014; Casillas et al. 2022; El Zokm et al. 2022). Thus, boron and sulfur in sediments were determined from the equilibrium partitioning method (EPM) using PNECWater as follows (Eqs. (1) and (2) and Table S5; EC 2003; El Zokm et al. 2022):
KOC values were estimated from the use of Kd for each element as indicated (Eqs. (3) and (4) and Table S5; El Zokm et al. 2022):
Kd, the sediment/liquid partition coefficient (L kg−1) and KOC for boron and sulfur were calculated using their contents in sediment (ToxinSediment, mg kg−1) and in deep water, (ToxinWater, mg L−1). The current averaged values of KOC and KOW for boron and sulfur in the sediments are 119,908 mg kg−1 and 342,593 and 12,831 mg kg−1 and 36,658, respectively, with logKOW/KOC values of approximately 0.46; less than 5. However, the EPM method is not usually recommended for substances that may pose a significant risk to sediment organisms with logKOW/KOC values > 5 (ECHA European Chemicals Agency 2016).
Both the boron and sulfur mixture risk characterization ratios (RCRmix) were calculated through two paths (Casillas et al. 2022). First, the human health risks based on reference doses (Eq. (5); Casillas et al. 2022).
The sum of toxic units (STU) for the most sensitive trophic level was the basis for the second computational assumption (Eq. (6); Casillas et al. 2022).
where i refer to the trophic level analyzed.
For boron and sulfur, the PNECAcute-sediment values were calculated was estimated by dividing the 50% lethal concentration (EC50 or LC50) of boron and sulfur by an assessment factor (AF) of 1000 (one short term for each trophic level) for three aquatic organisms: fish, invertebrates, and algae or plants. PNECChronic-sediment values were obtained by dividing the no observed effect concentration (NOEC) and the 5% lethal concentration (EC05) of boron and sulfur by an AF of 100 (one long-term for each trophic level), an AF of 50 (long-term for two trophic levels), and an AF of 10 (long-term for three trophic levels) (Eqs. (7) and (8); EC 2003; Kosma et al. 2014; Li et al. 2016). The EC50, LC50, EC05, and NOEC data for boron and sulfur were obtained from previous studies (Table S6).
Ecotoxic effects of toxicants can be characterized by extrapolating the PNEC based on EC50 or LC50 values obtained from the acute toxicity test or NOEC or EC05 from the chronic toxicity test dataset of three taxonomic groups (Table S6 and Eqs. (7) and (8); Arisekar et al. 2022; Casillas et al. 2022).
In the present work, to estimate the worst-case scenario implying lower toxicity values for boron and sulfur in the sediments, the maximum bioavailable fraction (MEC) values were used in the risk quotient (RQ) calculations. Also, RQ values were calculated using acute and chronic data as previously reported (Reis et al. 2021). The risk quotient (RQ) for an individual toxicant in the sediment for each aquatic organism was the ratio of the bioavailable fraction content (MEC) and PNECAcute or PNECChronic for a specific aquatic organism (Eq. (9); Arisekar et al. 2022; Casillas et al. 2022; Zhang et al. 2022).
The different ecological risk levels were determined to be as follows: low risk to organisms with an RQ value below 0.1, medium risk for a value between 0.1 and 1, and high risk for a value greater than 1 (El Zokm et al. 2022).
The cumulative RCRmix (∑RQi) was used to classify the sampling locations (n) using the RQ values for each aquatic organism (Eq. (10); Arisekar et al. 2022):
RCRmix value was the sum of the RQi values of aquatic organisms at each site (n). When an RCRmix ≤ 1 value indicated sites were classified as having low ecological risk, and RCRmix > 1 indicated a high ecological risk for aquatic organisms grown in a given site (Arisekar et al. 2022).
The relative contribution (RC) of an individual toxicant was computed separately for the RCRmix values for each organism (Eq. (11); Arisekar et al. 2022). RC was used to estimate the lowest and highest ecological toxicity risks contributing to each organism in the sediment (Arisekar et al. 2022; Casillas et al. 2022).
The toxic unit (TU) as ecotoxicological risk estimation of a substance to particular organism can be calculated by the following formula (Eq. (12); Reis et al. 2021)
where TU ≥ 1 represents high risk, 0.1 = TU < 1 indicates medium risk, and 0.01 = TU < 0.1 gives low risk to the trophic level (Arisekar et al. 2022).
The sum of toxic units (STU) was calculated for each sensitive trophic level (algae or plants, invertebrates, and fish) within each sampling site during the entire sampling period, considering both acute and chronic data (Eq. (13); Reis et al. 2021).
where STU > 1 indicates high risk and STU < 1 indicates low risk at the trophic level.
2.8 Quality assurance
The extraction procedure was performed in triplicate, and the values presented are the means of three replications. Three replicate analyses of one sample were used to calculate the coefficient of variation (CV) to assess precision (with 10% accuracy). Five replicates of standard reference material (IAEA/356 polluted sea sediments from Austria) were used to test the accuracy of the method. The standard reference material with the studied sediments was analyzed to obtain quality control results, which showed an acceptable range (88 to 112%).
2.9 Statistical analyses
Pearson correlation and cluster analyses were performed using GraphPad Prism 9.3.1 and Past4.03 software. Additionally, principal component analysis (PCA) and boxplot analysis were carried out using SPSS 15 software.
3 Results and discussion
3.1 Sediment characteristics
The mean sediment grain size (Mz) in Lake Burullus ranges from 2.07 (sand) to 7.02 φ (silt), with an average of 5.07 φ. Fine sediments were located in the southeast and west of the lake, and coarse sediments were located in the central basin (Fig. 1). Silty sand and sandy silt make up the majority of the lake bed (> 90%). Sand averaged 43.20%, ranging from 13.66% (site 3) to 96.79% (site 1), while silt averaged 45.77%, ranging from 2.80% (site 1) to 69.45% (site 11), and clay averaged 11.04%, ranging from 0.41% (site 1) to 22.08% (site 15).
The lowest levels of sand, silt, and clay were detected at sites 3, 1, and 1, respectively (eastern basin), while the largest values were measured at sties 1 (eastern basin), 11, and 15, respectively (western basin). The median diameter is between fine sand and sandy silt. Fine sand, silt, sandy silt, and silty sand cover 3, 7, 42, and 48% of the total area of the lake bottom, respectively, meaning that silty sand and sandy silt dominate the majority of the research area by about 90% (Folk 1974).
The total organic carbon (TOC) ranged from 0.76 to 5.11%, with an average of 3.36%. The highest value was recorded at site 15, while the lowest value was recorded at site 1. The highest total carbonate content (54.66%) was detected at site 11, while the lowest concentration (3.17%) was observed at site 14, with an average of 28.35%.
3.2 Total boron (BT)
The total boron concentration (BT) and boron fraction levels in the sediments of Lake Burullus are presented in Table 1 and Fig. 3. The lowest boron concentration was reported at site 3 (86.2 μg g−1) and the maximum was found in site 16 (1130 μg g−1), with an average concentration of 436.7 ± 288.3 µg g−1. The horizontal distribution of BT concentration in Lake Burullus is characterized by its high concentration in the central and western regions of the lake, where it ranges from 386 to 980 µg g−1 (Fig. 3).
Horizontal distribution of the geochemical fractions of boron (µg g−1) in the sediments of Lake Burullus. BT is the total boron content and BF1, BF2, BF3, BF4 and BF5 are the boron fractions including water-soluble and exchangeable, specifically adsorbed, oxide-bound, organic-bound, and residual, respectively
Faragallah et al. (2010), Moneer et al. (2012) and Soliman et al. (2018) reported (125.45–267 μg g−1), (127–277 μg g−1), and (151.50–413.76 μg g−1) in El Mex Bay, Abu Qir Bay, and Lake Qarun, respectively lower than this study. Although the concentration range is more comparable to that of Lake Edku (300 to 910 μg g−1) and the average concentration was considerably lower than that in Taijinar Salt Lake in China (3004 µg g−1) (Li et al. 2021). The high level of BT in Lake Burullus can be attributed to boron-laden wastewater from the surrounding areas located on the southern side of Lake Burullus and fish farms as well as the drainage of boron-laden agricultural silty clays from the Nile River into the lake canals.
3.3 Boron fractions
Boron fractions exhibited variations across the sites in the study area with different boron mobility.
3.3.1 Water soluble boron and exchangeable forms (BF1)
This fraction is readily bioavailable and indicator for toxicity. The boron content extracted by 0.01 M CaCl2 ranges from ND µg g−1 at sites 3, 4, 12, and 13 to 26.81 µg g−1 at site 2, with an average of 8.31 ± 9.02 µg g−1 (Table 1). The boron content in water-soluble and exchangeable fractions of Lake Burullus recorded a lower average concentration compared to Taijinar Salt Lake in China (450.6 μg g−1 and 15%; Li et al. 2021) and a higher concentration than the average concentration of Lake Qarun, Egypt (0.9 μg g−1 and 0.36%; Soliman et al. 2018). Sites 1 and 2 record high fraction values due to sewage water discharged from the surrounding area. The fraction can be remobilized at low pH (Thomas et al. 1994), so the boron content in the exchangeable fraction was recorded at a high concentration in the drainage point sources in the eastern parts of Lake Burullus due to its low pH. Figure S1 shows regional differences in the relative percentage of water-soluble boron and exchangeable forms of boron (BF1) in the Lake Burullus sediments, with a range of ND-8.06% and an average of 2.20% (Table 1).
3.3.2 The adsorbed fraction (BF2)
The range of boron content for the carbonate fraction fluctuates from 15.22 μg g−1 at site 3 to 225.36 μg g−1at site 16, with an average concentration of 61.21 ± 66.99 μg g−1 (Table 1). The relative percentage specifically adsorbed fraction ranges from 3.79 to 32.35% of the total content, with an average of 13.34 ± 9.85% (Fig. S1). The average boron concentration in the carbonate fraction records higher values compared to Lake Qarun, Egypt (14.3 μg g−1; Soliman et al. 2018) and lower values than the concentration range compared to Taijinar Salt Lake, China (95.8–759.1 μg g−1; Li et al. 2021). The horizontal distribution of boron concentration in the carbonate fraction (BF2) shows a different pattern from that recorded in the exchangeable fraction (BF1), as it was found that the higher concentrations were in the westernmost region of Lake Burullus (sites 13, 14, 15, and 16), and their concentrations range between 15.22 and 225.36 μg g−1 (Table 1 and Fig. S1). The highest concentrations in the carbonate fraction correspond to a higher pH compared to other parts of the lake. However, it was stated that the concentration can be refilled in the carbonate fraction when the pH of the environment decreases (Li et al. 2020).
3.3.3 Fraction bound to oxides (BF3)
The boron content in the oxide fraction (BF3) ranges from 6.88 μg g−1 at site 12 to 214.86 μg g−1 at site 16, with an average of 72.04 ± 70.51 μg g−1 (Table 1). It is evident that the boron contents in the oxide fraction (BF3) are higher than those in the water-soluble boron and the exchangeable forms (BF1) in this study. The relative percentage fraction of oxides ranges from 2.96 and 31.06% of the total content, with an average of 15.47 ± 9.64% (Table 1 and Fig. S1). The average boron concentration in the oxide fraction is higher than that recorded in El-Max Bay (13.62–20.26 μg g−1; Faragallah et al. 2010) and Abu Qir Bay (11.85–38.6 μg g−1; Faragallah et al. 2010) and in Lake Qarun; 0.09 to 10.79% with an average of 5.76% (Soliman et al. 2018). High reducibility and/or pH, which are indicators of anthropogenic activities, can lead to remobilization of the boron content in the oxide fractions (Shibini Mol and Sujatha 2020). As shown in Fig. 3, which displays the horizontal distribution of boron content in Lake Burullus, it was found that the boron concentrations are higher in the central region as well as in the far western part of the lake, with an average 72.04 ± 70.05 μg g−1. Boron levels at the sampling sites indicate significant disparities in Lake Burullus sediments, probably resulting from varying mineral compositions in the sediments, which serve as the primary transporters of boron.
3.3.4 Bound organic (BF4)
In this study, the boron content bound to the organic fraction (BF4) ranges from ND in the six sites (1, 3, and 13–16) to 139.86 μg g−1 at site 8, showing an average of 37.30 ± 53.14 μg g−1 (Table 1). The relative percentage of boron in the organic fraction ranges from ND to 31.77% of the total content (Table 1 and Fig. S1), with an average of 9.85 ± 12.40%. The average boron concentration in the organic fraction records lower values compared to Lake Qarun, Egypt (19.88%; Soliman et al. 2018). Figure 3 shows the horizontal distribution of boron concentration in the organic-bound fraction of Lake Burullus. However, it was found that the values of boron concentrations are characterized by their height in the central and western regions of the lake, where they range from ND to 139.86 μg g−1. A significant amount of boron is adsorbed on organic materials, mainly on the carboxylic and phenolic sites of humic acid (Lemarchand et al. 2005).
3.3.5 Residual form (BF5)
The residual boron fraction (BF) ranges from 52.51 μg g−1 at site 3 to 678.72 μg g−1 at site 16, with an average of 265.90 ± 174.51 μg g−1 (Table 1). This fraction may be concentrated in fine sediments. Its relative percentage of BF5 ranges from 59.87 to 72.33% of the total content, with an average of 61.48 ± 3.84% (Table 1 and Fig. S1). Figure 3 shows the horizontal distribution of boron concentration in the residual fraction of Lake Burullus; which was characterized by its height in the central and western regions of the lake. The average percentage of residual boron in Lake Burullus (61.48 ± 3.84%) is lower compared to the average in Lake Qarun, Egypt (74%; Soliman et al. 2018). The residual boron represents the largest fraction of the total boron (61.48%), indicating less interaction between total boron, and aquatic organisms in Lake Burullus. This may also indicate that boron tends to be incorporated into the lattice structure of the sediment, and thus the boron content in the residual fraction is generally less bioavailable to aquatic organisms. Clay minerals adsorb boron on surface sites at low temperatures, and replace boron with tetrahedral silicon during leaching. BF5 (61.48 ± 3.84%) > BF3, (15.47 ± 9.64%) > BF2 (13.34 ± 9.85%) > BF4 (9.85 ± 12.40%) > BF1 (2.2 ± 2.66%) (Table 1) were achieved.
The Box and Whisker plot of total boron contents (BT) and residual boron fraction (BF5) shows that about 50% of the sampling sites had values below the median value of 386.55 μg g−1 for BT and more than the median value of 200 μg g−1 for BF5, and that of all the studied sites, site 16 had the highest boron content (Fig. S2). About 50% of the sampling sites have BF2 and BF3 fractional values greater than the median values of 25 μg g−1 and 32.79 μg g−1, respectively.
The heat map shows the relationship between total boron content and its fractional values with sediment properties (silt %, clay %, sand %, TOC, TCO3%, and TP) (Fig. 4). BF4 (boron bound to organic matter) has a negative correlation with BF3 (boron bound to oxides) (r = − 0.70) as the adsorption capacity of the oxides of boron decreases in the presence of high molecular weight organic acids (Prodromou 2004). BF4 also has such a negative correlation with BF2 (r = − 0.55) since carbonates have little or no ability to adsorb boron (Li et al. 2020).
Heatmap of the Pearson correlation matrix of total boron, total sulfur, and their fractions, and other geochemical parameters in Lake Burullus sediments. Blue color in the heatmap represents positive correlation; red color indicates negative correlation, and white color indicates no correlation. A color change from dark to pale indicates a change from stronger to weaker correlation. Total B and total S are total boron and sulfur contents; BF1, BF2, BF3, BF4, and BF5 are water-soluble and exchangeable, specifically adsorbed, oxide-bound, organic-bound, and residual boron fractions; SF1, SF2, SF3, SF4 and SF5 are water-soluble, phosphate extractable sulfur, CaCl2-extractable sulfur, organic sulfur, and residual sulfur fractions; TP is the total phosphorus content; sand, silt, clay, TOC, TCO3 are the percentage of sand, silt, clay, total organic carbon and total carbonate, respectively
3.4 Total sulfur (ST)
The total sulfur concentrations (ST) in the sediments of Lake Burullus are recorded in Table 1. Concentrations range from 1.87 to 20.95 mg g−1 at sites 11 and 9, respectively, with an average content of 13.41 ± 5.93 mg g−1 (Table 1). Figure 5 shows relatively high concentrations are in the central and eastern parts of the lake. Sulfur was introduced to Lake Burullus in the form of sulfate due to human activities such as agriculture, industry, and dry/wet atmospheric deposition. Sulfur deposition in lake sediments is higher in eutrophic lakes (Holmer and Storkholm 2001). Burullus Lake is classified as a hypereutrophic water body owing to the excessive nutrient load stemming from wastewater discharge (Elsayed et al. 2019). Holmer and Storkholm (2001) reported high sulfate reduction, organic sulfur sedimentation with less re-oxidation, lack of animal activity, and control of sulfur dynamics by root macrophytes.
The current results for sulfur are much lower compared to Lake Edward (Uganda/Congo), which ranged from 8.500 to 15.90 mg g−1 (Russell and Werne 2009). Results of total sulfur concentration in the sediments of five lakes located in southwest China contained high sulfur content ranging from 36.5 to 88.9% (Jingfu et al. 2016), which were much higher compared to the concentrations of the current study.
3.5 Sulfur fractions
The different fractions of sulfur concentrations determined in the sediments of Lake Burullus and their relative contribution to total sulfur are listed (Table 1 and Fig. S2).
3.5.1 Water-soluble fraction (SF1)
It is the best estimate of readily available sulfur in sediments (Table 1). The range and average of the released sulfur in SF1 vary from 0.08 to 0.22 mg g−1 at site 12 and site 8, respectively, with an average of 0.12 ± 0.04 mg g−1. Figure 5 shows the horizontal distribution of sulfur associated with the SF1 fraction in the surface sediments of Lake Burullus. It was found that the relatively high sulfur concentrations are in the central part of the lake, ranging from 0.12 to 0.22 mg g−1 (Fig. 5). SF1 represented 0.5% and 6.77% from total S, which corresponds to an average of 1.34 ± 1.52% (Table 1 and Fig. S3). However, the highest contribution at site 11 (6.77%) revealed ecological hazards.
3.5.2 Phosphate extractable fraction (SF2)
The adsorbed sulfur fraction is represented by the phosphate extractable SF2 fraction (Table 1 and Fig. S3). Site 7 has the highest SF2 value (3.90 mg g−1), while site 12 has the lowest content (0.34 mg g−1), and the average is 1.85 ± 1.13 mg g−1. The contribution of the SF2 fraction to the total sulfur content ranges from 3.24% at site 14 to 28.93% at site 11, with an average contribution of 15.47 ± 9.34% (Fig. S3). Figure 5 shows the horizontal distribution of the SF2 fraction in the Lake Burullus sediments. The SF2 fraction is highest in the central and eastern regions of the lake, ranging from 1.50 to 3.90 mg g−1.
3.5.3 CaCl2 -extractable fraction (SF3)
The results indicate that the CaCl2-extractable sulfur fraction (SF3) in the sediments of Lake Burullus is relatively high compared to that recorded in SF1, ranging from 0.28 to 0.96 mg g−1 with an average of 0.57 ± 0.21 mg g−1 (Table 1). The minimum and maximum values are recorded at stations 10 and 1, respectively. The horizontal distribution of sulfur associated with the SF3 fraction in the surface sediments of Lake Burullus gives relatively high concentrations in the central and eastern parts of the lake, ranging from 0.50 to 0.90 mg g−1 (Fig. 5). The low average SF3 fraction in Lake Burullus could be related to the higher sulfate reduction rates due to the reduction conditions of anoxic environment (Smolders et al. 2006; Elsayed et al. 2019). This fraction represents a fluctuating percentage between 2.16 and 17.12%, with an average percentage of 4.48 ± 3.90% (Fig. S3).
3.5.4 Organic sulfur (SF4)
The current study revealed that the organic fraction extracted with H2O2 ranks third in terms of abundance among the sulfur fractions extracted in the study, ranging from 0.16 mg g−1 at station 12 to 1.56 mg g−1 at station 4, with an average value of 0.81 ± 0.48 mg g−1 (Table 1). The relative percentage of organic sulfur from total sulfur in Lake Burullus showed a wide range between 1.09% at station 1 and 21.77% at station 11, with an average of 7.20 ± 5.35% (Fig. S3). The horizontal distribution of SF4 concentration in the sediments of Lake Burullus is characterized by high concentrations in the central and eastern regions of the lake, ranging from 0.76 to 1.36 mg g−1 (Fig. 5). There are several processes that affect the concentration of sulfur in the sediments of Lake Burullus, the biological reduction of sulfate is the most important process in the sulfur cycle in sediment, as it is part of the transformation of organic carbon during the process of mineralization into mineral form and the production of hydrogen sulfide (Katsev et al. 2006).
3.5.5 Residual sulfur (SF5)
SF5 constitutes the largest form of total sulfur, ranges from 0.47 mg g−1 in station 11 to 17.42 mg g−1 in station 3, with an average concentration of 10.07 ± 5.14 mg g−1 (Table 1). The relative percentage of residual sulfur fraction ranges from 25.41 to 89.71%, with an average of 70.52 ± 16.92% (Fig. S3). Residual sulfur in Lake Burullus comprised a significant portion of the total sulfur concentration, indicating that reactive iron was relatively abundant for the formation of disulfide compounds (pyrite and marcasite) under primarily anoxic (reduced) conditions (Smolders et al. 2006). The presence of sulfur in the residual form of 70.52% indicates low toxicity of sulfur to biological life directly in Lake Burullus as the residual form is inactive and not exchangeable with water (Soliman et al. 2018). The residual sulfur fraction may cause internal eutrophication in Lake Burullus by reducing the P-binding capacity of the sediments and increasing the movement of phosphate (Smolders et al. 2006). SF5 (70.52 ± 16.92%) > SF2 (15.47 ± 9.34%) > SF4 (7.20 ± 5.35%) > CaCl2- SF3 (9.85 ± 12.40%) > SF1 (1.34 ± 1.52%) were identified.
The box and whisker plot of total sulfur contents (ST) and residual sulfur fraction (BF5) shows that about 50% of the sampling sites had values below the median value of 13.87 mg g−1 for ST and more than the median value of 77.13 mg g−1 for SF5, and that of all the studied sites, site 8 had the highest SF1 content (Fig. S2). About 50% of the sampling sites had SF3 and SF4 fractional values higher than the median values of 3.99 mg g−1 and 6.25 mg g−1, respectively.
In general, sulfur loaded with organic matter consists of two sources: originating from different terrestrial sources (allochthonous) or being formed within the same environment from autochthonous biogeochemical reactions. Sulfur enters plankton, organic residues, and minerals and precipitates (Couture et al. 2016).
The heat map shows the relationship of total sulfur (r = − 0.37), and sulfur content of the fractions SF1 (r = 0.6), SF3 (r = 0.69), SF4 (r = 0.75), and TP (r = 0.6) (Fig. 4). These associations may reflect inland eutrophication in Lake Burullus by reducing the P-binding capacity of the sediments and increasing the phosphate release (Smolders et al. 2006).
3.6 Principal component analysis
PCA/FA was performed on variables characterizing the sediment samples, ST, and BT, and different fractions for 16 sites along Lake Burullus (Table S7). The five PCs explained the total variance of 81.03%. PC1 (26.047%) had a high positive load on silt% (0.908), clay% (0.897), and grain size (0.938) (sediment texture). The PC2 (22.02%) had a high positive load with BT, BF2, BF3 and BF5; 0.888, 0.775, 0.788, and 0.874, respectively (boron factor). PC3 (14.59%) was loaded with ST and SF3, SF4 and SF5 with 0.724, 0.701, 0.896, and 0.704, respectively (sulfur factor). PC2 and PC3 reflected the controlling effect on the mobility of boron and sulfur in Lake Burullus. At low reactive Fe, H2S reacts with organic matter, forming organosulfur compounds (Basafa and Hawboldt 2019). PC4 (9.61%) expressed the biological activities of marine organisms with a high negative load (− 0.832) for TP and − 0.627 for BF4, which may be accompanied by boron adsorption on organic matter at some active sites, such as carboxylic and phenolic sites (Lemarchand et al. 2005). PC5 (8.76%) showed the inverse relationship between TCO3% and BF1, with − 0.787 and 0.799, respectively.
3.7 Cluster analysis
The cluster of total boron, total sulfur, and their geochemical-fractions and different geochemical parameters (silt %, clay %, sand %, TOC, TCO3%, and TP) in Lake Burullus shows similar properties between sites 3 and 12, 5 and 6, 2 and 10, 7 and 15, 8 and 9, and 13 and 14 (Fig. S4).
3.8 Pollution situation
3.8.1 Sediment contamination classification
EF, CF, ICF, GCF and RAC were applied for each site. The boron enrichment factor (BFE) and the sulfur enrichment factor (SEF) have range values of 0.001–0.010 and 0.07–0.56, respectively, which classify the boron and sulfur sources in the lake sediment contents as natural sources (EF, 0.5–1.5; Sattarova et al. 2022). However, the boron contamination factor (BCF) ranges from 1.15 to 15.07, indicating that approximately 57% of the sampling sites have significant boron content and about 30% are highly polluted with boron, and almost all high-pollution sites are located on the western side of the lake. Conversely, the sulfur contamination factor (SCF) shows a range of 0.44–4.93, which categorizes approximately 63% of lake sampling sites as highly polluted (3 ≤ CF < 6; Liu et al. 2020) located in the eastern and central parts of the lake, while 25% of sampling sites are of medium risk (1 ≤ CF < 3; Liu et al. 2020), and the remaining area of the lake is of low risk (CF < 1; Liu et al. 2020).
The individual contamination factor for boron (BICF) and sulfur (SICF) fall under the low contamination category with the exception of sites 11 and 16, which have moderate sulfur contamination (Fig. S5a). On the other hand, calculating GCF for boron and sulfur gives a range of 0.63–3.64, lower than for low contamination (GCF < 6; Soliman et al. 2018), indicating that there is no sum of the potential hazards of boron and sulfur present to the lake and aquatic organisms (Fig. S5b). The present study utilized the Risk Assessment Code (RAC) as a key evaluation index for determining the environmental risks associated with boron and sulfur in the lake environment based on the interaction of the geochemical fracture with sediment particles that are potentially absorbed by organisms (Soliman et al. 2018). The calculation of the boron (BRAC) and sulfur (SRAC) The Risk Assessment Code reports that approximately 60% of the sampling sites have a moderate risk of boron and sulfur (11–30%; Passos et al. 2011). While the rest of the sites are low-risk, except for a few sites on the western side of the lake area (Fig. S5c).
3.8.2 Ecological risk assessment
The RQ and RCRmix(PEC/PNEC) values of different trophic levels for acute and chronic outcomes presented in boron and chronic sulfur show values less than one. In contrast, the RQ and RCRmix(PEC/PNEC) values of acute current data from sulfur raised a value higher than one for all sampling sites (Fig. 6). The RQ and RCRmix(PEC/PNEC) values of acute and chronic outcomes for boron take an order of plants (av. 1.19E-01) > fish (av. 4.44E-02) > invertebrates (av. 2.40E-02) and invertebrates (av. 2.81E-02) > algae (av. 3.23E-03) > fish (av. 1.85E-03), respectively. These values reflect that this lake remains ecologically characterized by low boron risk. The RC for boron indicates a high acute boron % contribution for plants (63.5%), followed by fish (23.7%) and invertebrates (12.8%), while, the highest chronic boron % contribution is for invertebrates (84.7%), followed by algae (9.8%) and then fish (5.6%) (Fig. S6). According to the LC05 values for boron documented for the acute (18.8 mg L−1) and chronic (1.18 mg L−1) lethal end points (Soucek et al. 2011), none of the aquatic organisms at the sites examined are expected to show acute positive risk (bioavailable boron fraction, 0.76–11.85 mg L−1). However, 11 sites (1–2, 5, 7–8, and 11–16) appear to be suffering from agricultural wastes that have adverse chronic effects on aquatic trophic levels.
The TU and STU of boron ranged between 0.01 and 0.1 for most invertebrate and fish species, except for those at sites 12–14 in the eastern part of the lake. Additionally, most plants species show higher values that exceed the risk limits for medium and high risks, especially at the extreme ends of the eastern and western sides, beside the central lake area (Fig. 7). The chronic TU and STU of boron give values within intermediate risk limits between 0.1 and 1 for most algae and fish species at the investigated sites, except for fish species on the eastern side of the highly terminated lake and all invertebrate species that exceed the risk limits for high risks (Fig. 7).
The acute and chronic TU values for three trophic levels of sulfur along the lake sites show ranges of 0.03–1.12 and 0.02–1.24, respectively (Fig. 7). Most algae and fish species have moderately acute sulfur hazards from sediments (0.1 = TU < 0.1), except for sites 11–14 and 16 that are low risk (0.01 = TU < 0.1). At most sites in the lake, the values of TU for sulfur for invertebrates range from medium to high risk for acute (0.12–1.12) risk and chronic risk (0.13–1.24). Interestingly, sulfur exposure for the three trophic levels shows a similar symmetry trend with relatively low TU and STU values, i.e., the effect of sulfur on aquatic organisms is affected by agricultural waste disposed into lake sediments. STU values for acute and chronic sulfur along all sampling sites range from low to high risk, in the eastern (1–2), central (4 and 7), and western (10 and 14) sites of the lake. The acute and chronic RC values and the sulfur trend for the three trophic levels are relatively similar for acute and chronic %TUalgae or plants, %TUinvert, and %TUfish with orders of invertebrates (64.9%) > fish (21.2%) > algae (14.0%), and invertebrates (73.1%) > fish (15.0%) > algae (11.9%), respectively (Fig. S6b). Comparing the acute and chronic RCRmix and STU values of boron and sulfur, it appears that, like any toxicant, boron shows high risk in the long term, but sulfur shows high risk to aquatic organisms in the short term, as documented before (Soucek et al. 2011).
4 Conclusions
The objectives of this study were to characterize boron and sulfur geochemistry in Lake Burullus sediments. Sequential and non-sequential extraction techniques were applied to identify boron and sulfur fractions, respectively. The ICF, GCF and RAC revealed that 60% of sites had moderate boron/sulfur hazards. The RQ and RCRmix(PEC/PNEC) were below one, acute sulfur values exceeded one. The BCF and SCF indicated around 57–63% of sites were contaminated, located mainly in the west. Remarkably, highly polluted sites were located on the western side of the lake. As the first study reporting boron and sulfur fractionation in Lake Burullus, these findings lay groundwork to aid management through informing future research and decision-making.
Availability of data and materials
The datasets used and/or analyzed during the current study are available in this published paper.
References
Abreu IM, Cordeiro RC, Soares-Gomes A, Abessa DM, Maranho LA, Santelli RE (2016) Ecological risk evaluation of sediment metals in a tropical Euthrophic Bay, Guanabara Bay, Southeast Atlantic. Mar Pollut Bull 109:435–45. https://doi.org/10.1016/j.marpolbul.2016.05.030
Al-Afify ADG, Abdo MH, Othman AA, Abdel-Satar AM (2023) Water quality and microbiological assessment of Burullus Lake and its surrounding drains. Water Air Soil Pollut 234:385. https://doi.org/10.1007/s11270-023-06351-3
APHA-AWWA-WPCF (1999) Standard Methods for the Examination of Water and Waste Water, twentieth ed., American Public Health Association, Washington, DC
Arisekar U, Shakila RJ, Shalini R, Jeyasekaran G, Arumugam AN, Almansour AI, Keerthana M, Perumal K (2022) Bioaccumulation of organochlorine pesticide residues (OCPs) at different growth stages of pacific white leg shrimp (Penaeus vannamei): first report on ecotoxicological and human health risk assessment. Chemosphere 308:136459. https://doi.org/10.1016/j.chemosphere.2022.136459
Aspila KI, Agemian H, Chau ASY (1976) A semi-automated method for the determination of nitrogen, organic and total phosphorus in sediments. Analyst 101:187–197
Basafa M, Hawboldt K (2019) Reservoir souring: sulfur chemistry in offshore oil and gas reservoir fluids. J Petrol Explor Prod Technol 9:1105–1118. https://doi.org/10.1007/s13202-018-0528-2
Black CA (1965) Methods of soil analysis: part I physical and mineralogical properties. American Society of Agronomy, Madison, Wisconsin
Casillas A, de la Torre A, Navarro I, Sanz P, Martínez MA (2022) Environmental risk assessment of neonicotinoids in surface water. Sci Total Environ 809:151161. https://doi.org/10.1016/j.scitotenv.2021.151161
Chen J, Zhang H, Liu L, Zhang J, Cooper M, Mortimer RJG, Pan G (2021) Effects of elevated sulfate in eutrophic waters on the internal phosphate release under oxic conditions across the sediment-water interface. Sci Total Environ 790:148010. https://doi.org/10.1016/j.scitotenv.2021.148010
Couture RM, Fischer R, Van Cappellen P, Gobeil C (2016) Non-steady state diagenesis of organic and inorganic sulfur in lake sediments. Geochim Cosmochim Acta 194:15–33. https://doi.org/10.1016/j.gca.2016.08.029
Datta SP, Rattan RK, Suribabu K, Datta SC (2002) Fractionation and colorimetric determination of boron in soils. J Plant Nutr Soil Sci 165(2):179–184. https://doi.org/10.1002/1522-2624(200204)165:2%3c179::AID-JPLN179%3e3.0.CO;2-Q
Davies TD, Hall KJ (2007) Importance of calcium in modifying the acute toxicity of sodium sulphate to Hyalella azteca and Daphnia magna. Environ Toxicol Chem 26(6):1243–1247. https://doi.org/10.1897/06-510R.1
EC (European Commission) (2003). Technical Guidance Document in support of Commission Directive 93/67/EEC on Risk Assessment for new notified substances, Commision Regulation (EC) No 1488/94 on Risk Assessment for existing substances and Directive 98/9/ECof the European Parliament and of the Council concerning the placing of biocidal products on the market. Part II. Ispra, Italy: European Chemicals Bureau, Institute for Health and ConsumerProtection. Report no. EUR 20418 EN/2
ECHA European Chemicals Agency (2016) Guidance on information requirements and Chemical Safety Assessment, Chapter R.16: Environmental exposure assessment. Helsinki (FI): European Chemicals Agency
El Zokm GM, El-Said GF, El Ashmawy AA (2022) A comparative study, distribution, predicted no-effect concentration (PNEC) and contamination assessment of phenol with heavy metal contents in two coastal areas on the Egyptian Mediterranean Sea coast. Mar Environ Res 179:105687. https://doi.org/10.1016/j.marenvres.2022.105687
El Zokm G, Masoud M, El-Shorbagi E, Elsamra R, Okbah M (2023) Reactive sulfide dynamic models for predicting metal hazardous in sediments of two northern Egyptian Lakes. Mar Pollut Bull 188:114694. https://doi.org/10.1016/j.marpolbul.2023.114694
El-Naggar NA, Rifaat AE (2022) AQUASEA hydrodynamic and transport model: salinity and dissolved oxygen simulation in El-Burullus Lake (Nile Delta, Egypt) considering different boundary conditions. Egypt J Aquat Res 48:107–113. https://doi.org/10.1016/j.ejar.2022.01.004
Elsayed FA, Okbah MA, El-Syed SM, Eissa MA, Goher ME (2019) Nutrient salts and eutrophication assessment in northern delta lakes: case study Burullus lake. Egypt. Egypt J Aquat Biol Fish 23:145–163. https://doi.org/10.21608/ejabf.2019.30239
Faragallah HM, Khalil MK, Okbah MA (2010) Distribution of boron fractions in Mediterranean Sea sediments (El-Max and Abu-Qir bays). Egypt Fresenius Environ Bull 19:461–468
Finizio A, Vighi M (2014) Predicted no effect concentration (PNEC). Encyclopedia of Toxicology 3:1061–1065. https://doi.org/10.1016/B978-0-12-386454-3.00572-8
Folk RL (1974) Petrology of sedimentary rocks. Hemphill Publishing Company, Austin, TX
Holmer M, Storkholm P (2001) Sulphate reduction and sulphur cycling in lake sediments: a review. Freshw Biol 46(4):431–451. https://doi.org/10.1046/j.1365-2427.2001.00687.x
Hou J, Evans LJ, Spiers GA (1994) Boron fractionation in soils. Commun Soil Sci Plant Anal 25(9–11):1841–1853. https://doi.org/10.1080/00103629409369157
Jingfu W, Jingan C, Zhihui D, Haiquan Y, Chenyan M (2016) Sulfur speciation in the surface sediments of lakes from different regions, China: Characterization by S K-Edge XANES Spectroscopy. J Chem Volume 2016: Article ID 3672348. https://doi.org/10.1155/2016/3672348
Katsev S, Sundby B, Mucci A (2006) Modeling vertical excursions of the redox boundary in sediments: application to deep basins of the Arctic Ocean. Limnol Oceanogr 51(4):1581–1593. https://doi.org/10.4319/lo.2006.51.4.1581
Kosma CI, Lambropoulou DA, Albanis TA (2014) Investigation of PPCPs in wastewater treatment plants in Greece: occurrence, removal and environmental risk assessment. Sci Total Environ 466–467:421–438. https://doi.org/10.1016/j.scitotenv.2013.07.044
Lemarchand E, Schott J, Gaillardet J (2005) Boron isotopic fractionation related to boron sorption on humic acid and the structure of surface complexes formed. Geochim Cosmochim Acta 69:3519–3533. https://doi.org/10.1016/j.gca.2005.02.024
Li B, Liu R, Gao H, Tan R, Zeng P, Song Y (2016) Spatial distribution and ecological risk assessment of phthalic acid esters and phenols in surface sediment from urban rivers in Northeast China. Environ Pollut 219:409–415. https://doi.org/10.1016/j.envpol.2016.05.022
Li L, Wu J, Lu J, Xu J (2020) Speciation, risks and isotope-based source apportionment of trace elements in soils of the northeastern Qinghai-Tibet Plateau. Geochem Explor Environ Anal 20:315–322. https://doi.org/10.1144/geochem2019-042
Li B, Wu J, Li L (2021) Speciation and correlation of boron and lithium in surficial sediments of the eastern and western Taijinar Salt Lake. Environ Earth Sci 80:1–10. https://doi.org/10.1007/s12665-021-09689-1
Likens GE, Driscoll CT, Buso DC, Mitchell MJ, Lovett GM, Bailey SW, Siccama TG, Reiners WA, Alewell C (2002) The biogeochemistry of sulfur at Hubbard Brook. Biogeochemistry 60:235–316. https://doi.org/10.1023/A:1020972100496
Liu J, Han G (2021) Tracing riverine sulfate source in an agricultural watershed: constraints from stable isotopes. Environ Pollut 288:117740. https://doi.org/10.1016/j.envpol.2021.11774
Liu M, Xu Y, Nawab J, Rahman Z, Khan S, Idress M, din ZU, Ali A, Ahmad R, Khan SA, Khan M, Khan Q, Tang Y-T, Li G, (2020) Contamination features, geo-accumulation, enrichments and human health risks of toxic heavy metal (loids) from fish consumption collected along Swat River. Pakistan Environ Technol Innov 17:100554. https://doi.org/10.1016/j.eti.2019.100554
Loring DH, Rantala RTT (1992) Manual for the geochemical analysis of marine sediments and suspended particulate matter. Earth Sci Rev 32(4):235–283
Mahanta PL, Singh AK, Radhamani R, Rao DP (2017) Determination of total sulfur and sulfate sulfur in geological materials of rocks, soils, and sediments by ICP-OES after sample digestion using alkali flux. At Spectrosc 38(4):99–105
Molnia BF (1974) A rapid and accurate method for the analysis of calcium carbonate in small samples. J Sediment Res 44(2):589–590. https://doi.org/10.1306/74D72A9F-2B21-11D7-8648000102C1865D
Moneer AA, El-Sadawy MM, El-Said GF, Radwan AA (2012) Boron human health risk assessment relative to the environmental pollution of Lake Edku. Egypt J King Abdulaziz Univ Mar Sci 23:41–55. https://doi.org/10.4197/Mar.23-2.3
Morche L (2008) S-fluxes and spatial alterations of inorganic and organic sulphur fractions in soil as well as their accumulation and depletion in the rhizosphere of agricultural crops by partial use of the radioisotope 35S. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2008. (In German)
Passos EDA, Alves JDPH, Garcia CAB, Costa ACS (2011) Metal fractionation in sediments of the Sergipe River, Northeast. Brazil J Braz Chem Soc 22(5):828–835. https://doi.org/10.1590/S0103-50532011000500004
Prodromou KP (2004) Boron adsorption by amorphous Al(OH)3 in the presence of low molecular weight organic acids. Agrochimica 48:172–176
Raitzsch M, Bijma J, Benthien A, Richter K-U, Steinhoefel G, Kučera M (2018) Boron isotope-based seasonal paleo-pH reconstruction for the Southeast Atlantic – a multispecies approach using habitat preference of planktonic foraminifera. Earth Planet Sci Lett 487:138–150. https://doi.org/10.1016/j.epsl.2018.02.002
Reis ED, Santos LVS, Lange LC (2021) Prioritization and environmental risk assessment of pharmaceuticals mixtures from Brazilian surface waters. Environ Pollut 288:117803. https://doi.org/10.1016/j.envpol.2021.117803
Russell JM, Werne JP (2009) Climate change and productivity variations recorded by sedimentary sulfur in Lake Edward. Uganda/d r Congo Chem Geol 264:337–346. https://doi.org/10.1016/j.chemgeo.2009.03.020
Sattarova VV, Aksentov KI, Ivanov MV, Alatortsev AV, Kim DV, Obrezko MS (2022) Distribution and assessment of trace metals in modern bottom sediments in the southwestern Chukchi Sea. Mar Pollut Bull 180:113797. https://doi.org/10.1016/j.marpolbul.2022.113797
Shan XQ, Chen B (1995) Determination of carbon-bonded sulfur in soils by hydriodic acid reduction and hydrogen peroxide oxidation. Fresenius J Anal Chem 351:762–767. https://doi.org/10.1007/BF00323633
Shibini Mol PA, Sujatha CH (2020) Distribution and geochemical speciation of sediment bound heavy metals in the specific zones of central Kerala. India Environ Nanotechnol Monit Manag 14:100358. https://doi.org/10.1016/j.enmm.2020.100358
Smolders AJP, Lamers LPM, Lucassen ECHET, Van Der Velde G, Roelofs JGM (2006) Internal eutrophication: how it works and what to do about it - a review. Chem Ecol 22(2):93–111. https://doi.org/10.1080/02757540600579730
Soliman NF, El Zokm GM, Okbah MA (2018) Risk assessment and chemical fractionation of selected elements in surface sediments from Lake Qarun, Egypt using modified BCR technique. Chemosphere 191:262–271. https://doi.org/10.1016/j.chemosphere.2017.10.049
Soucek DJ, Dickinson A, Koch BT (2011) Acute andchronic toxicity of boron to a variety of freshwater organisms. Environ ToxicolChem 30(8):1906–1914
Thomas RP, Ure AM, Davidson CM, Littlejohn D, Rauret G, Rubio R, López-Sánchez JF (1994) Three-stage sequential extraction procedure for the determination of metals in river sediments. Anal Chim Acta 286:423–429. https://doi.org/10.1016/0003-2670(94)85088-7
Xiao-quan S, Bin C, Long-zhu J, Yan Z, Xiao-ping H, Shi-fen M (1992) Determination of sulfur fractions in soils by sequential extraction, inductively coupled plasma-optical emission spectroscopy and ion chromatography. Chem Spec Bioavailab 4(3):97–103. https://doi.org/10.1080/09542299.1992.11083186
Zhang J, Shi J, Ge H, Tao H, Guo W, Yu X, Zhang M, Li B, Xiao R, Xu Z, Li X (2022) Tiered ecological risk assessment of nonylphenol and tetrabromobisphenol A in the surface waters of China based on the augmented species sensitivity distribution models. Ecotoxicol Environ Saf 236:113446. https://doi.org/10.1016/j.ecoenv.2022.113446
Acknowledgements
The authors would like to thank the National Institute of Oceanography and Fisheries for providing the capabilities and facilities available for a research plan entitled “Fractionation of Sulfur and Boron as a Risk Assessment Tool for Sediments of lake Brrullus 2019-2020” from which this work was produced. Many thanks to our colleagues at NIOF for providing us with samples required to perform this work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Gehan M. El Zokm: Conceptualization, methodology, data processing, formal analysis, writing—original draft, investigation and validation, supervision, and writing—review & editing. Mohamed A. Okbah: Conceptualization, methodology, data processing, formal analysis, writing—original draft, investigation and validation, supervision, and writing—review & editing. Ghada F. El-Said: Conceptualization, methodology, data processing, formal analysis, writing—original draft, investigation and validation, supervision, and writing—review & editing. Khaled S. Sinoussy: Conceptualization, data processing, formal analysis, writing—original draft, investigation and validation, supervision, and writing—review & editing.
Corresponding author
Ethics declarations
Consent for publication
It is a pleasure for all the authors to publish our paper in this valuable journal.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Klara Hilscherova
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Zokm, G.M., Okbah, M.A., El-Said, G.F. et al. Ecological implications of boron and sulfur in sediments from a lake in the Nile Delta: Remote sensing, fractionation and ecotoxicity. J Soils Sediments 24, 1361–1376 (2024). https://doi.org/10.1007/s11368-024-03750-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-024-03750-9