- 1Department of Neurosurgery, Center for Malignant Brain Tumors, National Glioma MDT Alliance, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2China Anti-Cancer Association Specialty Committee of Glioma, Beijing, China
- 3Eight-year Medical Doctor Program, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 4"4+4" Medical Doctor Program, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 5Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: The 2021 World Health Organization Classification of Central Nervous System Tumors updates glioma subtyping and grading system, and incorporates EGFR amplification (Amp) as one of diagnostic markers for glioblastoma (GBM).
Purpose: This study aimed to describe the frequency, clinical value and molecular correlation of EGFR Amp in diffuse gliomas based on the latest classification.
Methods: We reviewed glioma patients between 2011 and 2022 at our hospital, and included 187 adult glioma patients with available tumor tissue for detection of EGFR Amp and other 59 molecular markers of interest. Clinical, radiological and pathological data was analyzed based on the status of EGFR Amp in different glioma subtypes.
Results: 163 gliomas were classified as adult-type diffuse gliomas, and the number of astrocytoma, oligodendroglioma and GBM was 41, 46, and 76. EGFR Amp was more common in IDH-wildtype diffuse gliomas (66.0%) and GBM (85.5%) than IDH-mutant diffuse gliomas (32.2%) and its subtypes (astrocytoma, 29.3%; oligodendroglioma, 34.8%). EGFR Amp did not stratify overall survival (OS) in IDH-mutant diffuse gliomas and astrocytoma, while was significantly associated with poorer OS in IDH-wildtype diffuse gliomas, histologic grade 2 and 3 IDH-wildtype diffuse astrocytic gliomas and GBM.
Conclusion: Our study validated EGFR Amp as a diagnostic marker for GBM and still a useful predictor for shortened OS in this group.
Introduction
The most prevalent primary malignant brain tumors are gliomas, most of which grow invasively and lack a clear boundary with normal brain tissue, and hence are defined as diffuse gliomas. Despite a low average annual incidence of about 8 per 100,000, gliomas have a grave prognosis, with a 5-year survival rate of 6.9% for the most aggressive subtype of glioblastoma (GBM; Ostrom et al., 2022). Gliomas are characterized by various histopathology and genetic heterogeneity. In pursuit of more precise glioma diagnosis and prognostic prediction, the World Health Organization (WHO) Classification of Central Nervous System (CNS) Tumors has been updated to the fifth edition (WHO CNS5) in 2021 (Louis et al., 2021). Compared with the WHO CNS4 classification in 2016 which mostly relies on tumor histology (Louis et al., 2016), the latest version makes major changes in the categorization scheme of gliomas and incorporates several molecular markers related to different subtypes. Currently, diffuse gliomas are divided into adult-type, pediatric-type low-grade and pediatric-type high-grade. Adult-type diffuse gliomas, as the predominant pathological type, include astrocytoma, IDH-mutant; oligodendroglioma, IDH-mutant and 1p/19q-codeleted; and glioblastoma, IDH-wildtype.
Epidermal growth factor receptor (EGFR) gene, located on the short arm of human chromosome 7 (chr 7p11.2), encodes a transmembrane glycoprotein that is a member of receptor tyrosine kinases (RTKs) and regulates cell proliferation (Voldborg et al., 1997). EGFR amplification (Amp) and mutations have been identified as driving events for multiple cancers, non-small cell lung cancer (NSCLC), breast cancer and GBM in particular (da Cunha et al., 2011; Brennan et al., 2013; Hsu and Hung, 2016; Sigismund et al., 2018). EGFR Amp was reported to occur in nearly two-thirds of primary GBM, and almost half of those positive for EGFR Amp harbored the mutant EGFRvIII and EGFR single nucleotide variants (SNVs; Brennan et al., 2013; Furnari et al., 2015; Eskilsson et al., 2018; Munoz-Hidalgo et al., 2020). As shown in Figure 1, EGFR Amp leads to overexpression of EGFR protein in glioma cells, contributing to tumor proliferation, angiogenesis and invasion via RAS and PI3K/AKT signaling pathway. Besides, especially in GBM, genomic rearrangement caused by EGFR Amp increases the occurrence of EGFRvIII, which could activate PI3K/AKT and other downstream pathways independent of extracellular ligand, exerting pro-tumorigenic effects (Yang et al., 2017). Studies in recent years have found that the prognosis of IDH-wildtype diffuse lower grade gliomas with EGFR Amp overlapped with that of GBM (Aibaidula et al., 2017; Stichel et al., 2018; Petersen et al., 2021). Therefore, the WHO CNS5 classification has added EGFR Amp as a key molecular marker for diagnosing IDH-wildtype GBM in the absence of microvascular proliferation (MVP) and necrosis (Louis et al., 2021). However, EGFR Amp was less investigated in other diffuse gliomas, particularly those of IDH-mutant. It remains unknown about the landscape of EGFR Amp in diffuse gliomas under the current classification and whether it has new clinical roles in IDH-mutant diffuse gliomas. Therefore, it is necessary to restudy the frequency and prognostic value of EGFR Amp in different subtypes of diffuse gliomas defined by the WHO CNS5 classification.
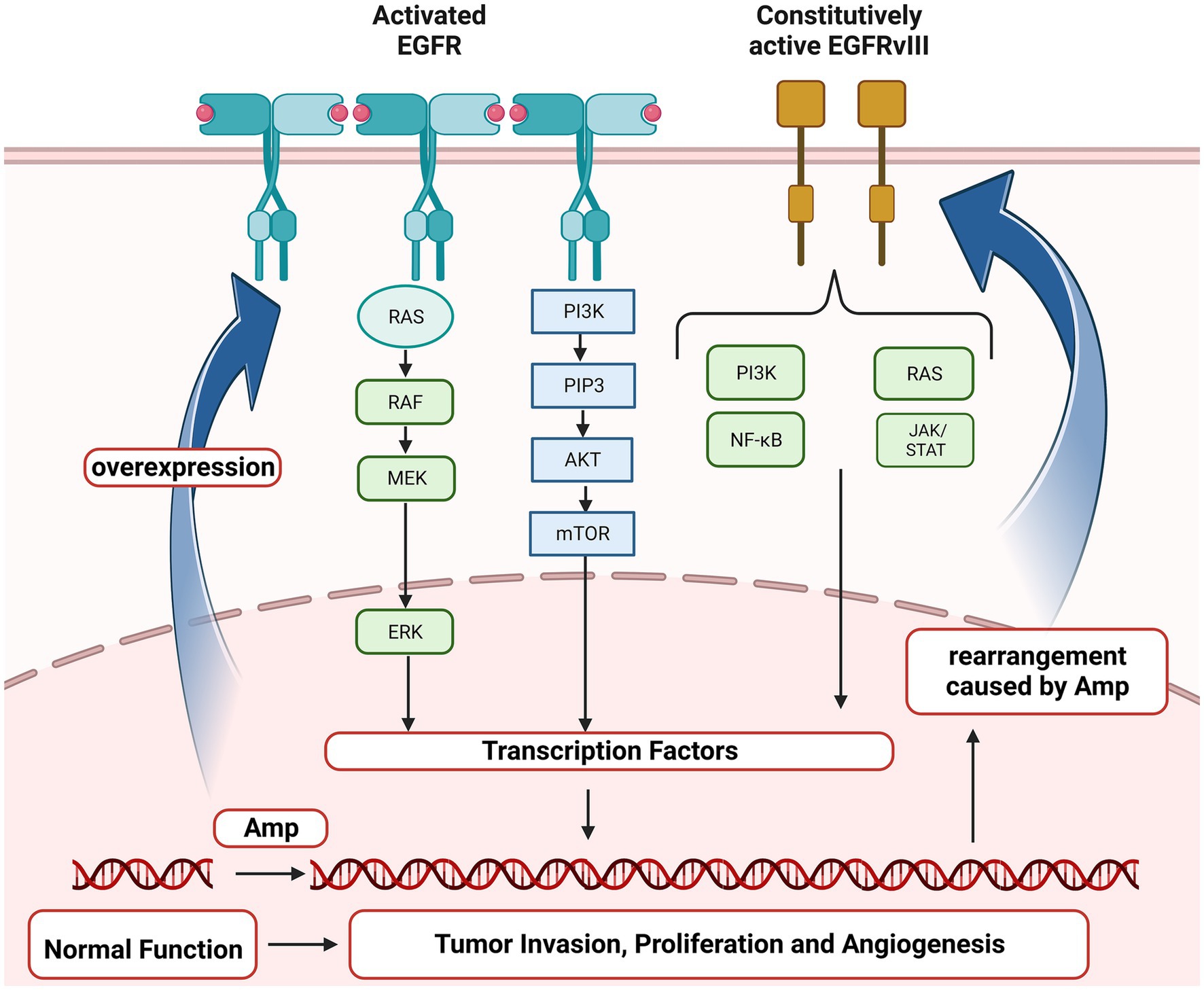
Figure 1. Oncogenic EGFR signaling pathway in glioma. EGFR, Epidermal growth factor receptor; EGFRvIII, Epidermal growth factor receptor variant III; RAS, a family of genes (HRAS, KRAS and NRAS) involved in signaling pathways of cell growth and death; RAF, Serine/threonine-protein kinases; MEK, Mitogen-activated protein kinase; ERK, Extracellular signal-regulated kinases; PI3K, Phosphoinositide 3-kinase; PIP3, Phosphatidylinositol (3,4,5)-trisphosphate; AKT, Protein kinase B; mTOR, mammalian target of rapamycin; NF-κB, Nuclear factor kappa-light-chain-enhancer of activated B cells; JAK/STAT, Janus kinase/signal transducers and activators of transcription; Amp, amplification.
This study enrolled 187 adult patients diagnosed with diffuse gliomas from January 2011 to April 2022 at our hospital and reclassified them according to the present WHO classification. We analyzed the frequency of EGFR Amp, and its association with patient prognosis and other genetic alterations in different subgroups, aiming to offer more knowledge about the application of EGFR Amp in the 2021 WHO classification.
Materials and methods
Patient population
605 patients with glioma, resected or biopsied between January 2011 and January 2022 at the Department of Neurosurgery at Peking Union Medical College Hospital (PUMCH), were screened for this study (Guo et al., 2023). Exclusion criteria were: (i) age under 18 years; (ii) missing data for diagnostic molecular markers (i.e., IDH1/2 mutation, CDKN2A/B mutation, 1p/19q codeletion, TERT promoter mutation, EGFR amplification and combined whole chromosome 7 gain and whole chromosome 10 loss); (iii) circumscribed astrocytic gliomas, glioneuronal and neuronal tumors and ependymal tumors according to the 2021 WHO classification of CNS tumors. 187 patients were included for further analysis. This study was approved by the Institutional Ethics Review Board of PUMCH (ID: S-424), and all participants provided written informed consent.
Data collection
Clinical information of all patients was collected from the medical records, including gender, age at diagnosis, body mass index (BMI), disease duration before admission, baseline Karnofsky Performance Score (KPS), primary or recurrent tumors, clinical symptoms, the extent of resection (EOR) and postoperative treatments. The survival status was collected via outpatient and telephone follow-ups. The overall survival (OS) was defined as the time from operation to the patient’s death or last follow-up (censored).
Baseline MRI images of all patients were retrieved from the Picture Archiving and Communication System (PACS) to extract radiological features, including number of tumors, tumor locations, involvement of eloquent areas, signal intensity on T1WI and T2WI, presence of contrast enhancement, peritumoral edema and necrotic center, and maximal diameter of tumor, edema and necrosis. Two junior neuroradiologists evaluated specific features separately, and one neuroradiologist with over-10-year working experience examined the results.
Histopathological data were acquired from the pathological reports by the department of pathology at PUMCH, including Ki-67 index and histological WHO grade. Formalin-fixed, paraffin-embedded tumor tissue sections were subjected to next-generation sequencing (NGS), polymerase chain reaction (PCR)-based assays, and fluorescence in situ hybridization methods (FISH) for the detection of 60 molecular markers (Supplementary Table S1). These markers were chosen based on recent studies on tumorigenesis and prognosis of glioma. DNA was extracted with QIAGEN 56404 Kit, and DNA concentration and purity were determined by Qubit 4.0 Fluorometer (Thermo Fisher Scientific) and Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific) respectively. After DNA fragmentation and PCR amplification, double-ended sequencing was performed by NovaSeq 6000. Copy number variation was identified from DNA sequencing results using CNVkit. The objective criteria for deletion were the ratio of copy number ≤ 1.5 while the number of bin ≥ 0.3, and the objective criteria for amplification were the ratio of copy number ≥ 2.5 while the number of bin ≥ 0.3. FISH was applied to verify EGFR amplification, with the EGFR probe to chromosome 7 probe ratio ≥ 2.0. Histopathological and molecular pathological data were integrated to determine the subtypes of gliomas according to the 2021 WHO classification of CNS tumors.
Statistical analyses
Continuous variables were presented as the mean ± standard deviation (SD) or median plus interquartile range (IQR) based on data distribution, while categorial variables were presented as number plus percentage. Each variable was compared between EGFR amplification (Amp) and non-amplification (Non-amp) in different subtypes. Normally distributed continuous variables were compared by Student’s t-test, while non-normally distributed continuous variables were compared by Mann–Whitney U test. The comparison of categorical variables was performed using the chi-squared test. The difference of OS between EGFR Amp and Non-amp in different groups was evaluated with the Kaplan–Meier method and the log-rank test. Besides, Fisher’s exact test was performed to analyze the correlation between EGFR Amp and other genes’ alteration, and the results were illustrated by the heatmap of -1og10(p-value). p < 0.05 was considered as statistically significantly for all statistical analyses. SPSS (version 26.0, IBM, United States) statistical software and R software (version 4.2.1) were used for data analysis, and GraphPad Prism (9, GraphPad Software, United States) software was used for graphic drawing.
Results
Subtyping of diffuse gliomas using the WHO CNS5 classification and frequency of EGFR amplification
Figure 2 illustrates the subtyping flow of 187 diffuse gliomas with intact diagnostic molecular markers. Initially, they were screened for IDH1/2 mutation, and divided into 87 IDH-mutant and 100 IDH-wildtype diffuse gliomas. Of 87 IDH-mutant diffuse gliomas, 41 astrocytoma, IDH-mutant and 46 oligodendroglioma, IDH-mutant and 1p/19q-codeleted were confirmed based on the absence or presence of 1p/19q-codeletion. 44 of 100 IDH-wildtype diffuse gliomas presented microvascular proliferation (MVP) or necrosis and were defined as histological glioblastoma (GBM). 56 of 100 IDH-wildtype diffuse gliomas without MVP or necrosis were defined as diffuse astrocytic glioma, IDH-wildtype, and further screened for EGFR amplification (Amp), +7/−10 and TERT promotor mutation, with 32 of them defined as molecular GBM. 44 histological and 32 molecular GBM made up the group of glioblastoma, IDH-wildtype under the WHO CNS5 classification.
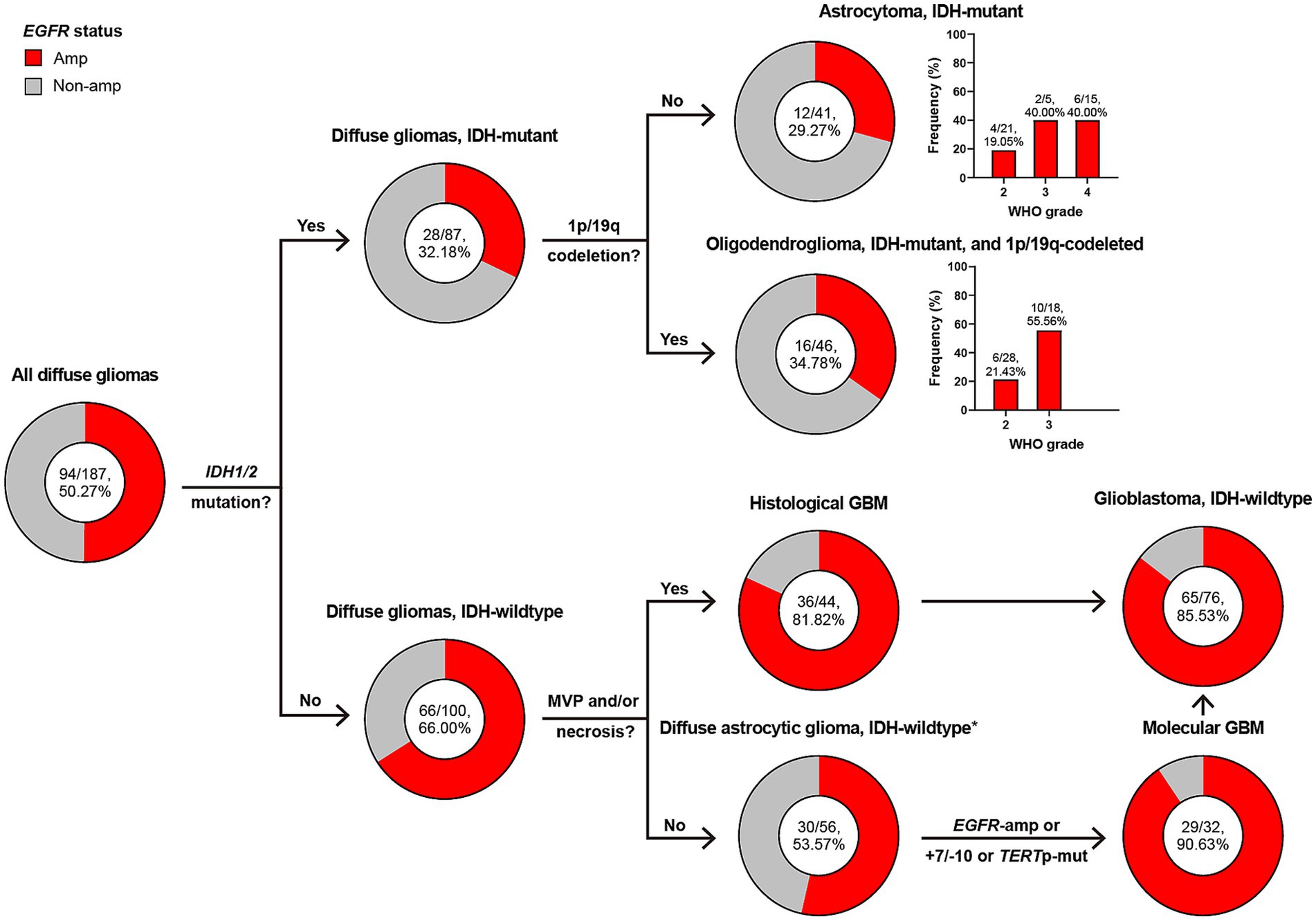
Figure 2. Classification of diffuse gliomas according to histological and molecular markers, and frequency of EGFR amplification in different subgroups. The total patient number, and the number plus percentage of patients with EGFR amplification in each group are presented. *Diffuse astrocytic glioma, IDH-wildtype refers to histologic grade 2 and 3 IDH-wildtype diffuse astrocytic gliomas, as is stated in cIMPACT-NOW update 3 (Brat et al., 2018). Amp, amplification; Non-amp, non-amplification; MVP, microvascular proliferation; GBM, glioblastoma, IDH-wildtype; +7/−10, combined whole chromosome 7 gain and whole chromosome 10 loss; TERTp, TERT promoter; Mut, mutation.
EGFR Amp appeared in 50% of all diffuse gliomas, but was more common in those of IDH-wildtype (66% vs. 32%). Astrocytoma and oligodendroglioma shared similar frequency of EGFR Amp (29% and 34%), with higher in WHO grade 3 or 4. The presence of EGFR Amp was consistent in histological, molecular and all GBM, with a frequency of 81%, 90%, and 85%, respectively.
Clinical, radiological, and pathological features based on EGFR amplification and non-amplification
Baseline information of three types of adult-type diffuse gliomas, namely astrocytoma, oligodendroglioma and GBM, was detailed in Table 1. Clinical, radiological and histopathological differences between EGFR Amp and Non-amp were explored. Less GBM patients with EGFR Amp developed symptoms of intracranial hypertension (headache and/or vomiting; 40.0% vs. 90.9%). Oligodendroglioma with EGFR Amp tended to have larger maximal tumor diameter (5.83 ± 1.81 vs. 4.30 ± 2.13). Besides, oligodendroglioma with EGFR Amp was more likely to manifest as higher histological grade.
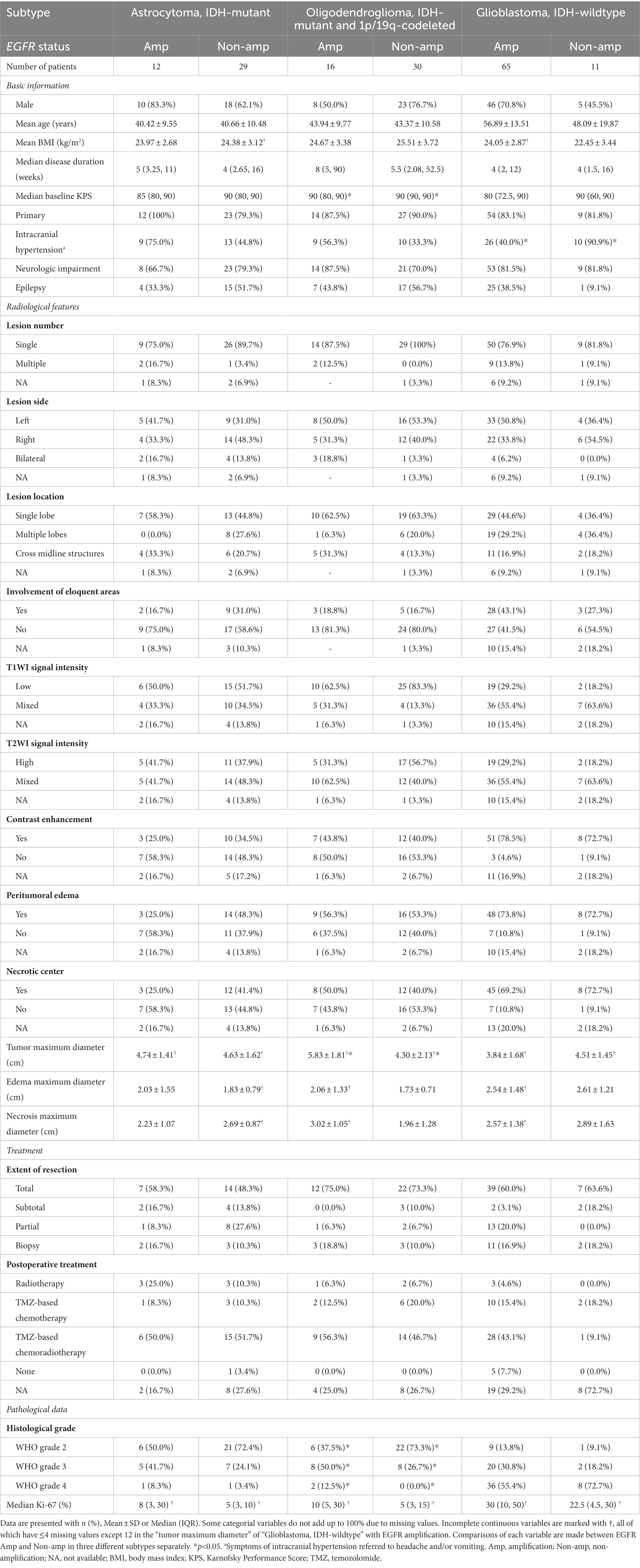
Table 1. Clinical, radiological, and pathological characteristics of adult-type diffuse gliomas based on EGFR status.
Baseline information of other IDH-wildtype diffuse gliomas was detailed in Supplementary Table S2. Since there was only EGFR Amp or Non-amp in each subtype, no comparison was made.
Overall survival differences between EGFR amplification and non-amplification in different subtypes of diffuse gliomas
EGFR Amp manifested as an unfavorable molecular marker for median overall survival (mOS) in all diffuse gliomas [24.2 months vs. 83.6 months, hazard ratio (HR) = 2.76, p < 0.001] and IDH-wildtype diffuse glioma (18.4 months vs. 75.3 months, HR = 2.94, p < 0.001), while not for IDH-mutant diffuse glioma (75.9 months vs. 83.6 months, HR = 1.14, p = 0.781) and astrocytoma (66.2 months vs. 59.7 months, HR = 1.28, p = 0.624). EGFR Amp seemed not to discriminate mOS in astrocytoma of different WHO grades, either (Supplementary Figure S1). Histological GBM with EGFR Amp tended to have a shorter mOS than those of EGFR Non-amp (17.5 months vs. 43.3 months, HR = 1.93, p = 0.175). The mOS of both histologic grade 2 and 3 IDH-wildtype diffuse astrocytic gliomas and molecular GBM was significantly stratified by EGFR Amp (18.5 months vs. 83.8 months, HR = 3.30, p < 0.01, 16.1 months vs. NA, p = 0.044). Taken histological and molecular GBM together, namely GBM under the WHO CNS5 classification, EGFR Amp was still associated with significantly worse survival, with a mOS of 17.5 months (HR = 2.75, p = 0.039; Figure 3).
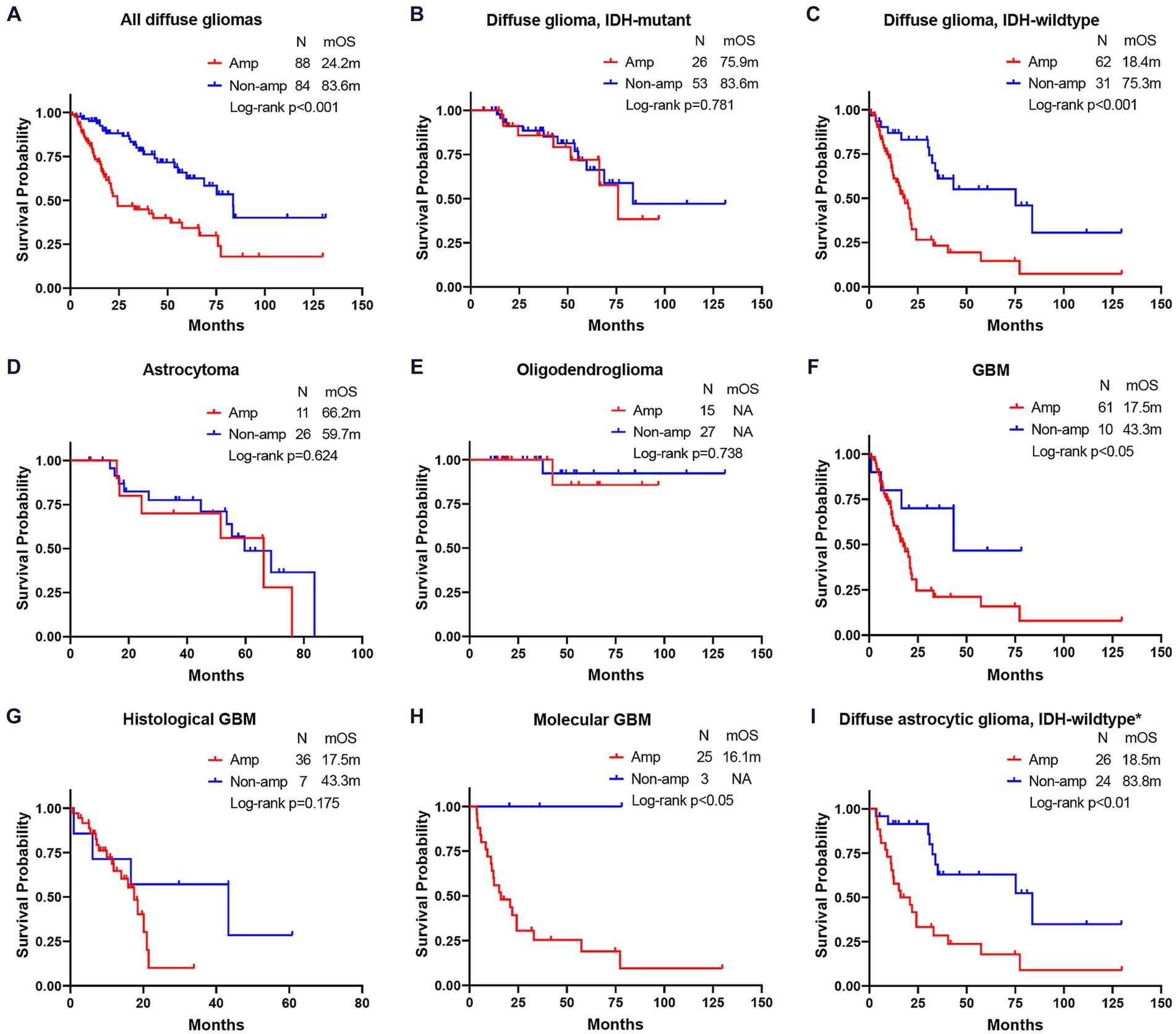
Figure 3. Comparison of overall survival between EGFR amplification and non-amplification in different subgroups of diffuse gliomas. (A) all diffuse gliomas; (B) diffuse glioma, IDH-mutant; (C) diffuse glioma, IDH-wildtype; (D) astrocytoma; (E) oligodendroglioma; (F) glioblastoma (GBM); (G) histological GBM; (H) molecular GBM; (I) diffuse astrocytic glioma, IDH-wildtype. The patient number, mOS and Log-rank p-value are presented. *Diffuse astrocytic glioma, IDH-wildtype refers to histologic grade 2 and 3 IDH-wildtype diffuse astrocytic gliomas, as is stated in cIMPACT-NOW update 3 (Brat et al., 2018). Amp, amplification; Non-amp, non-amplification; NA, not available; mOS, median overall survival.
Correlations between EGFR amplification and other genes’ alterations
Given the prognostic value of EGFR Amp and in order to show the patterns of alterations in other genes, we analyzed the correlations between EGFR Amp and a set of selected genes in IDH-mutant and IDH-wildtype diffuse glioma and their subtypes (Figure 4).
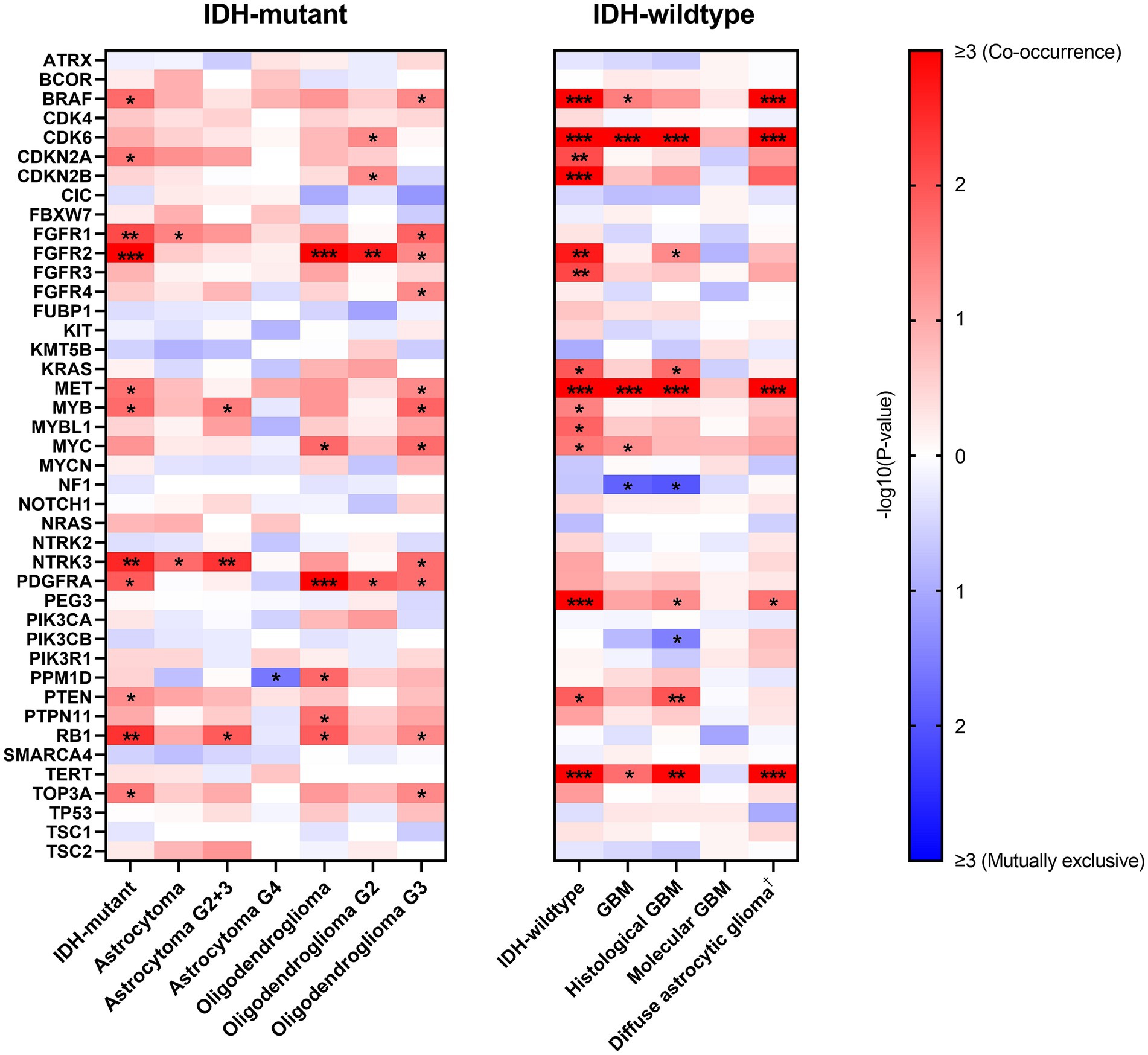
Figure 4. Correlations between EGFR amplification and other genes’ alterations in IDH-mutant and IDH-wildtype diffuse gliomas. −log10(p-value) is calculated to show correlation between paired genes, with red and blue indicating co-occurrence and mutually exclusive, respectively. Only genes with at least one correlation result are shown here (ACVR1, HIST1H3B, HIST1H3C, H3F3A, MAP2K1, SMARCB1, YAP1 excluded) and white indicates no computed result for specific gene pairs. *p < 0.05; **p < 0.01; ***p < 0.001. †Diffuse astrocytic glioma, IDH-wildtype refers to histologic grade 2 and 3 IDH-wildtype diffuse astrocytic gliomas, as is stated in cIMPACT-NOW update 3 (Brat et al., 2018). G2, WHO grade 2; G3, WHO grade 3; G2 + 3, WHO grade 2 and 3; G4, WHO grade 4; GBM, glioblastoma, IDH-wildtype.
In IDH-mutant diffuse glioma, EGFR Amp tended to co-occur with FGFR1, FGFR2, NTRK3 and RB1 alterations. This result was not completely consistent in astrocytoma and oligodendroglioma. However, IDH-wildtype diffuse glioma had a distinct pattern, with BRAF, CDK6, CDKN2A/B, FGFR2, FGFR3, MET, PEG3 and TERT alterations more likely co-occurring with EGFR Amp. The co-occurrence of CDK6 and MET alterations with EGFR Amp was consistent in all GBM, histological GBM and histologic grade 2 and 3 IDH-wildtype diffuse astrocytic gliomas, whereas no possibly correlated genetic alterations were found in molecular GBM.
Discussion
In this study, we investigated the distribution of EGFR amplification (Amp) in different subtypes of diffuse gliomas based on the WHO CNS5 classification (Louis et al., 2021), its value for prognosis, and its relationship with other genetic changes. We found that EGFR Amp mainly occurred in IDH-wildtype diffuse gliomas, accounting for 66%, which was twice as high as that in IDH-mutant diffuse gliomas. In IDH-mutant diffuse gliomas, EGFR Amp tended to indicate higher WHO grade. In IDH-wildtype diffuse gliomas, EGFR Amp was mostly distributed in GBM, particularly molecular GBM. Additionally, EGFR Amp was linked to significantly worsened prognosis in all diffuse gliomas, IDH-wildtype diffuse gliomas, GBM, molecular GBM, and histologic grade 2 and 3 IDH-wildtype diffuse astrocytic gliomas. This result was consistent with the role of EGFR Amp in diffuse glioma in the WHO CNS5 classification (Louis et al., 2021). Finally, the correlation between EGFR Amp and other molecular alterations was found various among different subgroups and grades of diffuse gliomas.
The frequency of EGFR Amp was seldom depicted in IDH-mutant diffuse gliomas. Several previous studies included IDH-mutant GBM which is defined as IDH-mutant astrocytoma (WHO grade 4) currently, and examined EGFR Amp, with the ratio ranging from 3% to 16% (Verhaak et al., 2010; Brennan et al., 2013; Li et al., 2019; Wong et al., 2021; Umphlett et al., 2022). Besides, a study by Bai et al. found that 16 out of 86 (18%) IDH-mutant grade 2 to 3 gliomas were EGFR-amplified (Bai et al., 2016). However, description of EGFR Amp in oligodendroglioma is rare, with only one study in 2001 reporting a frequency of 31% in anaplastic oligodendrogliomas (WHO grade III) (Hoang–Xuan et al., 2001). Our study demonstrated that EGFR Amp was present in 29% of astrocytoma and 34% of oligodendroglioma, with higher frequency in WHO grade 3 or 4. The clinical recognition of EGFR Amp in our IDH-mutant glioma patients was close to previous reports, and the slightly higher frequency may be accounted for by the inclusion of EGFR gene in routine molecular tests for glioma since the publication of cIMPACT-NOW update 3 (Brat et al., 2018). As for GBM, 85% had amplified EGFR gene, with elevated ratio in molecular ones, reaching over 90%. These results were in line with the finding that EGFR Amp was more prevalent in IDH-wildtype gliomas, especially GBM (Brennan et al., 2013). Higher distribution of EGFR Amp in our GBM patients was due to the addition of IDH-wildtype lower grade astrocytoma with amplified-EGFR into the GBM of the WHO CNS5 classification.
We explored clinical, radiological, and pathological differences between EGFR Amp and Non-amp and most of comparisons yielded no significant difference. In oligodendroglioma, EGFR-amplified tumor had larger maximal tumor diameter. Although few articles reported similar finding, it could be explained by higher histological grade in EGFR-amplified oligodendroglioma in our study. In addition, less EGFR-amplified GBM patients developed symptoms of intracranial hypertension (headache and/or vomiting), but there were no differences in maximal tumor and edema diameter. Such discrepancy may be related to missing radiological data in our research. Therefore, further verification through complete clinical information is necessary.
EGFR, as an oncogenic gene, has been extensively investigated for its prognostic value in gliomas, especially IDH-wildtype gliomas. EGFR Amp has been established as an independent marker for poor overall survival (OS) in IDH-wildtype lower grade gliomas, which was close to that of GBM (Aibaidula et al., 2017; Stichel et al., 2018; Petersen et al., 2021). Our study verified EGFR Amp as an unfavorable marker for OS in IDH-wildtype histologic grade 2 and 3 astrocytic gliomas, with a median OS (mOS) of 18.5 months. This result indicated the accuracy of the 2021 WHO classification of CNS tumors. Besides, we also implied that EGFR Amp was potentially meaningful in all GBM and histological GBM, although the difference in the latter group was not statistically significant. Two recent studies on prognostic molecular markers in GBM yielded different results about EGFR. In the retrospective study by Sirui Ma et al., univariable analysis of 367 adult patients with IDH-wildtype GBM (both histological and molecular) showed that EGFR Amp was not significantly associated with OS (Ma et al., 2020). In the study by Peter H. Yang et al., EGFR mutation was associated with decreased OS in the subset analysis of 167 patients with IDH-wildtype GBM (Yang et al., 2022). However, Peter H. Yang et al.’s study was based on the 2016 WHO criteria and did not reveal the specific result on EGFR Amp (Yang et al., 2022). Therefore, the prognostic role of EGFR Amp in the new entity of GBM under the 2021 WHO criteria needs further investigation. On the contrary, in IDH-mutant gliomas, EGFR alterations were less common and its prognostic value was under evaluated (Bai et al., 2016; Umphlett et al., 2022). A study by Craig Horbinski et al. used EGFR immunohistochemistry (IHC) and showed that EGFR expression failed to discriminate survival among astrocytic tumors (Horbinski et al., 2011). Additionally, strong EGFR expression was associated with reduced survival in WHO grade II oligodendrogliomas, but was a favorable marker for survival in WHO grade III anaplastic oligodendrogliomas (Horbinski et al., 2011). A recent study suggested that EGFR Amp in WHO grade 4 IDH-mutant astrocytoma was not related to worse OS, unless CDKN2A/B homozygous deletion were also detected (Li et al., 2019). In our study, OS did not differ significantly among astrocytoma and its different WHO grades. Meanwhile, owing to insufficient endpoints, we were unable to determine the role of EGFR Amp in predicting oligodendroglioma patients’ OS. Thus, further exploration of EGFR Amp in IDH-mutant gliomas should be considered in tumors of specific grade or together with other genetic alterations.
Furthermore, we calculated the correlations between EGFR Amp and other genetic changes. In previous studies, oligodendroglioma was found associated with CIC and FUBP1 due to the close chromosomal location of these genes and 1p/19q co-deletion (Sahm et al., 2012; Yip et al., 2012), while astrocytoma mostly presented TP53 and ATRX mutations (Cancer Genome Atlas Research Network et al., 2015). In our cohort of IDH-mutant diffuse glioma, EGFR Amp was found to co-occur with FGFR1, FGFR2, NTRK3 and RB1 alterations, which was not completely consistent in astrocytoma and oligodendroglioma. These results corresponded to the comparatively low frequency of EGFR Amp in IDH-mutant diffuse glioma and its subtypes. Besides, given the relatively small number of IDH-mutant glioma patients in our cohort, these co-occurrence results need to be validated in larger group. In IDH-wildtype diffuse glioma, a different pattern was seen, with BRAF, CDK6, CDKN2A/B, FGFR2, FGFR3, MET, PEG3 and TERT alterations most co-occurring with EGFR Amp. EGFR Amp co-occurred with CDK6 and MET alterations in all GBM, histological GBM and histologic grade 2 and 3 IDH-wildtype diffuse astrocytic gliomas. These genes have been reported to link with tumorigenesis and progression of GBM, especially CDK6, CDKN2A/B, MET and TERT (Xu and Li, 2018; Cheng and Guo, 2019). Nevertheless, in molecular GBM, EGFR Amp did not have significantly correlated genetic alterations. Such difference in molecular pattern between histological GBM and molecular GBM suggested that EGFR-amplified GBM with or without histological malignancy is different in oncogenesis. Further researches should be conducted to explore the value of these biomarkers.
However, our study results must be interpreted while considering some limitations. Firstly, statistical analyses were only conducted in patients with intact molecular data, and thus selection bias should be taken into account. Secondly, our patient cohort was not large enough and the follow-up of some subgroups (e.g., oligodendroglioma) was not long enough, which potentially interfere with the analysis. Thirdly, forms of molecular alteration are various, in which simply classifying EGFR and other genes as amplified and non-amplified or normal and altered may cover up some meaningful changes. Besides, our panel was pre-designed with certain molecular markers, meaning that more molecular correlations may not be accounted for.
Conclusion
In this real-world study of 187 adult patients, we described the frequency of EGFR Amp, and explored its clinical, radiological and pathological characteristics in diffuse gliomas under the 2021 WHO classification of CNS tumors. EGFR Amp was confirmed as a significant prognostic biomarker for all IDH-wildtype diffuse glioma, histologic grade 2 and 3 IDH-wildtype diffuse astrocytic gliomas and GBM, but a limited one for IDH-mutant diffuse glioma and its subtypes. However, molecular correlations indicated that further classification may be required for some types. Our findings further verified the clinical implications of EGFR Amp in diffuse gliomas, and suggested future research should be undertaken on its association with other molecular alterations to offer more precise diagnosis, treatment and prognostic prediction of glioma.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Peking Union Medical College Hospital (S-424). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HW: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. XZ: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. JiL: Writing – review & editing. WC: Conceptualization, Data curation, Writing – review & editing. XG: Conceptualization, Data curation, Writing – review & editing. YanW: Data curation, Writing – review & editing. YueW: Data curation, Writing – review & editing. HX: Data curation, Writing – review & editing. TL: Data curation, Writing – review & editing. YS: Data curation, Writing – review & editing. DL: Data curation, Writing – review & editing. TY: Data curation, Writing – review & editing. YX: Data curation, Writing – review & editing. JuL: Data curation, Writing – review & editing. JW: Data curation, Writing – review & editing. QL: Data curation, Writing – review & editing. TQ: Data curation, Writing – review & editing. SG: Data curation, Writing – review & editing. HL: Data curation, Writing – review & editing. KZ: Data curation, Writing – review & editing. YL: Data curation, Writing – review & editing. SJ: Data curation, Writing – review & editing. DZ: Data curation, Writing – review & editing. YuW: Funding acquisition, Supervision, Writing – review & editing. WM: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the CAMS Innovation Fund for Medical Sciences (2021-I2M-1-014), the National High-Level Hospital Clinical Research Funding (2022-PUMCH-A-019) and the Beijing Municipal Natural Science Foundation (7202150) for YuW, the National High-Level Hospital Clinical Research Funding (2022-PUMCH-B-113), the Tsinghua University-Peking Union Medical College Hospital Initiative Scientific Research Program (2019ZLH101), and the Beijing Municipal Natural Science Foundation (19JCZDJC64200[Z]) for WM.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2024.1308627/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Comparison of overall survival between EGFR amplification and non-amplification in different grades of astrocytoma. The patient number, mOS and Log-rank p value are presented. G2+3, WHO grade 2 and 3; G4, WHO grade 4; Amp, amplification; Non-amp, non-amplification; mOS, median overall survival.
References
Aibaidula, A., Chan, A. K., Shi, Z., Li, Y., Zhang, R., Yang, R., et al. (2017). Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro Oncol. 19, 1327–1337. doi: 10.1093/neuonc/nox078
Bai, H., Harmancı, A. S., Erson-Omay, E. Z., Li, J., Coşkun, S., Simon, M., et al. (2016). Integrated genomic characterization of IDH1-mutant glioma malignant progression. Nat. Genet. 48, 59–66. doi: 10.1038/ng.3457
Brat, D. J., Aldape, K., Colman, H., Holland, E. C., Louis, D. N., Jenkins, R. B., et al. (2018). cIMPACT-NOW update 3: recommended diagnostic criteria for "diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV". Acta Neuropathol. 136, 805–810. doi: 10.1007/s00401-018-1913-0
Brennan, C. W., Verhaak, R. G., McKenna, A., Campos, B., Noushmehr, H., Salama, S. R., et al. (2013). The somatic genomic landscape of glioblastoma. Cell 155, 462–477. doi: 10.1016/j.cell.2013.09.034
Cancer Genome Atlas Research NetworkBrat, D. J., Verhaak, R. G., Aldape, K. D., Yung, W. K., Salama, S. R., et al. (2015). Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 372, 2481–2498. doi: 10.1056/NEJMoa1402121
Cheng, F., and Guo, D. (2019). MET in glioma: signaling pathways and targeted therapies. J. Exp. Clin. Cancer Res. 38:270. doi: 10.1186/s13046-019-1269-x
da Cunha, S. G., Shepherd, F. A., and Tsao, M. S. (2011). EGFR mutations and lung cancer. Annu. Rev. Pathol. 6, 49–69. doi: 10.1146/annurev-pathol-011110-130206
Eskilsson, E., Røsland, G. V., Solecki, G., Wang, Q., Harter, P. N., Graziani, G., et al. (2018). EGFR heterogeneity and implications for therapeutic intervention in glioblastoma. Neuro Oncol. 20, 743–752. doi: 10.1093/neuonc/nox191
Furnari, F. B., Cloughesy, T. F., Cavenee, W. K., and Mischel, P. S. (2015). Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat. Rev. Cancer 15, 302–310. doi: 10.1038/nrc3918
Guo, X., Shi, Y., Liu, D., Li, Y., Chen, W., Wang, Y., et al. (2023). Clinical updates on gliomas and implications of the 5th edition of the WHO classification of central nervous system tumors. Front. Oncol. 13:1131642. doi: 10.3389/fonc.2023.1131642
Hoang–Xuan, K., He, J., Huguet, S., Mokhtari, K., Marie, Y., Kujas, M., et al. (2001). Molecular heterogeneity of oligodendrogliomas suggests alternative pathways in tumor progression. Neurology 57, 1278–1281. doi: 10.1212/wnl.57.7.1278
Horbinski, C., Hobbs, J., Cieply, K., Dacic, S., and Hamilton, R. L. (2011). EGFR expression stratifies oligodendroglioma behavior. Am. J. Pathol. 179, 1638–1644. doi: 10.1016/j.ajpath.2011.06.020
Hsu, J. L., and Hung, M. C. (2016). The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 35, 575–588. doi: 10.1007/s10555-016-9649-6
Li, K. K., Shi, Z. F., Malta, T. M., Chan, A. K. Y., Cheng, S., Kwan, J. S. H., et al. (2019). Identification of subsets of IDH-mutant glioblastomas with distinct epigenetic and copy number alterations and stratified clinical risks. Neurooncol Adv. 1:vdz015. doi: 10.1093/noajnl/vdz015
Louis, D. N., Perry, A., Reifenberger, G., von Deimling, A., Figarella-Branger, D., Cavenee, W. K., et al. (2016). The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131, 803–820. doi: 10.1007/s00401-016-1545-1
Louis, D. N., Perry, A., Wesseling, P., Brat, D. J., Cree, I. A., Figarella-Branger, D., et al. (2021). The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 23, 1231–1251. doi: 10.1093/neuonc/noab106
Ma, S., Rudra, S., Campian, J. L., Dahiya, S., Dunn, G. P., Johanns, T., et al. (2020). Prognostic impact of CDKN2A/B deletion, TERT mutation, and EGFR amplification on histological and molecular IDH-wildtype glioblastoma. Neurooncol Adv. 2:126. doi: 10.1093/noajnl/vdaa126
Munoz-Hidalgo, L., San-Miguel, T., Megias, J., Monleón, D., Navarro, L., Roldán, P., et al. (2020). Somatic copy number alterations are associated with EGFR amplification and shortened survival in patients with primary glioblastoma. Neoplasia 22, 10–21. doi: 10.1016/j.neo.2019.09.001
Ostrom, Q. T., Price, M., Neff, C., Cioffi, G., Waite, K. A., Kruchko, C., et al. (2022). CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 24, v1–v95. doi: 10.1093/neuonc/noac202
Petersen, J. K., Boldt, H. B., Sørensen, M. D., Blach, S., Dahlrot, R. H., Hansen, S., et al. (2021). Targeted next-generation sequencing of adult gliomas for retrospective prognostic evaluation and up-front diagnostics. Neuropathol. Appl. Neurobiol. 47, 108–126. doi: 10.1111/nan.12645
Sahm, F., Koelsche, C., Meyer, J., Pusch, S., Lindenberg, K., Mueller, W., et al. (2012). CIC and FUBP1 mutations in oligodendrogliomas, oligoastrocytomas and astrocytomas. Acta Neuropathol. 123, 853–860. doi: 10.1007/s00401-012-0993-5
Sigismund, S., Avanzato, D., and Lanzetti, L. (2018). Emerging functions of the EGFR in cancer. Mol. Oncol. 12, 3–20. doi: 10.1002/1878-0261.12155
Stichel, D., Ebrahimi, A., Reuss, D., Schrimpf, D., Ono, T., Shirahata, M., et al. (2018). Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol. 136, 793–803. doi: 10.1007/s00401-018-1905-0
Umphlett, M., Bilal, K. H., Martini, M. L., Suwala, A. K., Ahuja, S., Rashidipour, O., et al. (2022). IDH-mutant astrocytoma with EGFR amplification-genomic profiling in four cases and review of literature. Neurooncol Adv. 4:vdac067. doi: 10.1093/noajnl/vdac067
Verhaak, R. G., Hoadley, K. A., Purdom, E., Wang, V., Qi, Y., Wilkerson, M. D., et al. (2010). Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110. doi: 10.1016/j.ccr.2009.12.020
Voldborg, B. R., Damstrup, L., Spang-Thomsen, M., and Poulsen, H. S. (1997). Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann. Oncol. 8, 1197–1206. doi: 10.1023/a:1008209720526
Wong, Q. H., Li, K. K., Wang, W. W., Malta, T. M., Noushmehr, H., Grabovska, Y., et al. (2021). Molecular landscape of IDH-mutant primary astrocytoma grade IV/glioblastomas. Mod. Pathol. 34, 1245–1260. doi: 10.1038/s41379-021-00778-x
Xu, G., and Li, J. Y. (2018). CDK4, CDK6, cyclin D1, p16(INK4a) and EGFR expression in glioblastoma with a primitive neuronal component. J. Neurooncol 136, 445–452. doi: 10.1007/s11060-017-2674-7
Yang, P. H., Tao, Y., Luo, J., Paturu, M., Lu, H. C., Ramkissoon, S., et al. (2022). Multivariate analysis of associations between clinical sequencing and outcome in glioblastoma. Neurooncol Adv. 4:vdac002. doi: 10.1093/noajnl/vdac002
Yang, J., Yan, J., and Liu, B. (2017). Targeting EGFRvIII for glioblastoma multiforme. Cancer Lett. 403, 224–230. doi: 10.1016/j.canlet.2017.06.024
Keywords: diffuse gliomas, 2021 WHO classification of central nervous system tumors, EGFR amplification, integrated diagnosis, glioblastoma
Citation: Wang H, Zhang X, Liu J, Chen W, Guo X, Wang Y, Wang Y, Xing H, Liang T, Shi Y, Liu D, Yang T, Xia Y, Li J, Wu J, Liu Q, Qu T, Guo S, Li H, Zhang K, Li Y, Jin S, Zhao D, Wang Y and Ma W (2024) Clinical roles of EGFR amplification in diffuse gliomas: a real-world study using the 2021 WHO classification of CNS tumors. Front. Neurosci. 18:1308627. doi: 10.3389/fnins.2024.1308627
Edited by:
David Meredith, Brigham and Women's Hospital and Harvard Medical School, United StatesReviewed by:
Christina Wood-Wentz, Mayo Clinic, United StatesPrerana Jha, Karkinos Healthcare Private Limited, India
Copyright © 2024 Wang, Zhang, Liu, Chen, Guo, Wang, Wang, Xing, Liang, Shi, Liu, Yang, Xia, Li, Wu, Liu, Qu, Guo, Li, Zhang, Li, Jin, Zhao, Wang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence:Yu Wang, ywang@pumch.cn; Wenbin Ma, mawb2001@hotmail.com
†These authors have contributed equally to this work
 Hai Wang
Hai Wang Xin Zhang1†
Xin Zhang1† Wenlin Chen
Wenlin Chen Xiaopeng Guo
Xiaopeng Guo Yaning Wang
Yaning Wang Yuekun Wang
Yuekun Wang Hao Xing
Hao Xing Tingyu Liang
Tingyu Liang Yixin Shi
Yixin Shi Delin Liu
Delin Liu Yu Xia
Yu Xia Junlin Li
Junlin Li Jiaming Wu
Jiaming Wu Kun Zhang
Kun Zhang Yilin Li
Yilin Li Shanmu Jin
Shanmu Jin Dachun Zhao
Dachun Zhao Yu Wang
Yu Wang Wenbin Ma
Wenbin Ma