Abstract
Background
Thrombospondin-1 (THBS1) is a secretory adhesive glycoprotein involved in the progression of multiple malignancies, including breast cancer. However, the clinical significance and prognostic role of plasma THBS1 in breast cancer have yet to be clarified.
Methods
Plasma THBS1 levels in 627 breast cancer patients were analyzed by enzyme-linked immunosorbent assay. Bone marrow blood was drawn from the anterior/posterior superior iliac spine to detect the presence of disseminated tumor cells (DTCs). The effects of plasma THBS1 on the clinicopathological characteristics and survival prediction of breast cancer patients were explored.
Results
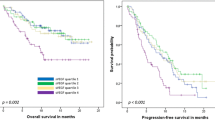
Plasma THBS1 did not correlate with overall survival, breast cancer-specific survival (BCSS), and distant disease-free survival (DDFS) in the entire breast cancer cohort. Notably, HER2-enriched patients with high-plasma THBS1 levels had significantly shorter BCSS (P = 0.027) and DDFS (P = 0.011) than those with low levels. Multivariate analyses revealed that plasma THBS1 was an independent prognostic marker of BCSS (P = 0.026) and DDFS (P = 0.007) in HER2-enriched patients. THBS1 levels were 24% higher in positive DTC patients than in negative DTC patients (P = 0.031), and high levels were significantly associated with poor BCSS in positive DTC patients (HR 2.08, 95% CI 1.17–3.71; P = 0.019). Moreover, high-plasma THBS1 levels were specifically associated with an increased occurrence of brain metastasis in HER2-enriched patients (P = 0.041).
Conclusion
These findings suggest that plasma THBS1 may be serving as an unfavorable prognosis predictor for HER2-enriched breast cancer and justifies the need for further research.






Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Achrol AS, Rennert RC, Anders C et al (2019) Brain metastases. Nat Rev Dis Primers 5:5
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14:48–54
Nik-Zainal S, Morganella S (2017) Mutational signatures in breast cancer: the problem at the DNA level. Clin Cancer Res 23:2617–2629
Harrell JC, Prat A, Parker JS et al (2012) Genomic analysis identifies unique signatures predictive of brain, lung, and liver relapse. Breast Cancer Res Treat 132:523–535
Muth M, Engelhardt BM, Kröger N et al (2011) Thrombospondin-1 (TSP-1) in primary myelofibrosis (PMF) - a megakaryocyte-derived biomarker which largely discriminates PMF from essential thrombocythemia. Ann Hematol 90:33–40
Khaiboullina SF, Morzunov SP, St Jeor SC et al (2016) Hantavirus infection suppresses thrombospondin-1 expression in cultured endothelial cells in a strain-specific manner. Front Microbiol 7:1077
Zhou L, Picard D, Ra YS et al (2010) Silencing of thrombospondin-1 is critical for myc-induced metastatic phenotypes in medulloblastoma. Cancer Res 70:8199–8210
Yee KO, Streit M, Hawighorst T et al (2004) Expression of the type-1 repeats of thrombospondin-1 inhibits tumor growth through activation of transforming growth factor-beta. Am J Pathol 165:541–552
Kazerounian S, Yee KO, Lawler J (2008) Thrombospondins in cancer. Cell Mol Life Sci 65:700–712
Martin-Manso G, Galli S, Ridnour LA et al (2008) Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells. Cancer Res 68:7090–7099
Incardona F, Lewalle JM, Morandi V et al (1995) Thrombospondin modulates human breast adenocarcinoma cell adhesion to human vascular endothelial cells. Cancer Res 55:166–173
Perez-Janices N, Blanco-Luquin I, Tuñón MT et al (2015) EPB41L3, TSP-1 and RASSF2 as new clinically relevant prognostic biomarkers in diffuse gliomas. Oncotarget 6:368–380
Borsotti P, Ghilardi C, Ostano P et al (2015) Thrombospondin-1 is part of a Slug-independent motility and metastatic program in cutaneous melanoma, in association with VEGFR-1 and FGF-2. Pigment Cell Melanoma Res 28:73–81
Lyu T, Jia N, Wang J et al (2013) Expression and epigenetic regulation of angiogenesis-related factors during dormancy and recurrent growth of ovarian carcinoma. Epigenetics 8:1330–1346
Nie S, Lo A, Wu J et al (2014) Glycoprotein biomarker panel for pancreatic cancer discovered by quantitative proteomics analysis. J Proteome Res 13:1873–1884
Ioachim E, Damala K, Tsanou E et al (2012) Thrombospondin-1 expression in breast cancer: prognostic significance and association with p53 alterations, tumour angiogenesis and extracellular matrix components. Histol Histopathol 27:209–216
Weinstat-Saslow DL, Zabrenetzky VS, VanHoutte K et al (1994) Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res 54:6504–6511
Mo D, He F, Zheng J et al (2021) tRNA-derived fragment tRF-17-79MP9PP attenuates cell invasion and migration via THBS1/TGF-β1/Smad3 axis in breast cancer. Front Oncol 11:656078
Wang T, Srivastava S, Hartman M et al (2016) High expression of intratumoral stromal proteins is associated with chemotherapy resistance in breast cancer. Oncotarget 7:55155–55168
Marcheteau E, Farge T, Pérès M et al (2021) Thrombospondin-1 silencing improves lymphocyte infiltration in tumors and response to anti-PD-1 in triple-negative breast cancer. Cancers (Basel) 13:4059
Rouanne M, Adam J, Goubar A et al (2016) Osteopontin and thrombospondin-1 play opposite roles in promoting tumor aggressiveness of primary resected non-small cell lung cancer. BMC Cancer 16:483
Zhu L, Li Q, Wang X et al (2019) THBS1 is a novel serum prognostic factors of acute myeloid leukemia. Front Oncol 9:1567
Hu XY, Ling ZN, Hong LL et al (2021) Circulating methylated THBS1 DNAs as a novel marker for predicting peritoneal dissemination in gastric cancer. J Clin Lab Anal 35:e23936
Suh EJ, Kabir MH, Kang UB et al (2012) Comparative profiling of plasma proteome from breast cancer patients reveals thrombospondin-1 and BRWD3 as serological biomarkers. Exp Mol Med 44:36–44
Byrne GJ, Hayden KE, McDowell G et al (2007) Angiogenic characteristics of circulating and tumoural thrombospondin-1 in breast cancer. Int J Oncol 31:1127–1132
Goldhirsch A, Winer EP, Coates AS et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24:2206–2223
Hayden K, Tetlow L, Byrne G et al (2000) Radioimmunoassay for the measurement of thrombospondin in plasma and breast cyst fluid: validation and clinical application. Ann Clin Biochem 37:319–325
Sutherland DR, Anderson L, Keeney M et al (1996) The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother 5:213–226
Cerami E, Gao J, Dogrusoz U et al (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–404
Behrens J, Frixen U, Schipper J et al (1992) Cell adhesion in invasion and metastasis. Semin Cell Biol 3:169–178
Huang T, Sun L, Yuan X et al (2017) Thrombospondin-1 is a multifaceted player in tumor progression. Oncotarget 8:84546–84558
Shen J, Cao B, Wang Y et al (2018) Hippo component YAP promotes focal adhesion and tumour aggressiveness via transcriptionally activating THBS1/FAK signalling in breast cancer. J Exp Clin Cancer Res 37:175
Murphy-Ullrich JE, Höök M (1989) Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol 109:1309–1319
Orr AW, Pallero MA, Murphy-Ullrich JE (2002) Thrombospondin stimulates focal adhesion disassembly through Gi- and phosphoinositide 3-kinase-dependent ERK activation. J Biol Chem 277:20453–20460
Cen J, Feng L, Ke H et al (2019) Exosomal thrombospondin-1 disrupts the integrity of endothelial intercellular junctions to facilitate breast cancer cell metastasis. Cancers (Basel) 11
Hartkopf AD, Wallwiener M, Fehm TN et al (2015) Disseminated tumor cells from the bone marrow of patients with nonmetastatic primary breast cancer are predictive of locoregional relapse. Ann Oncol 26:1155–1160
Braun S, Vogl FD, Naume B et al (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353:793–802
Hosseini H, Obradović MMS, Hoffmann M et al (2016) Early dissemination seeds metastasis in breast cancer. Nature 540:552–558
Liu X, Jin J, Liu Y et al (2021) Targeting TSP-1 decreased periodontitis by attenuating extracellular matrix degradation and alveolar bone destruction. Int Immunopharmacol 96:107618
Ghajar CM, Peinado H, Mori H et al (2013) The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15:807–817
Wen XF, Yang G, Mao W et al (2006) HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene 25:6986–6996
Widner DB, Park SH, Eber MR et al (2018) Interactions between disseminated tumor cells and bone marrow stromal cells regulate tumor dormancy. Curr Osteoporos Rep 16:596–602
Yang G, Cai KQ, Thompson-Lanza JA et al (2004) Inhibition of breast and ovarian tumor growth through multiple signaling pathways by using retrovirus-mediated small interfering RNA against Her-2/neu gene expression. J Biol Chem 279:4339–4345
del Barco BI, Nebreda AR (2012) Roles of p38 MAPKs in invasion and metastasis. Biochem Soc Trans 40:79–84
Wen HC, Avivar-Valderas A, Sosa MS et al (2011) p38α signaling induces anoikis and lumen formation during mammary morphogenesis. Sci Signal 4:ra34
Zhao HY, Ooyama A, Yamamoto M et al (2008) Molecular basis for the induction of an angiogenesis inhibitor, thrombospondin-1, by 5-fluorouracil. Cancer Res 68:7035–7041
Wang G, Wang J, Chang A et al (2020) Her2 promotes early dissemination of breast cancer by suppressing the p38 pathway through Skp2-mediated proteasomal degradation of Tpl2. Oncogene 39:7034–7050
Le XF, Lammayot A, Gold D et al (2005) Genes affecting the cell cycle, growth, maintenance, and drug sensitivity are preferentially regulated by anti-HER2 antibody through phosphatidylinositol 3-kinase-AKT signaling. J Biol Chem 280:2092–2104
Izumi Y, Xu L, di Tomaso E et al (2002) Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature 416:279–280
Yee KO, Connolly CM, Duquette M et al (2009) The effect of thrombospondin-1 on breast cancer metastasis. Breast Cancer Res Treat 114:85–96
Miller TW, Kaur S, Ivins-O’Keefe K et al (2013) Thrombospondin-1 is a CD47-dependent endogenous inhibitor of hydrogen sulfide signaling in T cell activation. Matrix Biol 32:316–324
Kennecke H, Yerushalmi R, Woods R et al (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28:3271–3277
Zagar TM, Van Swearingen AE, Kaidar-Person O et al (2016) Multidisciplinary management of breast cancer brain metastases. Oncology (Williston Park) 30:923–933
Wang TN, Qian X, Granick MS et al (1996) Thrombospondin-1 (TSP-1) promotes the invasive properties of human breast cancer. J Surg Res 63:39–43
Anastasi F, Greco F, Dilillo M et al (2020) Proteomics analysis of serum small extracellular vesicles for the longitudinal study of a glioblastoma multiforme mouse model. Sci Rep 10:20498
Kircher DA, Trombetti KA, Silvis MR et al (2019) AKT1(E17K) activates focal adhesion kinase and promotes melanoma brain metastasis. Mol Cancer Res 17:1787–1800
Priego N, Zhu L, Monteiro C et al (2018) STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med 24:1024–1035
Chen CY, Chao YM, Lin HF et al (2020) miR-195 reduces age-related blood-brain barrier leakage caused by thrombospondin-1-mediated selective autophagy. Aging Cell 19:e13236
Rege TA, Stewart J Jr, Dranka B et al (2009) Thrombospondin-1-induced apoptosis of brain microvascular endothelial cells can be mediated by TNF-R1. J Cell Physiol 218:94–103
Meijles DN, Sahoo S, Al Ghouleh I et al (2017) The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1. Sci Signal 10:eaaj1784
Alaoui-Jamali MA, Song DJ, Benlimame N et al (2003) Regulation of multiple tumor microenvironment markers by overexpression of single or paired combinations of ErbB receptors. Cancer Res 63:3764–3774
Hosonaga M, Saya H, Arima Y (2020) Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev 39:711–720
Acknowledgements
We sincerely thank the breast cancer patients who participated in this study.
Funding
This work was supported by the National Natural Science Foundation of China (82103630).
Author information
Authors and Affiliations
Contributions
YL: Conceptualization, Investigation, Methodology, Software, Validation, Resources, Funding acquisition, Writing (original draft, review, and editing). JQ: Conceptualization, Software, Resources, Validation. GC: Project administration, Visualization, Supervision, Investigation. WW: Conceptualization, Validation, Resources, Project administration, Formal analysis. XS: Conceptualization, Validation, Data curation, Visualization, Supervision, Project administration, Writing (review and editing).
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest or other disclosures.
Ethics approval and consent to participate
In the studies, all procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and in conformity with the 1964 Helsinki declaration, in addition to its later amendments or comparable ethical standards. No animal experiment was involved in the current study. The research protocol was reviewed and authorized by the Ethics Committee of the Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine. Informed consent was obtained from each participant.
Consent for publication
Patients signed informed consent to publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10147_2024_2472_MOESM1_ESM.tif
Fig. S1. The cBioPortal database was used to perform co-expression analysis of THBS1 and potential molecules on the basis of the mRNA expression (http://cbioportal.org/). The mRNA expression levels of THBS1 in breast cancer patients are found to be positively correlated with the expression of PIK3CA, TGFB1, MAPK14, STAT3, TNFRSF1A, and EGR1 (TIF 26208 KB)
About this article
Cite this article
Li, Y., Qin, J., Chen, G. et al. Plasma THBS1 as a predictive biomarker for poor prognosis and brain metastasis in patients with HER2-enriched breast cancer. Int J Clin Oncol 29, 427–441 (2024). https://doi.org/10.1007/s10147-024-02472-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02472-9