Abstract
Background
Obesity and liver cirrhosis represent significant health challenges, often leading to various complications.
Aims
This prospective study aimed to investigate the impact of a four-year bariatric intervention, focusing on adherence to the Mediterranean Diet, on anthropometric, hematologic, and biochemical parameters in obese patients with compensated liver cirrhosis. Additionally, the study evaluated the concurrent contribution of weight loss to these health indicators.
Methods
The study involved 62 patients with compensated liver cirrhosis (mean age 65.87 ± 6 years) and 44 healthy controls (mean age 59.11 ± 8 years), all with a BMI > 30 kg/m2. Both groups underwent a weight loss intervention based on the Mediterranean diet, with a four-year follow-up. Anthropometric, biochemical and hematologic parameters were evaluated at several time points during the study and their statistical significance was assessed.
Results
Anthropometric parameters, including weight, BMI, waist and hip circumference, percentage of fat mass, and handgrip strength, exhibited significant improvements (p < 0.05), particularly within the first year of the intervention. Liver function tests and lipid profiles of the patients also showed significant enhancements (p < 0.05). Hematological and biochemical indices, such as hematocrit and ferritin, experienced discreet improvements in the patient cohort (p < 0.05).
Conclusions
This study highlights the potential of a structured bariatric intervention rooted in the Mediterranean diet to positively influence the health of obese patients with compensated liver cirrhosis. The observed improvements in anthropometric, biochemical, and hematologic parameters, particularly within the first year of the intervention, suggest the importance of dietary modifications in managing the health of this patient population.
Similar content being viewed by others
Introduction
Obesity and malnutrition have been associated with a worse prognosis in patients with chronic liver disease. In recent decades, the proportion of overweight or obese patients with chronic liver diseases has increased even in cohorts on the waiting list for transplantation [1], and obesity has been identified as an independent risk factor for de novo liver disease, progressive liver fibrosis and cirrhosis, and adverse outcomes among patients with established cirrhosis [2, 3]. Moreover, malnutrition is also common in cirrhotic patients [4] and it is associated with ascites, hepatorenal syndrome, longer hospitalisations, higher healthcare costs, and higher mortality [4, 5]. According to the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines, nutritional therapy should be an integral part of the management of cirrhotic patients [6], since specialized nutrition counseling is related to improved survival in patients with liver cirrhosis [7]. Hence, obesity constitutes a major risk factor for the complication development in cirrhosis and weight loss should be an important goal for these patients [8].
Bariatric medicine aims at providing comprehensive care to patients with obesity and related conditions by helping them achieve and maintain a healthy weight by promoting changes in diet and lifestyle, as well as providing medical or surgical interventions when appropriate. Such an approach is the application of the Mediterranean diet (MEDD). The effect of this diet on patients with nonalcoholic fatty liver disease (NAFLD) and, in particular nonalcoholic steatohepatitis (NASH), has been widely studied [9, 10]. MEDD is a dietary pattern characterized by a high content of antioxidants and fiber, a balanced lipid profile with a focus on olive oil, moderate consumption of poultry, eggs, and dairy products and a low content of simple sugars, and processed and red meat. Cumulative data from seven interventional and five observational studies suggest that MEDD has beneficial effects on body weight, insulin sensitivity and hepatic steatosis, as well as fibrosis [11,12,13,14,15,16,17,18,19,20].
This prospective study aimed to evaluate the effects of a bariatric nutritional intervention based on the Mediterranean diet on anthropometric (e.g., body weight) and biochemical parameters in obese patients with compensated liver cirrhosis.
Methods
Description of Cohort
The study was conducted at the Department of Gastroenterology and Hepatology, University Hospital of Heraklion in Greece, the reference center for liver disease on the island of Crete (population 0.6 mi), from September 2010 until February 2015. The study protocol conformed to the principles of the declaration of Helsinki and was approved by the institutional review board of the School of Medicine, University of Crete. Participants were enrolled in this study after providing full informed consent; they were all informed that participation was voluntary and that they could withdraw at any time without consequences.
Patients
All outpatients with compensated liver cirrhosis assessed in the Hepatology clinic of our institution by liver biopsy (first patient enrolled: September 2010, last patient enrolled: February 2011), > 18 years old, and with a BMI > 30 kg/m2, without alcohol or illicit drug use, were eligible to participate in the study. Patients refusing to provide written informed consent, < 18 years old, pregnant women, and patients with decompensated cirrhosis were excluded.
Cirrhosis was determined as decompensated in patients with a history of ascites, variceal hemorrhage, hepatic encephalopathy, and/or jaundice (bilirubin > 3 mg/dl for hepatocellular and > 10 mg/dl for cholestatic jaundice). Liver cirrhosis severity was evaluated by means of the Child-Turcotte-Pugh (CTP) and Model for End-Stage Liver Disease (MELD) scores. During the study, all patients with cirrhosis were followed clinically and with, at least annual, ultrasound scans. Moreover, the SGA questionnaire was used during the different study visits to evaluate whether any patient presented an unfavorable progression due to cirrhosis and to rule out cases of initial state of malnutrition due to the disease.
Baseline Evaluation
Upon inclusion in the study, patients had their medical history taken, and physical examination was performed. Their nutritional status was evaluated using the subjective global assessment (SGA) score [21], where the following parameters are taken into consideration: (a) involuntary weight loss in the past six months, (b) dietary intake, as assessed by a three-day food record, (c) gastrointestinal symptoms for more than two weeks (nausea, vomiting, diarrhea, and anorexia), (d) physical status, and (e) metabolic needs due to their disease.
An alcohol intake history was obtained from patients as well as their accompanying relatives and medical records were scrutinized in order to ascertain the cause of the cirrhosis. Patients with alcohol-related liver cirrhosis or those with any alcohol consumption were excluded from the study. Patients were required not to consume any alcohol during the study period. Blood cell counts and biochemical indices, such as mean corpuscular volume, IgA, and γGT levels, were also taken into consideration in alcohol intake assessment.
Control Group
Control subjects were recruited through a call for participation to this study in the area of the institution and were required to be > 18 years old and have a medical history free from liver disease and any metabolic-associated dysfunction, and a BMI > 30 kg/m2. Controls were matched to patients by sex and body mass index (BMI > 30 kg/m2). Due to the high degree of difficulty in recruiting a sufficient number of healthy obese subjects, a slightly younger age group was recruited. Subjects refusing to provide written informed consent, < 18 years old, or pregnant women were excluded. They all underwent a liver ultrasound scan by a trained radiologist in order to exclude the possibility of fatty liver disease or other liver diseases. A full blood and biochemical work-up, including a complete blood count, liver function tests, electrolyte, and creatinine measurement together with thyroid function tests, was performed. Abnormal findings in any of these tests, as well as any reported or suspected (clinically, biochemically or during recruitment interviews) alcohol consumption, constituted exclusion criteria for control subjects. Controls were also required not to consume any alcohol during the study period.
Study Parameters
Anthropometric Measurements
The following anthropometric parameters were evaluated: height, weight, body mass index (BMI), waist circumference (WC), hip circumference (HC), skinfold thickness, and handgrip strength (HGS) for both groups at the following time points: upon inclusion (t = 0), and at 3, 6, 9, 12, 24, 36, and 48 months. Height was measured to the nearest millimeter at baseline while participants were standing with their heels in contact with the wall using a Seca wall-mounted height measure (SECA, Leicester, UK). All participants were also weighted without shoes and with very light clothing to the nearest 0.1 kg using a Seca 704 column scale (SECA, Leicester, UK). BMI was calculated as weight (in kilogram) divided by height squared (in m2). WC was determined, in duplicate, at the midpoint between the lowest costal ridge and the upper border of the iliac crest. HC was measured at the level of maximum extension of the hip in duplicate. A non-stretching and accurately calibrated measuring tape with 0.5-cm precision was used for the measurements.
A Harpenden skinfold caliper (Harpenden, West Sussex, UK) was used for determining fat distribution in the subcutaneous tissue. The sum of the values of the skinfold thickness of different body parts of the participants was used for the calculation of the percentage contribution of fat to the body composition based on the reference tables [22]. Different body parts were selected for skinfold measurements according to the sex of the subjects (triceps, iliac crest, and posterior thigh for women and abdomen, chest, and posterior thigh for men).
A Jamar Hydraulic Hand Dynamometer (Patterson Medical, Warrenville, IL, USA) was used for the evaluation of handgrip strength, as an indication of muscle strength. The subjects were asked to grasp the dynamometer handles with their hand and squeeze them with maximum strength, repeating the procedure three times with intervals of 15 s. Consecutive trials with each hand were evaluated and the best performance was recorded. Low skeletal muscle strength was defined as HGS < 27 kg for men and < 16 kg for women [23].
Laboratory Measurements
Venous blood samples were taken following 12 h’ fasting. Hemoglobin (Hgb), hematocrit (HCT), ferritin, and platelet count (PLT), glucose, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (γGT), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, alkaline phosphatase (ALP), total bilirubin, albumin, and glycosylated hemoglobin (HbA1c) were assessed at t = 0, 3, 6, 9, 12, 24, 36, and 48 months by standard in-house methods.
Proinflammatory Cytokine Analysis
Blood samples were drawn from all patients before breakfast between 8 and 9 AM, after an overnight bed rest, upon inclusion in the study (t = 0) and after 3 months. Blood plasma/serum was separated and stored at − 80 °C. Measurements using the serum samples were performed at the Laboratory of Experimental Endocrinology and Clinical Immunology, University Hospital of Heraklion, Heraklion, Greece by trained laboratory staff. Measurements of proinflammatory cytokines IL-6, IL-8, IL-10, and TNF-α were performed with commercially available ELISA kits, provided by the Invitrogen Corp. (Camarillo, CA) according to the manufacturer’s instructions. Normal values for the above cytokines are as follows: IL-6: 13.1–18.1 pg/ml, IL-8: < 30 pg/ml, IL-10: 0.08–0.28 pg/ml, TNF-α: 3.15–5.55 pg/ml. Triplicates of each sample were measured in the same analytical batch.
Nutrition Plan
The nutritional intervention lasted four years. Both study groups followed a specific four-week dietary plan based on the principles of the Mediterranean diet [24]. Dietary plans were issued by a gastroenterologist specialized in nutrition (ZK). The daily calorie intake was estimated to range between 1800 and 2400 kcal for men and 1400 and 1800 kcal for women. Nutritional plans were tailored individually for each participant based on their initial weight and BMI, with adjustments made to the percentage of intake for various macronutrients. Additionally, it's important to note that these nutritional plans aimed to create a caloric deficit, providing lower caloric values than the participants' daily energy requirements. The relationship between the different macronutrients in the plans was as follows: 50%–55% of carbohydrates of which 95%–100% complex carbohydrates, 15%–20% of proteins of which about 50% of animal origin (especially white meat, fish), and 30%–35% fat (mostly olive oil), with minimum to no salt intake. The nutritional plans were enriched in vitamins and omega-3 fatty acids. Diet adherence was estimated as high (score 6–9), moderate (4–5), or low (0–3) by questionnaires filled in by the participants at all evaluation time points, as previously described [25]. Assessment of the progress was done monthly. The plans would change according to seasonal changes or to the needs of each individual. Throughout the study all participants had access to phone or online support (ZK). Light exercise (e.g., walking) was suggested to all patients.
Statistical Analysis
Data are presented as the average and standard error of the mean (SEM) or n (%) as appropriate. Normality of distribution was mainly assessed via the Kolmogorov–Smirnov test, or, in certain analyses, by means of (a) the Shapiro–Wilk test, (b) the graphs Box plot, Normal Q–Q plot, and Detrended Normal Q–Q Plot (with visual checks), and (c) descriptive statistics (mean, median, skewness, kurtosis). Categorical baseline factors were compared between two different groups by using the χ2−test. For comparison of quantitative variables between groups (before–after intervention, control patients) the Wilcoxon and the Mann–Whitney U tests were used, as appropriate. For comparisons of quantitative variables between more than two groups, the Friedman test was used. Two-way or three-way ANOVA tests were used to compare the average values of variables between the two groups. A 2-tailed p-value less than 0.05 was considered statistically significant. IBM SPSS Statistics for Windows, version 25.0 (IBM Corp, Armonk, NY, USA) was used for statistical analysis.
Results
Baseline Characteristics
Overall, 106 subjects were enrolled and followed up for 48 months, 62 patients with liver cirrhosis and 44 control subjects (Table 1). The most common cause of liver cirrhosis was chronic hepatitis C infection (53%) followed by NASH (29%). None of the patients presented complications of liver cirrhosis or other conditions that could affect nutritional status (such as malignant disease, or other significant chronic illness). The same was true for controls who were also monitored closely during the study period by the lead author (ZK).
Anthropometric Parameters
At baseline, the patient group exhibited higher weight, BMI, percentage of body fat, WC, and HC and lower HGS compared to the control group (Table 2). The anthropometric indices of both groups during the time-course yielded statistically significant changes in all parameters (Fig. 1).
Anthropometric parameters during the study in patients with cirrhosis (continuous line) and controls (dotted line). The statistical significance, as calculated by the Friedman’s test, was p < 0.05 for all parameters. A Weight, B body mass index, C body fat, D handgrip strength, E waist circumference, F hip circumference
Implementation of the nutritional plan had a significant effect on the phenotype of all participants, based on their BMI (Fig. 1B and Fig. 2).
Biochemical Outcomes and Hematological Indices
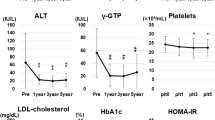
The time-course of all biochemical and hematological indices is depicted in Fig. 3 and in Supplementary Data, Table 1. Unsurprisingly, serum aminotransferases, ALP, γGT, and bilirubin values were higher in the patient group compared to controls throughout the study period. However, a significant reduction in these parameters was observed among patients with cirrhosis during the first year of the study. Mean MELD score was 6.85 upon enrollment and remained unchanged during the whole study period (Supplementary Data, Table 2).
Changes in biochemical parameters during the study in patients with cirrhosis (continuous line) and controls (dotted line). Statistical significance, as calculated by the Friedman’s test, was p < 0.05, unless otherwise stated in the graph. A Gamma-glutamyl transferase (γGT), B alanine transaminase (ALT), C aspartate transaminase (AST), D alkaline phosphatase (ALP), E albumin, F glucose, G creatinine, H hemoglobin A1c (HbA1c), I total bilirubin
A significant improvement in the lipid profile of the patients was observed, especially regarding triglyceride levels where a decremental trend was observed throughout the study (122.9 ± 3.9 mg/dl → 85.4 ± 2.5 mg/dl, p < 0.05; Fig. 4, Sup Data, Table 1). Concomitantly, the number of patients requiring treatment for dyslipidaemia dropped to 5 at 12 months from 23 at baseline, although by the end of the study, it increased to 12 patients.
Lipid profiles and hematological indices during the study in patients with cirrhosis (continuous line) and controls (dotted line). Statistical significance, as calculated by the Friedman’s test, was p < 0.05. A Total cholesterol, B high-density lipoprotein (HDL-C), C low-density lipoprotein (LDL) cholesterol, D triglycerides, E hematocrit (HCT), F ferritin, G platelet count
Diet Adherence
Adherence to the proposed diet, as assessed by means of questionnaires [25] completed annually by the study participants, was high for both groups during the first 12 months (Fig. 5). However, it deteriorated in both groups with 21% of cirrhotic patients showing low adherence at 48 months (Fig. 5). No alcohol consumption was recorded either in patients with cirrhosis or controls in the relevant subscale of the questionnaire (data not shown).
Clinical Evaluation at the End of the Study
At the end of the study, all patients remained in the compensated state (100%) and mean MELD score had not changed significantly compared to study enrollment (Supplementary data, Table 2). Throughout the study, there were no instances of patients decompensating and then reverting to a compensated state. Additionally, none of the participants developed portal vein thrombosis or diabetes.
Changes in Circulating Cytokine Concentrations in Cirrhotic Patients After Three Months of Nutritional Intervention
All cytokines showed a decrease in their concentration after three months of nutritional intervention in patients with cirrhosis (Fig. 6).
Discussion
The main findings of the current study are a positive outcome on weight control accompanied by an improved biochemical profile in obese patients with compensated cirrhosis that have followed a bariatric nutritional intervention based on the principles of the Mediterranean diet combined with close medical monitoring.
To date, the data on the possible health benefits of MEDD in patients with liver cirrhosis are very limited [26, 27]. This is the first prospective follow-up study, to our knowledge, investigating the role of the Mediterranean diet in obese patients with compensated liver cirrhosis using an extensive array of anthropometric, biochemical, and hematological indices.
The worldwide prevalence of obesity has been tripled over the last 40 years and is, respectively, very high in patients with compensated cirrhosis [3]. Since obesity has been identified as an independent risk factor for a worse clinical outcome in cirrhosis, ESPEN strongly recommends the application of a nutritional intervention aiming for beneficial effects of weight loss in obese cirrhotic patients [3, 6]. The majority of clinical trials, to date, have been performed in NAFLD and have shown improved steatosis and insulin sensitivity [28, 29]. In a meta-analysis of eight randomized controlled trials, patients achieving ≥ 5% weight loss showed improvement in hepatic steatosis while a ≥ 7% weight loss improved histological disease activity in NASH. However, most of these trials performed intensive lifestyle interventions that were plagued by weight gain after completion, since weight loss goals were achieved by less than 50% of the participants. Furthermore, most patients in these studies did not suffer from liver cirrhosis [30].
In the current study, patients with cirrhosis achieved an average weight loss of 17.3% at 12 months and managed to preserve an average weight loss of 15.7% at the end point of the study. Interestingly, after 12 months, a proportional increase in individuals with low adherence to the diet was observed only in the cirrhosis group (P). This can be explained if taken into consideration the demographic data of the two groups; cirrhotic patients were by a significant percentage from rural and semi-urban areas and more prone to incorrect eating habits due to culture and lifestyle, combined with the mild physiological deterioration of their health due to the disease. The controls were mainly from urban areas, with a stronger motivation to change their appearance and maintain the effect. This is still higher than a weight loss of > 5%–10% recommended by the European Association for the Study of the Liver guidelines for cirrhotic patients with BMI > 30 kg/m2 [31]. The applied intervention aimed at limiting the fatty infiltration of the liver and avoiding alcohol consumption, which were reinforced by regular surveillance and control. The improvement of liver enzymes values of cirrhotic patients corroborates with previous studies regarding the contribution of MEDD to the improvement of liver enzymes and contribute to the argument regarding the safety of the proposed lifestyle intervention [15, 20, 32]. Interestingly, during the four-year intervention, all cirrhotic patients (n = 62) remained in the compensated state despite that a percentage of 5–7% of patients with compensated cirrhosis is expected to transit to the decompensated state per year [33].
The duration of our study also provides useful insights into weight loss maintenance. Compared to baseline, cirrhotic patients had lost on average 18 ± 1.6 kg in 12 months after enrollment (p < 0.05) while they only regained 8.8% of the initial weight loss at the end of the follow-up period. Interestingly, obese control subjects continued to lose weight throughout the study period, despite the decline in diet adherence, resulting in an average loss of 26.2 ± 1.8 kg (p < 0.05). The majority of clinical trials studying lifestyle interventions have a duration of six to twelve months [34, 35]. Our long-term study indicates that a prolonged dietary intervention, based on MEDD is attainable in patients with early compensated cirrhosis and can result in maintained significant weight loss without major drop-out risks.
Sarcopenia, the combination of dynapenia (low muscle strength) and myopenia (low muscle mass), is one of the most common complications in advanced liver disease, even in obese cirrhotic patients [36]. Although sarcopenia was not assessed in the current study, one of the main goals of the designed individualized nutritional plans was adequate protein intake (15–20% of the daily macronutrient intake). No trials or intervention studies on the protein requirements of well-nourished compensated cirrhotic patients are available to our knowledge. Interestingly, HGS measurements exhibited a continuous increase in the first 12 months (31.1 ± 1.1 kg → 34 ± 1.2 kg, p < 0.05) and stabilized during the subsequent time points of the study, indicating increase or, at least, conservation of muscle strength, which is crucial for protecting from or improving sarcopenia.
Potential changes in the concentration of circulating cytokines in the cirrhotic patients were also assessed at three months from baseline. The results indicated a significant decrease in the concentration of IL-6 (p < 0.001), IL-8 (p < 0.05), and IL-10 (p < 0.05). Acute and chronic liver diseases are considered cytokine-driven as several proinflammatory cytokines (IL-1α, IL-1β, tumor necrosis factor-alpha (TNF- α), and IL-6) are critically involved in inflammation, steatosis, fibrosis, and cancer, as well as complication development [37]. Taken together with the fact that key inflammatory markers have been consistently associated with obesity and the risk of adverse outcomes in obesity-related diseases [38, 39], it is clear that obesity may be a further factor contributing to the systemic inflammation and, thus, cirrhosis progression. Unsurprisingly, previously published data have shown that obesity may be a risk factor for disease progression in cirrhosis [40, 41]. In a recent meta-analysis of 76 papers, an association of weight loss with reduction of IL-6 was established [42]. Our preliminary data show a decrease in proinflammatory cytokines in patients with cirrhosis during the first three months of dietary intervention. These data would need to be confirmed in further larger studies with follow-up cytokine measurements at longer intervals, investigating whether weight loss, in particular by means of following a MEDD, may be related to delaying cirrhosis progression.
Diet adherence was interpreted by a nutrition specialist (ZK) based on the questionnaires filled in by the subjects. The majority of the participants in both groups exhibited a very good level of adherence to the suggested nutritional programs for the first 12 months of the study (82.3% of the patient and 88.6% of the control group, respectively, had a score of 6–9 points), with the control group sustaining a high dietary adherence level for a longer period of time. These observations, along with previous reports, support the advantage of MEDD on adherence over most of other prescribed hypocaloric diets on adherence [29]. The variety of foods and flavors involved in the MEDD favors the achievement of the treatment objectives, especially in the long-term, which is potentially crucial in the case of chronic diseases.
Our study has several limitations. Ideally, an additional control group consisting of obese cirrhotic patients undergoing no intervention would be required. However, ethical issues were raised regarding leaving obese patients untreated while there were also concerns that patients residing in Crete were likely to follow a MEDD to some extent anyway, which could render comparisons difficult. Second, the cirrhotic and control groups had comparable gender distribution but the former were significantly older than the latter, which may have biased our findings. Another limitation of our study pertains to the fact that the cirrhosis group was heterogeneous with regard to the etiology of liver disease. The composition of our cirrhosis cohort, comprising mainly viral hepatitis patients, is consistent with that of other cirrhosis cohorts from southern Europe. Although there is no compelling reason to believe that the etiology of cirrhosis is contingent on the final study outcome, with increasing obesity and NASH rates, it seems reasonable to expect that future research would focus on the potential benefits of the Mediterranean diets on NASH patients with cirrhosis. An inherent limitation of our study was the disparity in the distribution of obesity severity between the study and control groups. This divergence stemmed from the notable challenge of enrolling a sufficient number of healthy obese participants, leading us to include a slightly younger age group with lower BMI values in the control cohort. Consequently, the degree of obesity among controls was less pronounced. It is important to emphasize, however, that statistically significant disparities were evident in both age and body type changes between the control and cirrhotic patient groups throughout the study stages (Mann–Whitney U = 706.00, p < 0.001 for age; χ2 = 278.13, b.e. = 28, p < 0.001 for control body type changes; χ2 = 123.38, b.e. = 28, p < 0.001 for cirrhotic patient body type changes). Third, a follow-up of patients and controls after the end of the intervention could provide insight into whether dietary modifications and its benefits may be maintained. Our data, however, provide strong evidence in support of the integration of dietary advice to the medical management of cirrhotic patients in general and those with obesity in particular [6]. Another limitation of our study is the absence of quantitative assessment of physical activity levels among participants. Although we inquired about physical activity during our monthly meetings and found no significant changes or unusual reports throughout the study, the absence of objective quantification remains a constraint. Future studies should include a more thorough investigation of participant physical activity. Hepatic steatosis was diagnosed using ultrasound scans due to the unavailability of methods such as controlled attenuation parameters at our institution. As conventional ultrasound scans may have limitations in sensitivity for diagnosing or excluding hepatic steatosis, particularly in cases of mild steatosis (< 30%), we cannot rule out that some participants may have had undetected mild steatosis. Lastly, we have no data on whether our suggested long-term intervention could be followed by the majority of obese cirrhotic patients or if an intervention based on the Mediterranean diet could be applicable in countries with different dietary habits. Therefore, although a clear beneficial effect of the nutritional intervention on the health outcomes of the subjects is demonstrated in our study, further and larger-scale studies are needed in order to establish the Mediterranean diet as a bariatric tool in the treatment of obese cirrhotic patients as well as other obesity-related comorbidities.
In conclusion, our findings suggest that a nutritional intervention based on the principles of the Mediterranean diet can be safely recommended to attain weight loss and improve biochemical and anthropometric outcomes in obese patients with compensated cirrhosis. Further prospective studies are warranted to assess the potential benefits of the Mediterranean diet in obese patients with compensated liver cirrhosis and its potential role as a non-pharmacological treatment in this population.
Abbreviations
- ESPEN:
-
European Society for Clinical Nutrition and Metabolism
- MEDD:
-
Mediterranean diet
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- CTP:
-
Child-Turcotte-Pugh
- MELD:
-
Model for End-Stage Liver Disease
- SGA:
-
Subjective global assessment
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- HC:
-
Hip circumference
- Hgb:
-
Hemoglobin
- HCT:
-
Hematocrit
- PLT:
-
Platelet count
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- γGT:
-
γ-Glutamyl transferase
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- ALP:
-
Alkaline phosphatase
- HbA1c:
-
Glycosylated hemoglobin
- SEM:
-
Standard error of the mean
References
Spengler EK, O’Leary JG, Te HS, Rogal S, Pillai AA, Al-Osaimi A et al. Liver Transplantation in the Obese Cirrhotic Patient. Transplantation 2017;101:2288–2296.
Everhart JE, Lok AS, Kim HY, Morgan TR, Lindsay KL, Chung RT et al. Weight-related effects on disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Gastroenterology 2009;137:549–557. https://doi.org/10.1053/j.gastro.2009.05.007.
Berzigotti A, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Morillas R, et al. Portal Hypertension Collaborative Group. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology 2011;54:555–61. doi: https://doi.org/10.1002/hep.24418.
Alberino F, Gatta A, Amodio P, Merkel C, Di Pascoli L, Boffo G et al. Nutrition and survival in patients with liver cirrhosis. Nutrition 2001;17:445–450.
Sam J, Nguyen GC. Protein calorie malnutrition as a prognostic indicator of mortality among patients hospitalized with cirrhosis and portal hypertension. Liver Int 2009;29:1396e402.
Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr 2019;38:485–521.
Iwasa M, Iwata K, Hara N et al. Nutrition therapy using a multidisciplinary team improves survival rates in patients with liver cirrhosis. Nutrition 2013;29:1418–1421. https://doi.org/10.1016/j.nut.2013.05.016.
Patton H, Heimbach J, McCullough A. AGA Clinical Practice Update on Bariatric Surgery in Cirrhosis: Expert Review. Clin Gastroenterol Hepatol 2021;19:436–445. https://doi.org/10.1016/j.cgh.2020.10.034.
Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol 2018;24:2083–2094. https://doi.org/10.3748/wjg.v24.i19.2083.
Kouvari M, Boutari C, Chrysohoou C et al. Mediterranean diet is inversely associated with steatosis and fibrosis and decreases ten-year diabetes and cardiovascular risk in NAFLD subjects: Results from the ATTICA prospective cohort study. Clin Nutr 2021;40:3314–3324. https://doi.org/10.1016/j.clnu.2020.10.058.
Gelli C, Tarocchi M, Abenavoli L, Di Renzo L, Galli A, De Lorenzo A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J Gastroenterol 2017;23:3150e62.
Katsagoni CN, Georgoulis M, Papatheodoridis GV, Fragopoulou E, Ioannidou P, Papageorgiou M, et al. Associations between lifestyle characteristics and the presence of nonalcoholic fatty liver disease: a case-control study. Metab Syndrome Relat Disord 2017;15:72e9.
Misciagna G, Del Pilar Diaz M, Caramia DV, Bonfiglio C, Franco I, Noviello MR, et al. Effect of a low glycemic index Mediterranean diet on non-alcoholic fatty liver disease. a randomized controlled clinici trial. J Nutr Health Aging 2017;21:404e12.
Perez -Guisado J, Munoz-Serrano A. The effect of the Spanish ketogenic Mediterranean diet on nonalcoholic fatty liver disease: a pilot study. J Med Food 2011;14:677e80.
Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol 2013;59:138e43.
Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359:229e41.
Trovato FM, Catalano D, Martines GF, Pace P, Trovato GM. Mediterranean diet and non-alcoholic fatty liver disease: the need of extended and comprehensive interventions. Clin Nutr 2015;34:86e8.
Aller R, Izaola O, de la Fuente B, De Luis Roman DA. Mediterranean diet is associated with liver histology in patients with non alcoholic fatty liver disease. Nutr Hosp 2015;32:2518e24.
Della Corte C, Mosca A, Vania A, Alterio A, Iasevoli S, Nobili V. Good adherence to the Mediterranean diet reduces the risk for NASH and diabetes in pediatric patients with obesity: the results of an Italian study. Nutrition 2017;39e40:8e14.
Kontogianni MD, Tileli N, Margariti A, Georgoulis M, Deutsch M, Tiniakos D, et al. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin Nutr 2014;33:678e83.
Baker JP, Detsky AS, Wesson DE, Wolman SL, Stewart S, Whitewell J et al. Nutritional assessment: a comparison of clinical judgement and objective measurements. N Engl J Med 1982;306:969–972.
Pollock ML, Schmidt DH, Jackson AS. Measurement of cardiorespiratory fitness and body composition in the clinical setting. Compr Ther 1980;6:12–27.
Alfonso J Cruz-Jentoft , Gülistan Bahat , Jürgen Bauer , Yves Boirie , et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; Jan 1;48:16–31. doi: https://doi.org/10.1093/ageing/afy169.
Keys A, Aravanis C. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease. Cambridge, MA: Harvard University Press; 1980.
Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608.
Cox IJ, Idilman R, Fagan A et al. Metabolomics and microbial composition increase insight into the impact of dietary differences in cirrhosis. Liver Int. 2020;40:416–427. https://doi.org/10.1111/liv.14256.
Aller R, Sigüenza R, Pina M et al. Insulin resistance is related with liver fibrosis in type 2 diabetic patients with non-alcoholic fatty liver disease proven biopsy and Mediterranean diet pattern as a protective factor. Endocrine 2020;68:557–563. https://doi.org/10.1007/s12020-020-02268-7.
Abenavoli L, Milic N, Peta V, Alfieri F, De Lorenzo A, Bellentani S. Alimentary regimen in non-alcoholic fatty liver disease: Mediterranean diet. World J Gastroenterol 2014;20:16831e40.
Suarez M, Boque N, Del Bas JM, Mayneris-Perxachs J, Arola L, Caimari A. Mediterranean diet and multi-ingredient-based interventions for the management of non-alcoholic fatty liver disease. Nutrients 2017;9:1052.
Musso G, Cassader M, Rosina F et al. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia 2012;55:885–904.
Merli M, Berzigotti A, Zelber-Sagi S et al. EASL clinical practice guidelines on nutrition in chronic liver disease. J Hepatol 2019;70:172–193.
Ma J, Hennein R, Liu C, Long MT, Hoffmann U, Jacques PF, et al. Improved diet quality associates with reduction in liver fat, particularly in individuals with high genetic risk scores for nonalcoholic fatty liver disease. Gastroenterology 2018;155:107e17.
Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis Hepatology. 2010; 51:1445–9.
Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367–378.
Berzigotti A, Albillos A, Villanueva C, Genescá J, Ardevol A, Augustín S, et al. Ciberehd SportDiet Collaborative Group. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology. 2017;65:1293–1305. doi: https://doi.org/10.1002/hep.28992.
Dhaliwal A, Armstrong MJ. Sarcopenia in cirrhosis: A practical overview. Clin Med (Lond). 2020;20:489–492. https://doi.org/10.7861/clinmed.2020-0089.
Del Campo JA, Gallego P, Grande L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J Hepatol. 2018;10:1–7. https://doi.org/10.4254/wjh.v10.i1.1.
Dalmas E, Clément K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol 2011;32:307–314. https://doi.org/10.1016/j.it.2011.04.008.
Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3:207–215. https://doi.org/10.1016/S2213-8587(14)70134-2.
Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, Naveau S. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–638.
Ortiz V, Berenguer M, Rayón JM, Carrasco D, Berenguer J. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408–2414.
Bianchi VE. Weight loss is a critical factor to reduce inflammation. Clin Nutr ESPEN. 2018;28:21–35. https://doi.org/10.1016/j.clnesp.2018.08.007.
Acknowldgement
I would like to express my sincere gratitude to the members of the research team who contributed to the successful completion of this study. Their dedication, expertise, and commitment were instrumental in the realization of our research objectives. I am thankful for their valuable insights, collaborative spirit, and unwavering support throughout the project (ZK).
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kalaitzakis, Z.E., Giahnakis, E., Koutroubakis, I.E. et al. Bariatric Nutritional Intervention in Obese Patients with Compensated Liver Cirrhosis: A Four-Year Prospective Study. Dig Dis Sci 69, 1467–1478 (2024). https://doi.org/10.1007/s10620-023-08223-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08223-6