Abstract
Objective
To describe hepatotoxicity due to amiodarone and dronedarone from the DILIN and the US FDA’s surveillance database.
Methods
Hepatotoxicity due to amiodarone and dronedarone enrolled in the U.S. Drug Induced Liver Injury Network (DILIN) from 2004 to 2020 are described. Dronedarone hepatotoxicity cases associated with liver biopsy results were obtained from the FDA Adverse Event Reporting System (FAERS) from 2009 to 2020.
Results
Among DILIN’s 10 amiodarone and 3 dronedarone DILIN cases, the latency for amiodarone was longer than with dronedarone (388 vs 119 days, p = 0.50) and the median ALT at DILI onset was significantly lower with amiodarone (118 vs 1191 U/L, p = 0.05). Liver biopsies in five amiodarone cases showed fibrosis, steatosis, and numerous Mallory-Denk bodies. Five patients died although only one from liver failure. One patient with dronedarone induced liver injury died of a non-liver related cause.
Nine additional cases of DILI due to dronedarone requiring hospitalization were identified in the FAERS database. Three patients developed liver injury within a month of starting the medication. Two developed acute liver failure and underwent urgent liver transplant, one was evaluated for liver transplant but then recovered spontaneously, while one patient with cirrhosis died of liver related causes.
Conclusion
Amiodarone hepatotoxicity resembles that seen in alcohol related liver injury, with fatty infiltration and inflammation. Dronedarone is less predictable, typically without fat and with a shorter latency of use before presentation. These differences may be explained, in part, by the differing pharmacokinetics of the two drugs leading to different mechanisms of hepatotoxicity.
Similar content being viewed by others
Introduction
Amiodarone is commonly used to treat patients with atrial and ventricular arrythmias with close to three million total prescriptions filled in 2017 in the United States [1]. Its efficacy and importance are further reflected by its listing on the World Health Organization’s List of Essential Medicines [2].
Dronedarone, a chemically modified derivative of amiodarone, also functions as a class III antiarrhythmic drug and is used in the treatment of atrial fibrillation and atrial flutter. Due to the absence of an iodine moiety, thyroid toxicity does not occur, and pulmonary toxicity is less frequent. In addition, the incidence of mild ALT elevation appears to be lower. These two medications have also been linked to a variety of adverse events. Amiodarone can cause pulmonary fibrosis, corneal microdeposits, peripheral neuropathy, and thyroid dysfunction of patients treated over prolonged periods of time. Additionally, numerous cases of amiodarone induced hepatotoxicity in the form of cirrhosis, chronic active hepatitis, or acute liver failure have been reported since the 1980s [3]. Dronedarone has also been implicated to cause a severe toxic hepatitis, though to date the number of reported cases is much more limited [4, 5, 19].
Consulting gastroenterologists are often asked to comment on questions regarding liver injury attributed to these agents, or about their use in patients who have underlying liver disease. In this report we aim to describe the presenting clinical features, liver histology and outcomes of liver injury attributed to amiodarone and dronedarone by reviewing consecutive cases enrolled into the Drug induced liver injury network (DILIN) prospective registry. DILIN is a multicenter registry founded by the NIDDK in 2004 to enhance knowledge of the etiologies and outcomes of DILI in the US and to collect biological samples for mechanistic studies [6]. In light of the paucity of literature on dronedarone hepatotoxicity and the few cases within the DILIN, we also analyzed cases of dronedarone hepatotoxicity reported to the FDA following its approval in 2009. These data provide a broader spectrum of liver injury presentation attributable to dronedarone. Our overarching goal with this study is to describe the clinical features of hepatotoxicity attributed to amiodarone and dronedarone, and to improve practicing physician knowledge of the hallmark laboratory, clinical and histological attributes which will, in turn, allow more timely recognition of potential DILI due to these agents going forward.
Methods
We identified cases of liver injury due to amiodarone and dronedarone from the DILIN Prospective Study between 2003 and 2021. Due to the small number of cases of dronedarone cases in the DILIN database, and lack of well-characterized published cases of attributable injury, we also identified cases of dronedarone liver injury from the Food Drug Administration (FDA) Adverse Event Reporting System (FAERS).
Founded in 2004, the DILIN Prospective Study is an ongoing cohort study of patients with suspected drug induced liver injury (DILI) within 6 months of onset. Patients eligible for enrollment meet pre-defined laboratory criteria of serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 5 times or serum alkaline phosphatase (ALP) levels > 2 times the upper limit of normal (or baseline before exposure) on two consecutive occasions. Those with a total serum bilirubin of greater than 2.5 mg/dL or an INR above 1.5 after an exposure are eligible as well. A more in-depth review of eligibility, evaluation, and enrollment in the DILIN Prospective Study has been described in a previous publication [6].
Causality assessment in the DILIN is based on consensus expert opinion. A panel of DILIN investigators score the case and implicated agents from 1 (definite) to 5 (unlikely) reflecting the likelihood of attribution for liver injury. Cases are graded as definite (> 95% likelihood), highly likely (75–95%), probable (50–74%), possible (25–49%), or unlikely (< 25%) DILI. In cases where more than one agent is implicated, each medication or product is scored separately for the likelihood that it was responsible for injury [6].
As stated previously, given the relatively few dronedarone hepatoxicity cases within the DILIN and the paucity of literature on the topic, we supplemented our dataset with dronedarone hepatotoxicity cases from the FDA. The FAERS database contains adverse event reports, medication error reports, and product quality complaints resulting in adverse events that were voluntarily submitted to the FDA [7]. This system was implemented to maintain post-marketing safety surveillance for drugs and therapeutic biologic products. Healthcare professionals, consumers, and manufacturers submit reports to FAERS. FAERS data has limitations. First, there is no certainty that the reported liver injury associated with dronaderone was due to the drug. FDA does not require that a causal relationship between a product and event be proven, and reports do not always contain enough detail to properly evaluate an adverse event. Furthermore, FDA does not receive reports for every adverse event or medication error that occurs with a product. Nonetheless, some reports of dronedarone injury contained detailed laboratory data and liver biopsy findings. For the purposes of this paper, the FAERS database was searched for reports received through January 30, 2021, using broad terms for drug-related hepatic disorders with serious outcomes. A total of 299 reports were identified; 9 unique (non-duplicate) cases were selected based upon suspicion of drug associated injury by a trained FDA abstractor and the availability of a liver biopsy description. The biopsy descriptions were reviewed by one author (DK) and summarized for this paper. Although formal causality assessment for the dronedarone FAERS cases could not be done in parallel to the DILIN process due to incomplete data, the FDA cases were assessed for causality using the DILIN expert opinion approach with available information by four authors (AP, VN, DH, MA). Liver biopsy material from the DILIN patients, when available, were reviewed and summarized by DK, using a standard scoring method [8].
Descriptive statistics were computed for demographic and patient characteristics by mean ± standard deviation, median (lowest, highest) for continuous variables, and as frequency (%) for categorical variables.
Results
DILIN Prospective Study Patients
From the DILIN database, amiodarone was implicated in 10 cases while dronedarone was implicated in 3. The cases were enrolled from 8 DILIN clinical sites between 2004 to 2016. Of the cases of DILI due to amiodarone, 6 had an overall causality score of highly likely and 4 of probable. One case of dronedarone toxicity was scored as definite, while 2 were considered highly likely. The clinical characteristics of several of these cases were reported in an earlier publication [18].
The clinical features and demographics of the DILIN cases are summarized in Table 1. The cases of amiodarone toxicity were split evenly between men and women. The median patient age was 70 (range; 59 to 89 years). Average BMI was 26 kg/m2 for both groups with a range of 22 to 33 kg/m2. Comorbidities included diabetes, heart disease, renal disease, and gastrointestinal disease. No patient had a history of viral hepatitis or other liver disease. Two patients reported alcohol use in each group.
The clinical characteristics of amiodarone and dronedarone hepatotoxicity identified by the DILIN are shown in Table 2. Amiodarone toxicity occurred after a longer period of use (latency) than dronedarone (387 vs 119 days, respectively). However, patients with amiodarone toxicity were also more likely to be symptomatic, with nausea and abdominal pain being most frequent.
The biochemical characteristics of liver injury differed between agents as well. The median ALT at DILI onset was higher with dronedarone-associated injury compared to injury due to amiodarone (1191 vs 387 U/L, respectively) while alkaline phosphatase and total bilirubin levels were similar. As a result, the cases involving dronedarone toxicity were characteristically hepatocellular as reflected in the pattern at onset with an R value greater than the amiodarone cases (100% vs 11%, p = 0.055). The R value is used to define the pattern of liver injury ([ALT/ULN] ÷ [Alk P/ULN]); as either hepatocellular as occurs with predominantly cellular injury, (R > 5), cholestatic as occurs with bile duct injury or impairment of bile flow (R value < 2), or mixed (R value 2–5).
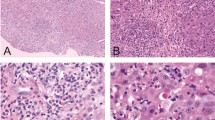
Liver biopsy results were available for 5 of the DILIN patients with amiodarone DILI. Four showed a variety of findings including fibrosis (advanced bridging fibrosis or cirrhosis in 3 cases), steatosis, and numerous Mallory-Denk bodies. In the one case with early fibrosis, the Mallory-Denk bodies were distinctly periportal, as has been previously described in the literature [13]. The fifth case showed a “burned-out” cirrhosis, with only mild inflammation and no steatosis or Mallory-Denk bodies.
In terms of severe liver injury, 9 patients with amiodarone injury were hospitalized compared to 1 from the dronedarone group. Five patients with amiodarone injury died at a median of 32 (range: 9 to 49) days after DILI onset, though only 1 from liver failure. The remaining 4 recovered from their liver injury. One patient with dronedarone induced liver injury was hospitalized and later died of a non-liver related cause at 30 days after DILI onset. This was the only patient in our cohort to be treated with corticosteroids. No patient went on to liver transplantation. The duration of illness did not differ between the groups, with the majority requiring more than 4 weeks for recovery. Chronic liver injury, defined as persistence of liver enzyme elevations for over 6 months from the onset of injury, developed in 1 patient with amiodarone injury.
FDA FAERS Database Patients
The 9 dronedarone cases were reported to the FDA between 2010 and 2012. Given a difference in reporting standards (passive FDA reports compared with prospective collected information in the DILIN), causality scores determined by four authors (AP, VN, DH, MA) was used to reflect the causal association between the reported injury and dronedarone: 3 cases were assessed as probable, and 6 as possible. As regards the possible cases; gallbladder disease, cholangitis, and alternative drugs were competing etiologies that reduced confidence in attributing the injury to dronedarone. By DILIN standards, cases scored as possible typically are not considered in analyses; however, due to the nature of the FAERS reports, with inconsistencies in reporting and incompleteness of data, we elected to include all cases in our analysis.
Table 3 displays the clinical features and demographic characteristics of the FAERS dronedarone cases. The median age was 69 (range: 54 to 89 years);7 (77%) were female. Race and BMI were not included in most of the reports. All patients took dronedarone for atrial fibrillation. Eight patients had been placed on a 400mg twice daily (BID) regimen while one took 200mg BID. Comorbidities included diabetes, cardiovascular disease, heart disease, pulmonary disease, and malignancy. No patient had a history of viral hepatitis. Two patients were anti-nuclear antibody positive and 1 was positive for an HFE gene mutation. Alcohol use and drug allergies were not reported. One patient was reported to have taken 6 tablets of undefined dosage of acetaminophen a day prior to liver injury. The biochemical characteristics of liver injury varied among the reported cases. The R value was available for 7 cases: 5 cases were hepatocellular, 1 was cholestatic, and 1 was mixed.
The clinical characteristics of suspected dronedarone hepatotoxicity are also shown in Table 3. Three patients developed liver injury within 1 month of starting the medication. Seven patients presented with jaundice. Nausea and abdominal pain were also commonly reported symptoms.
Seven reported cases of patients with dronedarone liver injury had documented hospitalizations. Only one was treated with steroids. Two developed acute liver failure and underwent urgent liver transplantation, and a third developed severe hepatocellular injury, was evaluated for liver transplantation, but spontaneously recovered. All other patients recovered from their liver injury except for 1 patient with cirrhosis who died at 30 days after DILI onset of non-liver related causes. The duration of symptoms was reported in 2 cases with improvement noted to have occurred in 4–7 weeks after drug cessation.
Based on the descriptions of liver biopsy findings in the FDA reports, dronedarone was not associated with a single pattern of injury. Three cases showed cholestatic hepatitis with moderate to severe hepatitis. The 2 patients who underwent transplant showed massive necrosis, likely related to a fulminant hepatitis. The remaining 4 cases showed mild hepatitis, with advanced fibrosis in 2 of those cases. None of the biopsies were reported to show a significant degree of fat/steatosis.
Discussion
Amiodarone is an important medication in the management of life-threatening arrhythmias, with many patients requiring it on a long-term basis. Cumulative experience from both Europe and North America indicates that mild abnormalities of liver function tests are seen in 15–55% of patients treated with amiodarone that frequently are non-progressive and resolve with continued drug administration [9]. Dronedarone, developed to mitigate the end-organ adverse effects of amiodarone, is less frequently used but reports of attributable liver injury do exist in the literature [13] Although approved for use in 2009, the drug is infrequently used now likely due to the non-hepatic adverse events seen in the PALLAS trial leading to early study discontinuation [10], as well as liver injury seen in post-marketing surveillance. Interestingly, all dronedarone FAERS cases were reported between 2010 and 2012. The basis for this apparent clustering of cases remains unclear. Although not substantiated, this could have been in part the result of more robust spontaneous reporting of serious dronedarone-associated liver injury events by practitioners in the early post-drug approval period. A critical observation that arises from our study is that the liver injury presentation due to amiodarone and dronedarone, termed the signature of hepatotoxicity, are remarkably different.
Adjudication of liver injury signals in patients receiving anti-arrhythmics as in all other causes of DILI requires the clinician to exclude other etiologies of liver injury. For example, a patient with severe atrial fibrillation with hypotension or symptoms and is hospitalized, ischemic hepatitis as well as passive congestion must be considered in the differential diagnosis. Furthermore, the possibility of cholestasis of sepsis or due to obstructive biliary tract disease often arises. Finally, many individuals on class III anti-arrhythmics have other co-morbidities and may be receiving other hepatotoxic drugs. The challenge in attribution of injury to a drug is reflected by our data wherein only 3 of the FAERS cases were considered probable with the other cases considered only possible. The liver histology in amiodarone hepatotoxicity has previously been described [14]. It is also like that seen in patients with diabetes mellitus and alcohol use, who frequently have simple steatosis or steatohepatitis, further confounding attribution given the frequency of this underlying condition. The presence of Mallory Denk bodies is also not specific to amiodarone toxicity and in fact is most commonly attributed to chronic alcohol consumption and/ or fatty liver.
The current study provides clinicians with a clearer picture of the presentation, course, and consequences of liver injury due to amiodarone and dronedarone. Liver injury caused by these medications can be severe and life threatening. However, as stated, the hepatotoxicity signature differs remarkably between the two drugs; amiodarone causes direct hepatotoxicity that resembles that seen in alcohol related liver injury, with fatty infiltration and inflammation, usually after long-term administration. Dronedarone, on the other hand is less predictable in presentation and onset, typically without fat and with a shorter latency of use before presentation with liver disease. Amiodarone, a long-recognized cause of serum ALT elevations on long-term therapy is more likely than dronedarone to cause a protracted liver injury that is associated with a longer latency period of use prior to the onset of injury. It has also been reported to cause an acute injury, soon after intravenous administration presumably due to the preservative/ solvent [11]. Interestingly, the majority of our cases did not receive high daily doses of amiodarone for prolonged periods of time with 60% receiving only 200 mg dose per day (Table 1). Dronedarone tends to cause injury after shorter periods of use but with no less severe outcomes. The need for hospitalization for liver injury associated with both drugs is common and injury may even lead to liver transplantation or death. Only amiodarone use was linked to chronic liver injury.
Some of the differences in liver injury presentation may be attributable to the drugs’ respective pharmacokinetic characteristics. Amiodarone has a large volume of distribution, and it is highly protein bound, accumulating in many organs leading to its prolonged half-life. It is metabolized by CYP 3A4 and CYP2C8 and has a long plasma terminal elimination half-life of 58 days. In addition, subjects over 65 years clear amiodarone more slowly than younger patients. As noted in the amiodarone drug label, hepatic failure has been a rare cause of death. Because amiodarone is an inhibitor of CYP3A4, there is also the potential for several drug interactions.
The hepatotoxicity of dronedarone has been linked to severe injury after up to 11 months of treatment in post-marketing experience. The clinical features of attributable hepatotoxicity have not been well defined. Although structurally similar, dronedarone includes chemical modifications that shorten the elimination half-life without significantly impacting its antiarrhythmic profile; these structural changes include deletion of the iodine component in the benzene ring as previously referenced, as well as addition of a methane sulfonamide group and replacement of ethyl groups by butyls. Dronedarone is extensively metabolized in the liver by CYP3A and is also an inhibitor of this enzyme, raising the potential for drug interactions. The drug has low bioavailability if taken without food and has a much shorter elimination half-life than amiodarone, 13–19 h. Like amiodarone, the drug and its metabolite are highly protein bound with a large volume of distribution. Females and patients over 65 also have higher blood concentration.
The mechanisms of hepatotoxicity of these two medications likely involve mitochondrial injury. Amiodarone toxicity is more direct in that it increases mitochondrial synthesis of reactive oxygen species (ROS) and uncouples oxidative phosphorylation by impairing mitochondrial bioenergetics [12]. While dronedarone shares a similar capacity to induce uncoupling of oxidative phosphorylation, it does so with less predictability, and thus is termed idiosyncratic. Dronedarone did not show any of the oxidative effects and did not impair mitochondrial function in mouse models [13]. Nonetheless, as both drugs inhibit mitochondrial function, accumulation of ROS and fatty acids plausibly explains the development of apoptosis and/or necrosis of hepatocytes [10, 14]. The differences in pharmacokinetics, predominantly the longer half-life associated with a large volume of distribution and associated tissue accumulation of amiodarone and the advanced age and predominantly female gender of the dronedarone patients, may explain some of the differences in presentation of liver injury between the two drugs.
In summary, our review confirms the well documented observation that amiodarone causes direct toxicity that can present either as acute or chronic liver injury [4, 9]. Dronedarone toxicity, in contrast occurs less predictably and after a shorter exposure period with chronic injury not being observed. Dronedarone injury is predominantly hepatocellular, in contrast to amiodarone, which may be appear as either a cholestatic or a mixed pattern. Both medications can present as a clinical spectrum ranging from isolated, asymptomatic, transaminase elevation to fulminant and fatal liver injury. Early recognition and monitoring of liver enzymes have been recommended in society guidelines, by specialist clinicians, and in the amiodarone (but not the dronedarone) package insert [11, 15,16,17]. Measuring liver tests prior to initiating either medication, particularly in patients who have risk factor for liver disease such as underlying metabolic syndrome, repeating liver tests every six months thereafter, and with the report of any symptoms that could suggest liver injury, however protean, is a reasonable approach. Further, a heightened awareness by health care providers of the potential for dronedarone-associated hepatotoxicity and the importance of testing liver enzymes when clinically indicated, seems prudent, even if the drug is used infrequently.
Key Messages
-
1.
Amiodarone and Dronedarone can cause liver injury.
-
2.
Amiodarone liver injury resembles that which occurs as the result of alcohol.
-
3.
Dronedarone injury occurs more quickly and may be more serious.
Abbreviations
- FAERS:
-
FDA Adverse Event Reporting System
- DILIN:
-
Drug Induced Liver Injury Network
- DILI:
-
Drug Induced Liver Injury
- FDA:
-
Food and Drug Administration
- AST:
-
Aspartate aminotransferase (AST)
- ALT:
-
Alanine aminotransferase
- ALP:
-
Alkaline phosphatase
References
Kane SP. About the ClinCalc DrugStats Database, Version 2022.08. ClinCalc: https://clincalc.com/DrugStats/About.aspx. Updated August 24, 2022. Accessed February 19, 2023.
World Health Organization Model List of Essential Medicines, 21st List, 2019. Geneva: World Health Organization; 2019. Licence: CCBY-NC-SA3.0IGO.
Flaharty KK, Chase SL, Yaghsezian HM, Rubin R. Hepatotoxicity associated with amiodarone therapy. Pharmacotherapy. 1989;9:39–44.
Jahn S, Zollner G, Lackner C, Stauber RE. Severe toxic hepatitis associated with dronedarone. Curr Drug Saf. 2013;8:201–202.
FDA Drug Safety Communication: Severe liver injury associated with the use of dronedarone (marketed as Multaq), FDA, 4 Aug. 2017, www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-severe-liver-injury-associated-use-dronedarone-marketed-multaq. Accessed November 26, 2023.
Fontana RJ, Watkins PB, Bonkovsky HL et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68.
FDA, 4 June 2018, www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers. Accessed November 26, 2023.
Kleiner DE. The pathology of drug induced liver injury. Semin Liver Dis 2009;29:364–372.
Rigas B. The evolving spectrum of amiodarone hepatotoxicity. Hepatology. 1989Jul;10:116–117.
Connolly S, Camm A, Halperin J, Joyner C, Alings M, Amerena J et al. Dronedarone in High-Risk Permanent Atrial Fibrillation. N Engl J Med 2011;365:2268–2276.
Epstein A.E., Olshansky B., Naccarelli G.V., et. al.: Practical management guide for clinicians who treat patients with amiodarone. Am J Med 2016; 129: pp. 468–475.
Serviddio G, Bellanti F, Giudetti AM et al. Mitochondrial oxidative stress and respiratory chain dysfunction account for liver toxicity during amiodarone but not dronedarone administration. Free Radic Biol Med. 2011;51:2234–2242.
Felser A, Blum K, Lindinger PW, Bouitbir J, Krähenbühl S. Mechanisms of hepatocellular toxicity associated with dronedarone–a comparison to amiodarone. Toxicol Sci. 2013;131:480–490.
Lewis JH, Mullick F, Ishak KG, Ranard RC, Ragsdale B, Perse RM, Rusnock EJ et al. Histopathologic analysis of suspected amiodarone hepatotoxicity. Hum Pathol 1990;21:59–67.
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society [Review]. J Am Coll Cardiol. 2018;72:e91–e220.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/018972s038s039lbl.pdf. Accessed November 26, 2023.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022425lbl.pdf. Accessed November 26, 2023.
Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, Watkins PB, Navarro V, Barnhart H, Gu J. Serrano, J for the United States Drug Induced Liver Injury Network. Gastroenterology 2015;148:1340.
FDA warning on dronedarone (Multaq). Med Lett Drugs Ther 2011; 53 (1359).
Acknowledgments
The authors wish to acknowledge Dr. Heather Le, Pharm D, Safety Evaluator, Office of Pharmacovigilance and Epidemiology, Center for Drug Evaluation and Research, Food and Drug Administration for assistance in obtaining Dronedarone liver injury cases reported to the FDA Adverse Reporting System (FAERS); and Dr. Sumeet Mainigi, Chair for the Division of Cardiovascular Medicine at Einstein Medical Center, Philadelphia, for his input on the clinical relevance of the content of this paper.
Funding
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health under award numbers U01DK065211 (Indiana University), U01DK065184 (University of Michigan), U01DK065201 (University of North Carolina-Chapel Hill), U01DK083020 (University of Southern California), U01DK083027 (Thomas Jefferson University/Albert Einstein Medical Center), U01DK100928 (Icahn School of Medicine at Mount Sinai), and U24DK065176 (Duke University). Additional support is provided by the intramural programs of the NIDDK and National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Pop, Alexander- No conflicts with the content of this paper. Halegoua-DeMarzio, Dina—No conflicts with the content of this paper. Barnhart, Huiman—No conflicts with the content of this paper. Kleiner, David—No conflicts with the content of this paper. Avigan, Mark I – No conflicts with the content of the paper. Chalasani, Naga – No conflicts with the content of this paper. For full disclosure, Dr Chalasani has paid consulting agreements with Madrigal, Zydus, Altimmune, Foresite, GSK and Merck. He has research support from DSM and Exact Sciences. Ahmad, Jawad. – No conflicts with the content of this paper. Fontana, Robert J.—No conflicts with the content of this paper. Gu, Jiezhun.—No conflicts with the content of this paper. Lee, William – Receives research funding from Intercept, Aurora, Gilead, NovoNordisk, Alexion, Camurus, Lipocine and Eiger; consultant for Glaxo Smith Klein, Veristat, SeaGen, Cortexyme, Seal Rock, and Forma. Barritt, A. Sidney—No conflicts with the content of this paper. Durazo, Francisco—No conflicts with the content of this paper. Hayashi, Paul H -No conflicts with the content of this paper. Navarro, Victor J – No conflicts with the content of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer: The content and opinions expressed in this article are the responsibility of the authors and do not necessarily reflect the position of the US Food and Drug Administration, the National Institutes of Health, US Department of Health and Human Services, or the US Government.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pop, A., Halegoua-DeMarzio, D., Barnhart, H. et al. Amiodarone and Dronedarone Causes Liver Injury with Distinctly Different Clinical Presentations. Dig Dis Sci 69, 1479–1487 (2024). https://doi.org/10.1007/s10620-023-08251-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08251-2