Abstract
Background
Intestinal mucosal barrier dysfunction plays a crucial role in the pathogenesis of irritable bowel syndrome with diarrhea (IBS-D). In order to explore the mechanism of electroacupuncture (EA) treatment on intestinal mucosal barrier, this study observed the effect of EA on aquaporins (AQPs), tight junctions (TJs), NF-κB pathway and the gut microbiota in IBS-D rats.
Methods
The IBS-D model was established by acetic acid enema combined with chronic restraint method. The effects of EA on the treatment of IBS-D were examined by the abdominal withdrawal reflex score, Bristol's fecal character score, fecal water content, small intestine propulsion rate and HE staining. AQPs, TJs and inflammation-related molecular mechanisms were explored. The fecal samples were applied for 16S rRNA sequencing to assess the effect of EA intervention to the intestinal bacterial abundance.
Results
EA reduced intestinal sensitization, restored intestinal motility and improved inflammatory cell infiltration. Furthermore, EA improved intestinal inflammation and flora environment significantly, inhibited NF-κB signaling and inflammatory factors (IL-1β and TNF-α). It can also increase the gene and protein expression of AQPs (AQP1, AQP3, and AQP8) and the gene levels of TJs (ZO-1 and Occludin).
Conclusion
EA has an inhibitory effect on the NF-κB signaling pathway, and regulates the proteins of AQP1, AQP3, AQP8, and TJs to restore the balance of water metabolism and intestinal permeability in IBS-D, which also restored the function of the intestinal mucosa by regulating the intestinal flora.
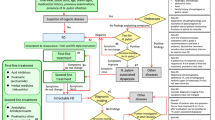
Graphical Abstract
Irritable bowel syndrome with predominant diarrhea (IBS-D) is a common gastrointestinal disease in clinical practice, which was affected by intestinal mucosal barrier dysfunction, intestinal mucosal inflammation production and abnormal intestinal flora, then there is currently no specific drug for treating IBS-D. Electroacupuncture (EA), as a non-pharmacological therapy, has good therapeutic effects in treating IBS-D. Aquaporins (AQPs) are distributed in the intestinal mucosa of the intestine and are important factors in mediating water–liquid transmembrane transport. Changes in AQPs expression have been identified as a common factor in the etiology of certain gastrointestinal diseases. AQP1, AQP3, and AQP8 are distributed in the distal colon. EA can inhibit NF-κB signaling pathway, and regulate the proteins of AQP1, AQP3, AQP8, and TJs to restore the balance of water metabolism and intestinal permeability in IBS-D, which also can restore the function of the intestinal mucosa by regulating the intestinal flora.









Similar content being viewed by others
References
Aziz I, Simrén M. The overlap between irritable bowel syndrome and organic gastrointestinal diseases. Lancet Gastroenterol Hepatol 2021;6:139–148.
Ghoshal UC. Pros and cons while looking through an Asian window on the Rome IV criteria for irritable bowel syndrome: Pros. J Neurogastroenterol Motil 2017;23:334–340.
Oka P, Parr H, Barberio B et al. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020;5:908–917.
Trinkley KE, Nahata MC. Medication management of irritable bowel syndrome. Digestion 2014;89:253–267.
Canavan C, West J, Card T. Calculating total health service utilisation and costs from routinely collected electronic health records using the example of patients with irritable bowel syndrome before and after their first gastroenterology appointment. Pharmacoeconomics 2016;34:181–194.
Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012;303:G775–G785.
Laforenza U. Water channel proteins in the gastrointestinal tract. Mol Aspects Med 2012;33:642–650.
Kalita A, Das M, Baro MR, Das B. Exploring the role of Aquaporins (AQPs) in LPS induced systemic inflammation and the ameliorative effect of Garcinia in male Wistar rat. Inflammopharmacology 2021;29:801–823.
Zhao GX, Dong PP, Peng R et al. Expression, localization and possible functions of aquaporins 3 and 8 in rat digestive system. Biotech Histochem 2016;91:269–276.
Singh SK, Binder HJ, Boron WF, Geibel JP. Fluid absorption in isolated perfused colonic crypts. J Clin Investig 1995;96:2373–2379.
Yano T, Kanoh H, Tamura A, Tsukita S. Apical cytoskeletons and junctional complexes as a combined system in epithelial cell sheets. Ann NY Acad Sci 2017;1405:32–43.
Vermette D, Hu P, Canarie MF, Funaro M, Glover J, Pierce RW. Tight junction structure, function, and assessment in the critically ill: a systematic review. Intensive Care Med Exp 2018;6:37.
Fischbarg J. Fluid transport across leaky epithelia: central role of the tight junction and supporting role of aquaporins. Physiol Rev 2010;90:1271–1290.
Yuan Y, Wang X, Huang S, Wang H, Shen G. Low-level inflammation, immunity, and brain–gut axis in IBS: unraveling the complex relationships. Gut Microbes 2023;15:2263209.
Vivinus-Nébot M, Frin-Mathy G, Bzioueche H et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut 2014;63:744–752.
Chao G, Zhang S. Aquaporins 1, 3 and 8 expression in irritable bowel syndrome rats’ colon via NF-κB pathway. Oncotarget 2017;8:47175–47183.
Rabitti S, Giovanardi CM, Colussi D. Acupuncture and related therapies for the treatment of gastrointestinal diseases. J Clin Gastroenterol 2021;55:207–217.
Zhu X, Liu Z, Qin Y et al. Analgesic effects of electroacupuncture at ST25 and CV12 in a rat model of postinflammatory irritable bowel syndrome visceral pain. Acupunct Med 2018;36:240–246.
Li J, Lu J, Sun J et al. Acupuncture with regulating mind and spleen for diarrhea irritable bowel syndrome and sleep quality: a randomized controlled trial. Zhongguo Zhen Jiu 2017;37:9–13.
Hou YJ, Wang K, Jiang HL et al. Study on the mechanism of electroacupuncture repairing intestinal barrier via regulating mast cell in rats with diarrhea-predominant irritable bowel syndrome. Zhen Ci Yan Jiu 2023;48:281–286.
Mengzhu S, Yujie Z, Yafang S et al. Electroacupuncture alleviates water avoidance stress-induced irritable bowel syndrome in mice by improving intestinal barrier functions and suppressing the expression of inflammatory cytokines. J Tradit Chin Med 2023;43:494–500.
Chao G, Wang Z, Yang Y, Zhang S. LncRNA H19 as a competing endogenous RNA to regulate AQP expression in the intestinal barrier of IBS-D patients. Front Physiol 2021;11:602076.
Williams CL, Villar RG, Peterson JM, Burks TF. Stress-induced changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology 1988;94:611–621.
La JH, Kim TW, Sung TS et al. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol 2003;9:2791–2795.
Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–924.
Camilleri M, Ford AC. Irritable bowel syndrome: pathophysiology and current therapeutic approaches. Handb Exp Pharmacol 2017;239:75–113.
Wu IXY, Wong CHL, Ho RST et al. Acupuncture and related therapies for treating irritable bowel syndrome: overview of systematic reviews and network meta-analysis. Ther Adv Gastroenterol 2019;12:1756284818820438.
Huang J, Lu M, Zheng Y et al. Quality of evidence supporting the role of acupuncture for the treatment of irritable bowel syndrome. Pain Res Manag 2021;2021:2752246.
Sun JW, Sun ML, Li D et al. Efficacy of acupuncture based on acupoint combination theory for irritable bowel syndrome: a study protocol for a multicenter randomized controlled trial. Trials 2021;22:719.
Zhou ZX, Ma HF, Yang YC, Chen JT, Feng YH. Effect of electroacupuncture on colonic motility and RhoA and ROCK protein expression in IBS-D rats. Zhen Ci Yan Jiu 2022;47:611–616.
Li X, Ren K, Hong X et al. Ameliorating effects of electroacupuncture on the low-grade intestinal inflammation in rat model of diarrhea-predominant irritable bowel syndrome. J Gastroenterol Hepatol 2022;37:1963–1974.
Su Q, Tun HM, Liu Q et al. Gut microbiome signatures reflect different subtypes of irritable bowel syndrome. Gut Microbes 2023;15:2157697.
Sun MZ, Zhang YJ, Song YF et al. Electroacupuncture at Tianshu (ST25) and Zusanli (ST36) alleviates stress-induced irritable bowel syndrome in mice by modulating gut microbiota and corticotropin-releasing factor. J Tradit Chin Med 2022;42:732–740.
Piche T. Tight junctions and IBS—the link between epithelial permeability, low-grade inflammation, and symptom generation? Neurogastroenterol Motil 2014;26:296–302.
Ivashkin V, Poluektov Y, Kogan E et al. Disruption of the pro-inflammatory, anti-inflammatory cytokines and tight junction proteins expression, associated with changes of the composition of the gut microbiota in patients with irritable bowel syndrome. PLoS ONE 2021;16:e0252930.
Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol 2018;16:457–470.
Salman MM, Kitchen P, Yool AJ, Bill RM. Recent breakthroughs and future directions in drugging aquaporins. Trends Pharmacol Sci 2022;43:30–42.
Wagner K, Unger L, Salman MM et al. Signaling mechanisms and pharmacological modulators governing diverse aquaporin functions in human health and disease. Int J Mol Sci 2022;23:1388.
Camilleri M, Carlson P, Chedid V et al. Aquaporin expression in colonic mucosal biopsies from irritable bowel syndrome with diarrhea. Clin Transl Gastroenterol 2019;10:e00019.
Wang JP, Hou XH. Expression of aquaporin 8 in colonic epithelium with diarrhoea-predominant irritable bowel syndrome. Chin Med J 2007;120:313–316.
Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol 2018;10:a029314.
Wang HY, Chi C, Xu YQ et al. Occludin endocytosis is involved in the disruption of the intestinal epithelial barrier in a mouse model of alcoholic steatohepatitis. J Dig Dis 2019;20:476–485.
Zhang W, Xu Y, Chen Z, Xu Z, Xu H. Knockdown of aquaporin 3 is involved in intestinal barrier integrity impairment. FEBS Lett 2011;585:3113–3119.
Dong LW, Chen YY, Chen CC et al. Adenosine 2A receptor contributes to the facilitation of post-infectious irritable bowel syndrome by γδ T cells via the PKA/CREB/NF-κB signaling pathway. World J Gastroenterol 2023;29:1475–1491.
Wang L, Lei J, Zhao Z, Jia J, Wang L. Therapeutic effects of paeoniflorin on irritable bowel syndrome in rats. J Vet Sci 2023;24:e23.
Zou L, Ruan JR, Chen JY et al. Moxibustion relieves colonic inflammation by up-regulating expression of miR-345-3p/miR-216a-5p and down-regulating NF-κB p65 in colonic tissue of rats with diarrhea-predominant irritable bowel syndrome. Zhen Ci Yan Jiu 2023;48:226–232.
Acknowledgments
This research was supported by New Teacher Initiation Fund Project of Beijing University of Chinese Medicine (No. 2020-JYB-XJSJJ-016).
Author information
Authors and Affiliations
Contributions
Yemao Chai, Xiaoxuan Ren and Honglin Zhang conceived and designed the study. Xueling Kang, Xiaying Li and Zhansheng Huang performed the experiments; Kai Zhang and Yuanyuan Li performed the statistical analysis of the data; Yemao Chai and Xueling Kang wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Disclosure of financial arrangements
Not applicable.
Ethical approval
The animal study was reviewed and approved by Animal Ethics Committee, Beijing University of Chinese Medicine, China (Permission number BUCM-1–2020100701-0013).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, X., Zhang, H., Li, X. et al. Electroacupuncture Improving Intestinal Barrier Function in Rats with Irritable Bowel Syndrome Through Regulating Aquaporins. Dig Dis Sci 69, 1143–1155 (2024). https://doi.org/10.1007/s10620-024-08288-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-024-08288-x