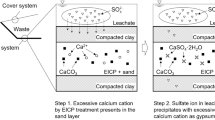
Abstract
Red gypsum (RG) is an industrial waste generated from the titanium dioxide process produced. For the low-cost and high-benefit utilization of RG, calcium sulfate (CaS) was prepared from recycled RG. Rod-like gypsum crystals with a rough surface, uniform size, 1 µm in diameter and 5 µm in length, were obtained, when the as-prepared CaS was divided into three equal portions and one portion was added at each interval of 2 h to neutralize the simulated acidic wastewater. CaS was prepared from RG to neutralize acidic wastewater to produce silicon-enriched RG for the next cycle. High silica residue (HSR) was obtained after 6 cycles. The effect of calcined temperature on the desulfurization of HSR was investigated. The sulfur content in the residue decreased by 97% after calcined at 1200 ℃ for 2 h. According to GB/T 12957-2005 “Industrial waste residue activity test method for cement admixture”, the activity index of desulfurized HSR was 90%, which it can be used as a cementing material.









Similar content being viewed by others
References
Rosli NA, Aziz HA, Selamat MR et al (2020) A mixture of sewage sludge and red gypsum as an alternative material for temporary landfill cover. J Environ Manag 263:110420. https://doi.org/10.1016/j.jenvman.2020.110420
Wu H, Fen Y, Li H et al (2019) Red gypsum utilization and acidic wastewater treatment based on metal self-enrichment process. Sci Total Environ 691:9–15. https://doi.org/10.1016/j.scitotenv.2019.07.080
Chen QJ, Ding WJ, Sun H et al (2020) Synthesis of anhydrite from red gypsum and acidic wastewater treatment. J Clean Prod 278:24026. https://doi.org/10.1016/j.jclepro.2020.124026
Perez-Moreno SM, Gazquez MJ, Barneto AG et al (2013) Thermal characterization of new fre-insulating materials from industrial inorganic TiO2 wastes. Thermochim Acta 552:114–122. https://doi.org/10.1016/j.tca.2012.10.021
Gázquez MJ, Bolívar JP, García-Tenorio R et al (2009) Physicochemical characterization of raw materials and co-products from the titanium dioxide industry. J Hazard Mater 166:1429–1440. https://doi.org/10.1016/j.jhazmat.2008.12.067
Zhang J, Yan Y, Hu ZH et al (2019) Properties and hydration behavior of Ti-extracted residues-red gypsum based cementitious materials. Constr Build Mater 218:610–617. https://doi.org/10.1016/j.conbuildmat.2019.05.099
Gázquez MJ, Bolívar JP, Vaca F et al (2013) Evaluation of the use of TiO2 industry red gypsum waste in cement production. Cem Concr Compos 37:76–81. https://doi.org/10.1016/j.cemconcomp.2012.12.003
Zhang JF, Yan Y, Hu ZH (2018) Preparation and characterization of foamed concrete with Ti-extracted residues and red gypsum. Constr Build Mater 171:109–119. https://doi.org/10.1016/j.conbuildmat.2018.03.072
Zhang YH, Wang F, Huang HW et al (2016) Gypsum blocks produced from TiO2 production by-products. Environ Technol 37(9):1094–1100. https://doi.org/10.1080/09593330.2015.1102329
Fauziah I, Zauyah S, Jamal S (1996) Characterization and land application of red gypsum: a waste product from the titanium dioxide industry. Sci Total Environ 188:243–251. https://doi.org/10.1016/0048-9697(96)05179-0
Rodriguez-Jorda MP, Garrido F, Garcia-Gonzalez MT (2010) Potential use of gypsum and lime rich industrial by-products for induced reduction of Pb, Zn and Ni leachability in an acid soil. J Hazard Mater 175:762–769. https://doi.org/10.1016/j.jhazmat.2009.10.074
Azdarpour A, Karaei MA, Hamidi H et al (2017) CO2 sequestration using red gypsum via pH-swing process: effect of carbonation temperature and NH4HCO3 on the process efficiency. Int J Miner Process 169:27–34. https://doi.org/10.1016/j.minpro.2017.09.014
Rahmani O (2018) Siderite precipitation using by-product red gypsum for CO2 sequestration. J CO2 Util 24:321–327. https://doi.org/10.1016/j.jcou.2018.01.020
Mittal A, Rakshit D (2020) Utilization of cement rotary kiln waste heat for calcination of phosphogypsum. Therm Sci Eng Prog 20:100729. https://doi.org/10.1016/j.tsep.2020.100729
Zheng DL, Ma LP, Wang RM et al (2018) Research on thermal decomposing properties of phosphogypsum with Fe addition under multi-atmosphere control. Thermochim Acta 661:59–66. https://doi.org/10.1016/j.tca.2018.01.011
Ma XL, Tan HB, Su XM et al (2022) Recovery elemental sulfur from calcium sulfide prepared by red gypsum in sulfuric acid wastewater treatment. J Mater Cycles Waste Manag 24(4):1542–1550. https://doi.org/10.1007/s10163-022-01419-4
Ma XL, Tan HB, Dong FQ et al (2020) Influence of troilite on the decomposition of ammonium jarosite and estimated activation energy. J Therm Anal Calorim 139:933–939. https://doi.org/10.1007/s10973-019-08480-6
Ma XL, Tan HB, Dong FQ et al (2022) Influence of carbon and pyrite on desulfurization behavior of red gypsum at high temperature. J Sustain Metall 8(1):409–418. https://doi.org/10.1007/s40831-021-00483-8
Kong B, Guan BH, Yates MZ et al (2012) Control of α-calcium sulfate hemihydrate morphology using reverse microemulsions. Langmuir 28(40):14137–14142. https://doi.org/10.1021/la302459z
Petrou AL, Terzidaki A (2014) Calcium carbonate and calcium sulfate precipitation, crystallization and dissolution: evidence for the activated steps and the mechanisms from the enthalpy and entropy of activation values. Chem Geol 381:144–153. https://doi.org/10.1016/j.chemgeo.2014.05.018
Liu ST, Nancollas GH (1973) Linear crystallization and induction-period studies of the growth of calcium sulphate dihydrate crystals. Talanta 20:211–216. https://doi.org/10.1016/0039-9140(73)80268-1
Liu ST, Nancollas GH (1970) The kinetics of crystal growth of calcium sulfate dihydrate. J Cryst Growth 6:281–289. https://doi.org/10.1016/0022-0248(70)90081-3
Touzo B, Scrivener KL, Glasser FP (2013) Phase compositions and equilibria in the CaO–Al2O3–Fe2O3–SO3 system, for assemblages containing ye’elimite and ferrite Ca2(Al, Fe)O5. Cement Concr Res 54:77–86. https://doi.org/10.1016/j.cemconres.2013.08.005
Zhao J, Su H, Zuo HB et al (2020) The mechanism of preparation calcium ferrite from desulfurization gypsum produced in sintering. J Clean Prod 267:959–6526. https://doi.org/10.1016/j.jclepro.2020.122002
Acknowledgements
This work was supported by the Research Fund of the State Key Laboratory of Solid Waste Reuse for Building Materials (SWR-2023-010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Ma, X., Zhong, W. et al. Preparation of calcium sulfate from recycled red gypsum to neutralize acidic wastewater and application of high silica residue. J Mater Cycles Waste Manag 26, 1588–1595 (2024). https://doi.org/10.1007/s10163-024-01914-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-024-01914-w