Abstract
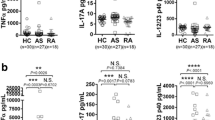
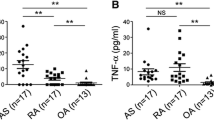
Ankylosing spondylitis (AS) is a chronic inflammatory disease, characterized by excessive new bone formation. We previously reported that the complement factor H-related protein-5 (CFHR5), a member of the human factor H protein family, is significantly elevated in patients with AS compared to other rheumatic diseases. However, the pathophysiological mechanism underlying new bone formation by CFHR5 is not fully understood. In this study, we revealed that CFHR5 and proinflammatory cytokines (TNF, IL-6, IL-17A, and IL-23) were elevated in the AS group compared to the HC group. Correlation analysis revealed that CFHR5 levels were not significantly associated with proinflammatory cytokines, while CFHR5 levels in AS were only positively correlated with the high CRP group. Notably, treatment with soluble CFHR5 has no effect on clinical arthritis scores and thickness at hind paw in curdlan-injected SKG, but significantly increased the ectopic bone formation at the calcaneus and tibia bones of the ankle as revealed by micro-CT image and quantification. Basal CFHR5 expression was upregulated in AS-osteoprogenitors compared to control cells. Also, treatment with CFHR5 remarkedly induced bone mineralization status of AS-osteoprogenitors during osteogenic differentiation accompanied by MMP13 expression. We provide the first evidence demonstrating that CFHR5 can exacerbate the pathological bone formation of AS. Therapeutic modulation of CFHR5 could be promising for future treatment of AS.
Key messages
-
Serum level of CFHR5 is elevated and positively correlated with high CRP group of AS patients.
-
Recombinant CFHR5 protein contributes to pathological bone formation in in vivo model of AS.
-
CFHR5 is highly expressed in AS-osteoprogenitors compared to disease control.
-
Recombinant CFHR5 protein increased bone mineralization accompanied by MMP13 in vitro model of AS.



Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
Abbreviations
- AS:
-
Ankylosing spondylitis
- HC:
-
Healthy controls
- CFHR5:
-
Complement factor H-related protein-5
- H&E:
-
Hematoxylin and eosin
- CRP:
-
C-reactive protein
- SO:
-
Safranin O
- ALP:
-
Alkaline phosphatase
- ARS:
-
Alizarin red S
- VON:
-
Von Kossa
- HA:
-
Hydroxyapatite
- MMP13:
-
Matrix metallopeptidase 13
- OCN:
-
Osteocalcin
References
Benjamin M, Toumi H, Suzuki D, Hayashi K, McGonagle D (2009) Evidence for a distinctive pattern of bone formation in enthesophytes. Ann Rheum Dis 68:1003–1010. https://doi.org/10.1136/ard.2008.091074
Tseng HW, Pitt ME, Glant TT, McRae AF, Kenna TJ, Brown MA, Pettit AR, Thomas GP (2016) Inflammation-driven bone formation in a mouse model of ankylosing spondylitis: sequential not parallel processes. Arthritis Res Ther 18:35. https://doi.org/10.1186/s13075-015-0805-0
Bleil J, Sieper J, Maier R, Schlichting U, Hempfing A, Syrbe U, Appel H (2015) Cartilage in facet joints of patients with ankylosing spondylitis (AS) shows signs of cartilage degeneration rather than chondrocyte hypertrophy: implications for joint remodeling in AS. Arthritis Res Ther 17:170. https://doi.org/10.1186/s13075-015-0675-5
Schett G, Lories RJ, D’Agostino MA, Elewaut D, Kirkham B, Soriano ER, McGonagle D (2017) Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol 13:731–741. https://doi.org/10.1038/nrrheum.2017.188
Ranganathan V, Gracey E, Brown MA, Inman RD, Haroon N (2017) Pathogenesis of ankylosing spondylitis - recent advances and future directions. Nat Rev Rheumatol 13:359–367. https://doi.org/10.1038/nrrheum.2017.56
Danve A, Deodhar A (2022) Treatment of axial spondyloarthritis: an update. Nat Rev Rheumatol 18:205–216. https://doi.org/10.1038/s41584-022-00761-z
Reveille JD (2015) Biomarkers for diagnosis, monitoring of progression, and treatment responses in ankylosing spondylitis and axial spondyloarthritis. Clin Rheumatol 34:1009–1018. https://doi.org/10.1007/s10067-015-2949-3
de Vries MK, van Eijk IC, van der Horst-Bruinsma IE, Peters MJ, Nurmohamed MT, Dijkmans BA, Hazenberg BP, Wolbink GJ (2009) Erythrocyte sedimentation rate, C-reactive protein level, and serum amyloid a protein for patient selection and monitoring of anti-tumor necrosis factor treatment in ankylosing spondylitis. Arthritis Rheum 61:1484–1490. https://doi.org/10.1002/art.24838
Braun J, Baraliakos X, Hermann KG, Xu S, Hsu B (2016) Serum C-reactive protein levels demonstrate predictive value for radiographic and magnetic resonance imaging outcomes in patients with active ankylosing spondylitis treated with golimumab. J Rheumatol 43:1704–1712. https://doi.org/10.3899/jrheum.160003
Inman RD (2021) Axial spondyloarthritis: current advances, future challenges. J Rheum Dis 28:55–59. https://doi.org/10.4078/jrd.2021.28.2.55
Baraliakos X, Gensler LS, D’Angelo S, Iannone F, Favalli EG, de Peyrecave N, Auteri SE, Caporali R (2020) Biologic therapy and spinal radiographic progression in patients with axial spondyloarthritis: a structured literature review. Ther Adv Musculoskelet Dis 12:1759720X20906040. https://doi.org/10.1177/1759720X20906040
Lucientes-Continente L, Marquez-Tirado B, Goicoechea de Jorge E (2023) The factor H protein family: the switchers of the complement alternative pathway. Immunol Rev 313:25–45. https://doi.org/10.1111/imr.13166
Rus H, Cudrici C, Niculescu F (2005) The role of the complement system in innate immunity. Immunol Res 33:103–112. https://doi.org/10.1385/IR:33:2:103
West EE, Woodruff T, Fremeaux-Bacchi V, Kemper C (2023) Complement in human disease: approved and up-and-coming therapeutics. Lancet. https://doi.org/10.1016/S0140-6736(23)01524-6
Brinch L, Vinje O, Teisberg P, Mellbye OJ, Aakesson I (1982) The in-vivo metabolism of C3 in ankylosing spondylitis. Ann Rheum Dis 41:86–89. https://doi.org/10.1136/ard.41.1.86
Kinsella TD, Espinoza L, Vasey FB (1975) Serum complement and immunoglobulin levels in sporadic and familial ankylosing spondylitis. J Rheumatol 2:308–313
Krauledat PB, Krapf FE, Manger B, Kalden JR (1985) Evaluation of plasma C3d and immune complex determinations in the assessment of disease activity of patients with rheumatoid arthritis, systemic lupus erythematosus, and spondylitis ancylopoetica. Rheumatol Int 5:97–101. https://doi.org/10.1007/BF00541327
Li T, Huang Z, Zheng B, Liao Z, Zhao L, Gu J (2010) Serum disease-associated proteins of ankylosing spondylitis: results of a preliminary study by comparative proteomics. Clin Exp Rheumatol 28:201–207
Vinje O, Moller P, Mellbye J (1984) Immunological variables and acute-phase reactants in patients with ankylosing spondylitis (Bechterew’s syndrome) and their relatives. Clin Rheumatol 3:501–513. https://doi.org/10.1007/BF02031273
Modinger Y, Loffler B, Huber-Lang M, Ignatius A (2018) Complement involvement in bone homeostasis and bone disorders. Semin Immunol 37:53–65. https://doi.org/10.1016/j.smim.2018.01.001
Alexander JJ, Sankaran JS, Seldeen KL, Thiyagarajan R, Jacob A, Quigg RJ, Troen BR, Judex S (2018) Absence of complement factor H alters bone architecture and dynamics. Immunobiology 223:761–771. https://doi.org/10.1016/j.imbio.2018.07.023
Liu S, Ji W, Lu J, Tang X, Guo Y, Ji M, Xu T, Gu W, Kong D, Shen Q et al (2020) Discovery of potential serum protein biomarkers in ankylosing spondylitis using tandem mass tag-based quantitative proteomics. J Proteome Res 19:864–872. https://doi.org/10.1021/acs.jproteome.9b00676
Panayi GS, Slaney J, Williams BD (1980) Circulating immune complexes in patients with ankylosing spondylitis. Ann Rheum Dis 39:445–448. https://doi.org/10.1136/ard.39.5.445
Rodahl E, Iversen OJ (1986) Analysis of circulating immune complexes from patients with ankylosing spondylitis by gel electrophoresis and immunoblotting using antiserum against a psoriasis associated retrovirus-like particle. Ann Rheum Dis 45:892–898. https://doi.org/10.1136/ard.45.11.892
Skerka C, Chen Q, Fremeaux-Bacchi V, Roumenina LT (2013) Complement factor H related proteins (CFHRs). Mol Immunol 56:170–180. https://doi.org/10.1016/j.molimm.2013.06.001
Walport MJ (2001) Complement. First of two parts. N Engl J Med 344:1058–1066. https://doi.org/10.1056/NEJM200104053441406
Lee JH, Jung JH, Kim J, Baek WK, Rhee J, Kim TH, Kim SH, Kim KP, Son CN, Kim JS (2020) Proteomic analysis of human synovial fluid reveals potential diagnostic biomarkers for ankylosing spondylitis. Clin Proteomics 17:20. https://doi.org/10.1186/s12014-020-09281-y
Chen Q, Manzke M, Hartmann A, Buttner M, Amann K, Pauly D, Wiesener M, Skerka C, Zipfel PF (2016) Complement factor H-related 5-hybrid proteins anchor properdin and activate complement at self-surfaces. J Am Soc Nephrol 27:1413–1425. https://doi.org/10.1681/ASN.2015020212
McRae JL, Duthy TG, Griggs KM, Ormsby RJ, Cowan PJ, Cromer BA, McKinstry WJ, Parker MW, Murphy BF, Gordon DL (2005) Human factor H-related protein 5 has cofactor activity, inhibits C3 convertase activity, binds heparin and C-reactive protein, and associates with lipoprotein. J Immunol 174:6250–6256. https://doi.org/10.4049/jimmunol.174.10.6250
Papp A, Papp K, Uzonyi B, Cserhalmi M, Csincsi AI, Szabo Z, Banlaki Z, Ermert D, Prohaszka Z, Erdei A et al (2022) Complement factor H-related proteins FHR1 and FHR5 interact with extracellular matrix ligands, reduce factor H regulatory activity and enhance complement activation. Front Immunol 13:845953. https://doi.org/10.3389/fimmu.2022.845953
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27:361–368. https://doi.org/10.1002/art.1780270401
Yoshii T, Sotome S, Torigoe I, Maehara H, Sugata Y, Yamada T, Shinomiya K, Okawa A (2010) Isolation of osteogenic progenitor cells from trabecular bone for bone tissue engineering. Tissue Eng Part A 16:933–942. https://doi.org/10.1089/ten.TEA.2009.0105
Jo S, Won EJ, Kim MJ, Lee YJ, Jin SH, Park PR, Song HC, Kim J, Choi YD, Kim JY et al (2021) STAT3 phosphorylation inhibition for treating inflammation and new bone formation in ankylosing spondylitis. Rheumatology (Oxford) 60:3923–3935. https://doi.org/10.1093/rheumatology/keaa846
Jo S, Wang SE, Lee YL, Kang S, Lee B, Han J, Sung IH, Park YS, Bae SC, Kim TH (2018) IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther 20:115. https://doi.org/10.1186/s13075-018-1582-3
Jo S, Nam B, Lee YL, Park H, Weon S, Choi SH, Park YS, Kim TH (2021) The TNF-NF-kB-DKK1 axis promoted bone formation in the enthesis of ankylosing spondylitis. J Rheum Dis 28:216–224. https://doi.org/10.4078/jrd.2021.28.4.216
Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, Sakihama T, Matsutani T, Negishi I, Nakatsuru S et al (2003) Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426:454–460. https://doi.org/10.1038/nature02119
Ruutu M, Thomas G, Steck R, Degli-Esposti MA, Zinkernagel MS, Alexander K, Velasco J, Strutton G, Tran A, Benham H et al (2012) Beta-glucan triggers spondylarthritis and Crohn’s disease-like ileitis in SKG mice. Arthritis Rheum 64:2211–2222. https://doi.org/10.1002/art.34423
Benham H, Rehaume LM, Hasnain SZ, Velasco J, Baillet AC, Ruutu M, Kikly K, Wang R, Tseng HW, Thomas GP et al (2014) Interleukin-23 mediates the intestinal response to microbial beta-1,3-glucan and the development of spondyloarthritis pathology in SKG mice. Arthritis Rheumatol 66:1755–1767. https://doi.org/10.1002/art.38638
Jo S, Lee SH, Park J, Nam B, Kim H, Youn J, Lee S, Kim TJ, Sung IH, Choi SH et al (2023) Platelet-derived growth factor B is a key element in the pathological bone formation of ankylosing spondylitis. J Bone Miner Res 38:300–312. https://doi.org/10.1002/jbmr.4751
Karpati E, Kremlitzka M, Sandor N, Hajnal D, Schneider AE, Jozsi M (2021) Complement factor H family proteins modulate monocyte and neutrophil granulocyte functions. Front Immunol 12:660852. https://doi.org/10.3389/fimmu.2021.660852
Ferreira VP, Pangburn MK, Cortes C (2010) Complement control protein factor H: the good, the bad, and the inadequate. Mol Immunol 47:2187–2197. https://doi.org/10.1016/j.molimm.2010.05.007
Jo S, Lee JS, Nam B, Lee YL, Kim H, Lee EY, Park YS, Kim TH (2022) SOX9(+) enthesis cells are associated with spinal ankylosis in ankylosing spondylitis. Osteoarthritis Cartilage 30:280–290. https://doi.org/10.1016/j.joca.2021.11.013
Bleil J, Maier R, Hempfing A, Sieper J, Appel H, Syrbe U (2016) Granulation tissue eroding the subchondral bone also promotes new bone formation in ankylosing spondylitis. Arthritis Rheumatol 68:2456–2465. https://doi.org/10.1002/art.39715
Chan AC, Iwashima M, Turck CW, Weiss A (1992) ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell 71:649–662. https://doi.org/10.1016/0092-8674(92)90598-7
Chan AY, Punwani D, Kadlecek TA, Cowan MJ, Olson JL, Mathes EF, Sunderam U, Fu SM, Srinivasan R, Kuriyan J et al (2016) A novel human autoimmune syndrome caused by combined hypomorphic and activating mutations in ZAP-70. J Exp Med 213:155–165. https://doi.org/10.1084/jem.20150888
Negishi I, Motoyama N, Nakayama K, Nakayama K, Senju S, Hatakeyama S, Zhang Q, Chan AC, Loh DY (1995) Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature 376:435–438. https://doi.org/10.1038/376435a0
Nakamura A, Zeng F, Nakamura S, Reid KT, Gracey E, Lim M, Leng L, Jo S, Park YS, Kusuda M et al (2021) Macrophage migration inhibitory factor drives pathology in a mouse model of spondyloarthritis and is associated with human disease. Sci Transl Med 13:eabg1210. https://doi.org/10.1126/scitranslmed.abg1210
Li X, Hao Z, Liu X, Li W (2020) Deficiency of mouse FHR-1 homolog, FHR-E, accelerates sepsis, and acute kidney injury through enhancing the LPS-induced alternative complement pathway. Front Immunol 11:1123. https://doi.org/10.3389/fimmu.2020.01123
Hu X, Liu H, Du J, Chen Y, Yang M, Xie Y, Chen J, Yan S, Ouyang S, Gong Z (2019) The clinical significance of plasma CFHR 1–5 in lupus nephropathy. Immunobiology 224:339–346. https://doi.org/10.1016/j.imbio.2019.03.005
Zhao J, Wu H, Khosravi M, Cui H, Qian X, Kelly JA, Kaufman KM, Langefeld CD, Williams AH, Comeau ME et al (2011) Association of genetic variants in complement factor H and factor H-related genes with systemic lupus erythematosus susceptibility. PLoS Genet 7:e1002079. https://doi.org/10.1371/journal.pgen.1002079
Malik TH, Gitterman DP, Lavin DP, Lomax-Browne HJ, Hiemeyer EC, Moran LB, Boroviak K, Cook HT, Gilmore AC, Mandwie M et al (2021) Gain-of-function factor H-related 5 protein impairs glomerular complement regulation resulting in kidney damage. Proc Natl Acad Sci USA 118. https://doi.org/10.1073/pnas.2022722118
Garam N, Cserhalmi M, Prohaszka Z, Szilagyi A, Veszeli N, Szabo E, Uzonyi B, Ilias A, Aigner C, Schmidt A et al (2021) FHR-5 serum levels and CFHR5 genetic variations in patients with immune complex-mediated membranoproliferative glomerulonephritis and C3-glomerulopathy. Front Immunol 12:720183. https://doi.org/10.3389/fimmu.2021.720183
Abrera-Abeleda MA, Nishimura C, Smith JL, Sethi S, McRae JL, Murphy BF, Silvestri G, Skerka C, Jozsi M, Zipfel PF et al (2006) Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease). J Med Genet 43:582–589. https://doi.org/10.1136/jmg.2005.038315
Besbas N, Gulhan B, Gucer S, Korkmaz E, Ozaltin F (2014) A novel CFHR5 mutation associated with C3 glomerulonephritis in a Turkish girl. J Nephrol 27:457–460. https://doi.org/10.1007/s40620-013-0008-1
Schmitz-Valckenberg S, Fleckenstein M, Zouache MA, Pfau M, Pappas C, Hageman JL, Agron E, Malley C, Keenan TDL, Chew EY et al (2022) Progression of age-related macular degeneration among individuals homozygous for risk alleles on chromosome 1 (CFH-CFHR5) or chromosome 10 (ARMS2/HTRA1) or both. JAMA Ophthalmol 140:252–260. https://doi.org/10.1001/jamaophthalmol.2021.6072
Menotti S, Donini M, Pessolano G, Tiro L, Cantini M, Croce J, Morandi M, Mazzi F, Donadello K, Olivieri O et al (2021) Atypical hemolytic uremic syndrome: unique clinical presentation linked to rare CFHR5 mutation. EJHaem 2:838–841. https://doi.org/10.1002/jha2.288
Ricklin D, Lambris JD (2007) Complement-targeted therapeutics. Nat Biotechnol 25:1265–1275. https://doi.org/10.1038/nbt1342
Hwang M, Assassi S, Zheng J, Castillo J, Chavez R, Vanarsa K, Mohan C, Reveille J (2023) Quantitative proteomic screening uncovers candidate diagnostic and monitoring serum biomarkers of ankylosing spondylitis. Arthritis Res Ther 25:57. https://doi.org/10.1186/s13075-023-03044-4
Holers VM, La Rosa FG, Banda NK (2021) A potential new mouse model of axial spondyloarthritis involving the complement system. Immune Netw 21:e45. https://doi.org/10.4110/in.2021.21.e45
Yang C, Ding P, Wang Q, Zhang L, Zhang X, Zhao J, Xu E, Wang N, Chen J, Yang G et al (2016) Inhibition of complement retards ankylosing spondylitis progression. Sci Rep 6:34643. https://doi.org/10.1038/srep34643
Poddubnyy D, Haibel H, Listing J, Marker-Hermann E, Zeidler H, Braun J, Sieper J, Rudwaleit M (2012) Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum 64:1388–1398. https://doi.org/10.1002/art.33465
Iglesias MJ, Sanchez-Rivera L, Ibrahim-Kosta M, Naudin C, Munsch G, Goumidi L, Farm M, Smith PM, Thibord F, Kral-Pointner JB et al (2023) Elevated plasma complement factor H related 5 protein is associated with venous thromboembolism. Nat Commun 14:3280. https://doi.org/10.1038/s41467-023-38383-y
Tam LS, Gu J, Yu D (2010) Pathogenesis of ankylosing spondylitis. Nat Rev Rheumatol 6:399–405. https://doi.org/10.1038/nrrheum.2010.79
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (Grant number 2021R1F1A1060970 to Chang-Nam Son, 2019R1A2C2004214 and 2021R1A6A1A03038899 to Tae-Hwan Kim, and 2020R1A2C1102386 to Sungsin Jo), the Korea Healthy Industry Development Institute (Grant number HI23C0661 to Tae-Hwan Kim), and the Medi-Start Up Program through Daegu-Gyeongbuk Medical Innovation Foundation funded by Daegu Metropolitan (Grant number B-A-H-21–02 to Chang-Nam Son).
Author information
Authors and Affiliations
Contributions
S Jo, J-H Lee, J-S Kim, T-H Kim, and C-N Son contributed to the study design and conception. S Jo, J-H Lee, SH Lee, and C Jeon performed and assisted with experiments. S–H Kim, Y-S Park, T-J Kim, T-H Kim, and C-N Son provided serum, PBMC, and surgical samples from patients with AS. J Youn provided SKG mice. J Han performed statistical analysis. S Jo and C-N Son drafted the manuscript. S Jo, J-H Lee, SH Lee, T-H Kim, and C-N Son wrote the revised version of the manuscript. C-N Son and T-H Kim supervised this study. All authors have read and approved the publication.
Corresponding authors
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University Hanyang University Hospital (2014–05-002) and approved by the Animals Ethics Screening Committee of Hanyang University (2021-0229A).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
Ji-Hyun Lee and Jinil Han are employed by Rheumarker Bio Inc. and Gencurix Inc., respectively. Chang-Nam Son is the chief executive officer (CEO) of Rheumarker Bio Inc. Other authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, JH., Lee, S.H., Jeon, C. et al. The complement factor H-related protein-5 (CFHR5) exacerbates pathological bone formation in ankylosing spondylitis. J Mol Med 102, 571–583 (2024). https://doi.org/10.1007/s00109-024-02428-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-024-02428-6