Abstract
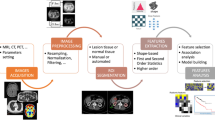
In this work, we propose a morphologic analysis by means of the construction of 3D models of the SARS-CoV-2 VP (viral particles) with algorithms in Python and Matlab based on the processing of frames. To this aim, we simulate the Takeda method to induce periodicity and apply the Fourier transform to obtain the phase of objects under analysis. To this aim, we analyze several research works focused on infected tissues by SARS-CoV-2 virus culture cells, highlighting the obtained medical images of the virus from microscopy and tomography. We optimize the results by performing image processing (segmentation and periodic noise removal) in order to obtain an accurate ROI (Region of Interest) segmentation containing only information on SARS-CoV-2 cells. We apply our algorithm to these images (3D tomographic medical images) to simulate the Takeda method (which also filters the image), considering the periodicity induced by us in the image to carry out a phase unwrapping process. Finally, we use the image phase to focus on the body, center (RNA, Protein M-N), and spikes (Protein S) of the SARS-CoV-2 cells to identify them as characteristic elements of the SARS-CoV-2 virion morphology to build a 3D model based only in the metadata of clinical studies on cell cultures. The latter results in the construction of a mathematical, physical, biological, and numerical model of the SARS-CoV-2 virion, a tool with volumes, or 3D non-speculative or animated models, based only on medical images (3D tomography) in clinical tests, faithful to the virus.











Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available in the Github repository.
Notes
We include all the algorithms in our proposal in the Github repository
We recall the two Matlab algorithms Results.m and FrameMask.m included in Github repository.
The Python code 2DFrame\(\_\)Fit is included in Github repository.
References
Organization WH (2023) SARS-CoV-2 variants. WHOhttps://www.who.int/es/activities/tracking-SARS-CoV-2-variants/
University JH (2022) Center of Systems Science and Engineering at Johns Hopkins University American. CSSE-JHUhttps://www.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6
for Disease Control C (2022) Prevention: Animals and COVID-19. CDChttps://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/animals.html
Behera AK, Kumar PR, Priya MM, Ramesh T, Kalle R (2022) The impacts of covid-19 lockdown on wildlife in deccan plateau. Sci Total Environ 822(20):153268. https://doi.org/10.1016/j.scitotenv.2022.153268
Baptista J, Blache D, Craddock N, Dalziel T, de Graaff N, Fernandes J, Green JR, H, S Kahn, Kelly, D, Lauber M, Maloney SK, Peachey B, Rodger I, Skuse J, Tilbrook AJ, Walker FR, Wall K, Zito S (2021) Impact of the covid-19 pandemic on the welfare of animals in australia. Front Vet Sci 7(28):621843 https://doi.org/10.3389/fvets.2020.621843
Jaramillo-Núñez A, Arriaga-Hernández JA, Cuevas-Otahola B, Pérez-Mesa M, Sánchez-Rinza B (2021) Díagnostic software proposal for bone scan follow-up using false color based on the gammagrams analysis from gray tone histograms. Biomed Phys Eng Express 7(3):035002–111. https://doi.org/10.1088/2057-1976/abe680
Arriaga-Hernández JA, Cuevas-Otahola B, Jaramillo-Núñez A, J, O-OJ, Morín-Castillo MM (2020) Optical-topological concepts in isomorphisms projecting bi-ronchi masks to obtain 3d profiles from objects in 2d images. App Opt 59(33):10464–10473. https://doi.org/10.1364/AO.401316
Malacara D (2006) Opt Shop Test. John Wiley & Sons, Inc., New Jersey, USA. https://doi.org/10.1002/9780470135976
Arriaga-Hernández JA, Cuevas-Otahola B, Jaramillo-Núñez A, J, O-OJ, Morín-Castillo MM (2020) Filter construction using ronchi masks and legendre polynomials to analyze the noise in aberrations by applying the irradíance transport equation. App Opt 59(13):3851–3860. https://doi.org/10.1364/AO.389716
Bhattacharya S, Sirohi RS (1997) Amplitude checker grating from one-dimensional ronchi grating and itsapplication to array generation. App Opt 36(16):3745–3752. https://doi.org/10.1364/AO.36.003745
Fornberg B (1998) A Practical Guide to Pseudospectral Methods. Cambridge University Press, Combridge, U.K
Zhang S (2010) Recent progresses on real-time 3d shape measurement using digital fringe projection techniques. Opt Lasers Eng 48(2):149–158. https://doi.org/10.1016/j.optlaseng.2009.03.008
Panguluri SK, Mohan L (2021) Otsu thresholding based image fusion framework using contour-let transform. In: 2021 6th International Conference on Inventive Computation Technologies (ICICT), vol 6695, pp. 686–693. IEEE, Coimbatore, India. https://doi.org/10.1109/ICICT50816.2021.9358794
Takeda M, Mutoh K (1983) Fourier transform profilometry for the automatic measurement of 3-d object shapes. App Opt 22(24):149–158. https://doi.org/10.1364/AO.22.003977
Pratt WK (2007) Digital Image Processing: PIKS Scientific Inside. John Wiley & Sons, New Jersey, USA. https://doi.org/10.1002/0470097434
Alassouli HM (2020) Signal, Audio and Image Processing. GmbH. & Co. KG, Munich, Germany
Cuevas-Otahola B, Arriaga-Hernández JA, Morín-Castillo MM, J, O-OJ, Vega-Salgado A (2021) Crystal 3D analysis in micrographs applied to cellular COVID anomalies identification. Paper presented at the MOMP 2021, Mexican Optics and Photonics Meeting, Puebla, Puebla, Mexico, 2021, pp 18–20 November 2021
Ullah I, Prévost J, Ladinsky MS, Stone H, Lu M, Anand SP, Beaudoin-Bussières G, Symmes K, Benlarbi M, Ding S, Gasser R, Fink C, Chen Y, Tauzin A, Goyette G, Bourassa C, Medjahed M, Mack M, Chung K, Wilen CB, Dekaban GA, Dikeakos JD, Bruce EA, Kaufmann DE, Stamatatos L, McGuire AT, Richard J, Pazgier M, Bjorkman PJ, Mothes W, Finzi A, Kumar P, Uchil PD (2021) Live imaging of sars-cov-2 infection in mice reveals that neutralizing antibodies require fc function for optimal efficacy. Immunity 54(9):2143–2158. https://doi.org/10.1016/j.immuni.2021.08.015
Turoǎová B, Sikora M, Schürmann C, Hagen WJH, Welsch S, Blanc FEC, Hörner C, van-Zandbergen G, Landry J, Doimo-de-Azevedo NT, Mosalaganti S, Schwarz A, Covino R, Mühlebach MD, Hummer G, Locker JK, Beck M (2020) Live imaging of sars-cov-2 infection in mice reveals that neutralizing antibodies require fc function for optimal efficacy. Science 370(6513):203–208. https://doi.org/10.1126/science.abd5223
Ke Z, Oton J, Qu K, Cortese M, Zila V, McKeane L, Nakane T, Zivanov J, Neufeldt CJ, Cerikan B, Lu JM, Peukes J, Xiong X, Kräusslich HG, Scheres SHW, Bartenschlager R, Briggs JAG (2020) Live imaging of sars-cov-2 infection in mice reveals that neutralizing antibodies require fc function for optimal efficacy. Nature 588:498–502. https://doi.org/10.1038/s41586-020-2665-2
Frese N, Schmerer P, Wortmann M, Schörmann M, König M, Westphal M, Weber F, Sudhoff H, Gölzhäuser A (2021) Imaging of sars-cov-2 infected vero e6 cells by helium ion microscopy. Beilstein J Nanotech 12(13):172–179. https://doi.org/10.3762/bjnano.12.13
AlZu’bi S, Shehab M, Al-Ayyoub M, Jararweh Y, Gupta B (2020) Parallel implementation for 3d medical volume fuzzy segmentation. Pattern Recognit Lett 130:312–318. https://doi.org/10.1016/j.patrec.2018.07.026
Chu V, Hamarneh G (2006) Matlab-itk interface for medical image filtering, segmentation, and registration. In: Reinhardt JM, Pluim JPW (eds) Medical Imaging 2006: Image Processing, vol 6144. SPIE, San Diego, California, United States, pp 61443–18. https://doi.org/10.1117/12.652628
George E, Liacouras P, Rybicki FJ, Mitsouras D (2017) Measuring and establishing the accuracy and reproducibility of 3d printed medical models. RadioGraphics 37(5):1424–1450. https://doi.org/10.1148/rg.2017160165
Ammanuel S, Brown I, Uribe J (2019) Creating 3d models from radiologic images for virtual reality medical education modules. J Med Syst 43(166):166. https://doi.org/10.1016/j.immuni.2021.08.015
González-Izard S, Sánchez-Torres R, Alonso-Plaza, Juanes-Méndez JA, García-Peñalvo FJ (2021) Nextmed: Automatic imaging segmentation, 3d reconstruction, and 3d model visualization platform using augmented and virtual reality. Sensors 20(2962):2962–119. https://doi.org/10.3390/s20102962
Smitha TV, Madhura S, Brundha R (2021) 2d image-based higher-order meshing for 3d modelling in matlab. IOP Conf Ser Mat Sci Eng 1070(1):012017–18. https://doi.org/10.1088/1757-899X/1070/1/012017
Mastronarde DN, Held SR (2017) Automated tilt series alignment and tomographic reconstruction in imod. J Struct Bio 197(2):102–113. https://doi.org/10.1016/j.jsb.2016.07.011
Pelt DM, Batenburg KJ, Sethian JA (2018) Improving tomographic reconstruction from limited data using mixed-scale dense convolutional neural networks. J Imag 4(11):128. https://doi.org/10.3390/jimaging4110128
Jähne B (2005) Digital Image Processing. Springer, Heidelberg, Germany. https://doi.org/10.1007/3-540-27563-0
Schultz SM (2007) Using matlab to help teach fourier optics. In: Awwal AAS, Iftekharuddin KM, Javidi B (eds) Optical Engineer- 895Optical Engineering + Applications, vol 6695. SPIE, San Diego, California, United States, pp 593–660. https://doi.org/10.1117/12.735889
Nagy JG, Palmer K, Perrone L (2004) Iterative methods for image deblurring: A matlab object-oriented approach. Numer Algorithms 36:73–93. https://doi.org/10.1023/B:NUMA.0000027762.08431.64
Takeda M, Ina H, Kobayashi S (1982) Fourier-transform method of fringe-pattern analysis for computer-based topography and interferometry. J Opt Soc Am 72(1):156–160. https://doi.org/10.1364/JOSA.72.000156
Fienup JR, Marron JC, Schulz TJ, Seldin JH (1993) Hubble space telescope characterized by using phase-retrieval algorithms. App Opt 32(10):1747–1767. https://doi.org/10.1364/AO.32.001747
Ichikawa K, Lohmann AL, Takeda M (1988) Phase retrieval based on the irradiance transport equation and the fourier transform method: experiments. App Opt 27(16):3433–3436. https://doi.org/10.1364/AO.27.003433
Arriaga-Hernández JA, Cuevas-Otahola B, Jaramillo-Núñez A, J O-OJ, Morín-Castillo MM (2019) Two-dimensional legendre polynomials as a basis for interpolation of data to optimize the solution of the irradíance transport equation analyzed as a boundary problem on surfaces testing. App Opt 58(18):5057–5066. https://doi.org/10.1364/AO.58.005057
Yin X, Ng BW-H, Abbott D (2012) Feature Extraction and Selection. In Terahertz Imaging for Biomedical Applications. Springer, Adelaide, Australia. https://doi.org/10.1007/978-1-4614-1821-4
Seeram E (2016) Computed Tomography: Physical Principles, Clinical Applications, and Quality Control. Elsevier, Sydney, Australia. https://doi.org/10.1148/radiology.194.3.782
Powsner RA, Palmer MR, Powsner ER (2022) Essentials of Nuclear Medicine Physics, Instrumentation, and Radíation Biology. Willey-Blackwell, Boston, USA. https://doi.org/10.1148/radiology.194.3.782
Vetterli M, Kovačević J, Goyal VK (2018) Foundations of Signal Processing. Cambridge University Press, Cambridge, USA. https://doi.org/10.1017/CBO9781139839099
Arendt W, Schleich WP (2009) Mathematical Analysis of Evolution, Information, and Complexity. Wiley-VCH Verlag GmbH & Co. KGaA, Ulm, Germany. https://doi.org/10.1002/9783527628025
Masters PS (2006) The molecular biology of coronaviruses. Adv Virus Res 66:193–292. https://doi.org/10.1016/S0065-3527(06)66005-3
Kadam SB, Sukhramani GS, Bishnoi P, Pable AA, Barvkar VT (2021) Sars-cov-2, the pandemic coronavirus: Molecular and structural insights. J Basic Microbio 61(3):180–202. https://doi.org/10.1002/jobm.202000537
Morín-Castillo MM, Arriaga-Hernández J, Cuevas-Otahola B, Oliveros-Oliveros JJ (2022) Analysis of dipolar sources in the solution of the electroencephalographic inverse problem. Mathematics 11(10):1926–122. https://doi.org/10.1016/j.patrec.2018.07.026
Acknowledgements
The authors thank CONACYT (Consejo Nacional de Ciencia y Tecnologia, México) and the Benemerita Universidad Autonoma de Puebla (BUAP) for the support given during this research work. Special thanks to Dra. Lilia Cedillo Ramírez, Dr. Ygnacio Martínez Laguna and Dr. Jorge Antonio Yáñez Santos for their collaboration and comments. We also thank the technical staff of the Biomolecular Detection Centre at BUAP: M.C. Jose Sergio Tepanecatl Xihuitl, M.C. Elda Carreon Moreno, M. C. Cristina Lara Ochoa, and David Zenteno Diaz for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arriaga-Hernández, J., Cuevas-Otahola, B., Oliveros-Oliveros, J.J. et al. Phase analysis simulating the Takeda method to obtain a 3D profile of SARS-CoV-2 cells. Pattern Anal Applic 27, 16 (2024). https://doi.org/10.1007/s10044-024-01225-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10044-024-01225-8