Abstract
Objective. Abundant lipid-laden macrophages are found at the injury site after spinal cord injury (SCI). These cells have been suggested to be pro-inflammatory and neurotoxic. AdipoRon, an adiponectin receptor agonist, has been shown to promote myelin lipid efflux from mouse macrophage foam cells. While it is an attractive therapeutic strategy, systemic administration of AdipoRon is likely to exert off-target effects. In addition, the pathophysiology after SCI in mice is different from that in humans, whereas rat and human SCI share similar functional and histological outcomes. In this study, we evaluated the effects of AdipoRon on rat macrophage foam cells and developed a drug delivery system capable of providing sustained local release of AdipoRon to the injured spinal cord. Approach. Rat macrophages were treated with myelin debris to generate an in vitro model of SCI foam cells, and the effects of AdipoRon treatment on myelin uptake and efflux were studied. AdipoRon was then loaded into and released from microparticles made from dextran sulfate and fibrinogen for sustained release. Main results. AdipoRon treatment not only significantly promotes efflux of metabolized myelin lipids, but also inhibits uptake of myelin debris. Myelin debris alone does not appear to be inflammatory, but myelin debris treatment potentiates inflammation when administered along with pro-inflammatory lipopolysaccharide (LPS) and interferon-γ. AdipoRon significantly attenuated myelin lipid-induced potentiation of inflammation. Bioactive AdipoRon can be released in therapeutic doses from microparticles. Significance. These data suggest that AdipoRon is a promising therapeutic capable of reducing lipid accumulation via targeting both myelin lipid uptake and efflux, which potentially addresses chronic inflammation following SCI. Furthermore, we developed microparticle-based drug delivery systems for local delivery of AdipoRon to avoid deleterious side effects. This is the first study to release AdipoRon from drug delivery systems designed to reduce lipid accumulation and inflammation in reactive macrophages after SCI.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Traumatic spinal cord injury (SCI) damages myelinated axons, resulting in generation and accumulation of myelin and other cellular debris. Myelin debris specifically has been shown to modulate macrophage behavior and promote inflammation after SCI [1], as well as limit regeneration by inhibiting oligodendrocyte differentiation [2, 3] and neurite outgrowth [4]. Myelin debris can be found as early as 24 h after injury [5], increases within the first week following SCI [6], and persists chronically [7]. At approximately 3–7 d after the initial injury, peripheral macrophages invade the injured spinal cord, where they encounter and begin clearing myelin debris [8]. When macrophages phagocytose and metabolize the lipid and cholesterol-rich myelin debris, they package the contents into organelles called lipid droplets [9]. Lipid droplets accumulate inside macrophages, resulting in a foamy appearance; these macrophages are subsequently referred to as foam cells.
In in vitro studies the consequences of myelin phagocytosis are highly context-dependent, and can result in inflammation or resolution of inflammation, as well as potentiation or mitigation of macrophage responses to pro-inflammatory stimuli [1]. In in vivo studies following SCI, however, foam cell formation results in increased expression of pro-inflammatory genes [10–12]. Foam cells first appear around 1 week after injury at the lesion epicenter, and progressively accumulate in the following weeks, forming a dense plaque-like structure [12]. Interestingly, this expansion of foam cell plaque parallels expression of pro-inflammatory M1 macrophage markers [13], suggesting that foam cells are likely a major driving force behind chronic inflammation after SCI.
In vitro, macrophages upregulate the membrane transport protein ATP-binding cassette subfamily member A1 (ABCA1) in response to myelin loading. The cells can then metabolize and efflux phagocytosed myelin debris within 72 h. In vivo, however, ABCA1 expression was found to decrease by 2 weeks post-injury [12]. Accordingly, Wang et al suggested that the accumulation of lipid droplets in macrophages drives pro-inflammatory myelin responses, and that inflammatory macrophage activation would likely subside upon efflux of lipid droplet contents. At the same time, foam cells have been shown to exhibit reduced phagocytic capacity for neutrophils, rendering them less efficient clearers of pro-inflammatory neutrophil debris [12]. Stimulating lipid efflux, therefore, represents a potential therapeutic intervention to reduce chronic inflammation after SCI. Notably, because macrophages take 3–7 d to invade injured spinal cord tissue and foam cells are not observed until 1 week after injury, strategies targeting lipid efflux need not be initiated immediately after injury. Instead, they offer an attractively wide window for therapeutic intervention, which may encourage clinical adoption.
The protein adiponectin has been shown to mediate a number of processes, including insulin sensitization, lipid handling, and inflammation [14]. Adiponectin stimulates ABCA-1 mediated efflux in macrophages, reducing lipid droplet accumulation and foam cell formation [15–18]. First described in 1995, adiponectin is a ∼30 kDa monomer synthesized by adipocytes and released into circulation as homo-oligomers of varying molecular weight [14]. Despite interest in targeting adiponectin signaling in diabetes, obesity, and cardiovascular disease, endogenous adiponectin administration is generally considered infeasible due to adiponectin's complex quaternary structure and short half-life (45–60 min in serum) [19]. In 2013, Okada-Iwabu et al screened a library of compounds and identified the aptly-named AdipoRon, a small-molecule agonist of both adiponectin receptors AdipoR1 and AdipoR2 [20]. A recent study by Zhou et al shows that AdipoRon administration stimulated lipid efflux from mouse foam cells in vitro and reduced myelin lipid accumulation in vivo in a mouse SCI model [21]. On the other hand, the pathology and recovery processes after SCI in mice is different from that in rats or humans [22, 23]. Instead, rat and human SCI have been shown to share similar functional, electrophysiological, and morphological outcomes [24]. For example, both rats and humans develop progressive necrosis and cavitation after contusion injury, the most clinically relevant SCI model [22, 23]. In contrast, mice do not develop cavitation after SCI; instead, the lesion site is usually filled with connective tissue [22, 23]. Because macrophages play a key role in post-injury inflammation and resulting cavitation [25], in this study we investigated the effect of AdipoRon on myelin lipid accumulation by rat macrophages. In addition, we not only studied the lipid efflux effect of AdipoRon, as reported by Zhou et al, but also studied its effect on myelin lipid uptake by macrophages. We found that AdipoRon was much more effective in reducing lipid accumulation in macrophages when it is administered during both lipid uptake and efflux stages, as compared to being administered in either stage alone. Moreover, Zhou et al found that lipid accumulation increased inflammation, which was inhibited by AdipoRon treatment. On the other hand, we found that treating rat macrophages with myelin debris only very slightly increased inflammatory response. Since SCI elicits a strong inflammatory response, we studied the anti-inflammatory effect of AdipoRon on lipid-laden macrophages in the presence of inflammatory stimuli, and we found that AdipoRon demonstrated anti-inflammatory activity even in the presence of strong inflammatory stimuli.
Although AdipoRon is orally available, increasing systemic adiponectin signaling may be associated with adverse off-target effects. For example, mice engineered to overexpress adiponectin exhibit reduced bone density and left ventricular hypertrophy [19]. Enhanced adiponectin signaling can also result in infertility [26] as well as adipogenesis and associated weight gain [27]. In lieu of systemic administration, local delivery of AdipoRon can bypass these off-target effects while still providing therapeutic benefit to the injured tissue. In the second part of this study, we loaded AdipoRon into microparticles made from dextran sulfate and fibrinogen (DS:Fb), and embedded the particles in agarose hydrogel, which can be implanted subdurally between the dura and the injured spinal cord, to provide controlled release of bioactive drug from a clinically relevant format.
2. Materials and methods
2.1. Generation and purification of myelin debris
All chemicals were purchased from Millipore Sigma, unless otherwise noted. All animal procedures were approved by the IACUC committee at Drexel University and followed National Institutes of Health guidelines for the care and use of laboratory animals. Myelin debris were collected and purified from adult Sprague Dawley rats (Charles River) according to a protocol modified from one generously provided by the lab of Dr John Gensel. The protocol was originally modified from those available in literature [28, 29]. Animals were euthanized via CO2 inhalation, and whole brains dissected out and immediately frozen at −80 °C. To generate myelin debris, brains were thawed on ice, mixed with 1x phosphate buffered saline (PBS) (Corning) containing 1% penicillin/streptomycin (P/S, Life Technologies), and crushed with seven strokes of a glass homogenizer. Crushed tissue was transferred to a 15 ml tube and sonicated with a Hielscher UP200H ultrasonic processor for 1 min on ice (amplitude 80%, frequency 1 Hz; tissue was sonicated for 15 sec, followed by a 15 sec rest period a total of four times). The resulting suspension was rinsed with PBS + P/S and debris collected via ultracentrifugation at 20 000 g for 10 min at 4 °C (Sorvall). After resuspension in fresh PBS + P/S, the suspension was underlaid with 30% Percoll solution and centrifuged again, trapping purified myelin above the Percoll layer. After lysing cells via subjection to 10 min of osmotic shock (20 mM TRIS, pH 7.45), the myelin preparation was further purified via another round of Percoll separation and rinsing in PBS + P/S. Finally, purified myelin was resuspended in 0.5 ml PBS + P/S per brain, aliquoted, and frozen at −80 °C. To ensure consistency between batches, myelin debris was isolated from three pooled rat brains each time. The concentration of myelin debris was determined using a bicinchoninic acid assay kit (Thermo Fisher Scientific) following the manufacturer's instructions. The concentration of the stock solution was then adjusted to 50 mg ml−1 protein content. For use in treating cells, stock myelin debris was thawed and diluted in cell culture media at 0.25–0.75 mg ml−1. Experiments were performed with myelin debris from multiple batches.
2.2. Primary rat bone marrow-derived macrophage isolation and culture
Primary rat bone marrow-derived macrophages were cultured according to established protocols [30, 31]. Femurs and tibias were dissected out from Sprague Dawley rats freshly euthanized via CO2 inhalation, cleared of overlying muscle and other tissue, and wiped with 70% ethanol. Each end of the bones were cut off, and loosely held with forceps. Using a 10 ml syringe outfitted with a 22 gauge needle, 7–10 ml of ice cold PBS was passed through the medullary cavity to flush out bone marrow. Bone marrow cells were collected via centrifugation at 1500 rpm for 10 min and resuspended in fetal bovine serum (FBS) (Life Technologies) for counting. Isolated bone marrow cells were diluted to 2.5*10^7 cells ml−1 in FBS containing 10% dimethyl sulfoxide (DMSO) (Corning), frozen for 24 h in the −80 °C freezer, and transferred to a liquid nitrogen cell bank for long-term storage.
To provide a source of macrophage colony-stimulating factor, Dulbecco's Modified Eagle Medium (DMEM) (life technologies) supplemented with 10% FBS and 1% P/S was incubated with L929 cells for 5 d, collected, and filtered through a 0.2 μm sterile filter to remove contaminating cells. For experiments, bone marrow cells were thawed and seeded onto 6 cm dishes in L929-conditioned media diluted to 30% with fresh DMEM + 10% FBS + 1% P/S. To ensure reproducibility, each study was repeated using cells isolated on different days from different rats. After 3 d, dishes were washed with DPBS containing calcium and magnesium ions to remove any nonadherent cells, and fed with an additional 5 ml of L929-conditioned media diluted to 30% in fresh media. After 5 d, dishes were washed once again, and fed with L929-conditioned media diluted to 10% in fresh media. After 7 d, macrophages were lifted from dishes with a cell scraper, counted, and seeded into 96 well plates at a density of 70 000 cells per well. At this stage, no conditioned media was included, and 100% of the cells stained positive for the macrophage marker CD68. After allowing macrophages to attach overnight, treatment was initiated.
2.3. Oil red O (ORO) staining and quantification
Macrophages were stained with ORO to identify lipid droplets characteristic of foam cells. A 10 mg ml−1 stock ORO solution was made by adding dye to isopropanol, shaking overnight, and filtering out undissolved solids with a P5 filter (Fisher). Immediately before use, stock ORO solution was diluted to 60% with deionized water, and the resulting solution filtered through a 0.45 μm filter. At the end of each experiment, cells were thoroughly washed with DPBS to remove any uningested myelin debris and then fixed with 4% paraformaldehyde (PFA) for 10 min at room temperature. After removing residual PFA with three five-minute DPBS washes, cells were equilibrated with 60% isopropanol for 5 min. Diluted and filtered ORO solution was applied to cells for 15 min, after which residual dye was removed with a brief 60% isopropanol wash. Nuclei were labelled with 1 μg ml−1 4ʹ,6-diamidino-2-phenylindole (DAPI) at room temperature for 10 min. The cells were imaged with an fluorescent inverted light microscope (Nikon). Images were analyzed using ImageJ software.
2.4. Fluorescein labeling of myelin debris
To fluorescently label myelin debris, fluorescein-NHS (Thermo Fisher Scientific) was dissolved in DMSO at a concentration of 10 mM. 100 μl of myelin debris purified as described in section 2.1 was thawed and mixed with 300 μl of 1x PBS, and spun at 15 000 g for 10 min at 4 °C to remove P/S. F-NHS was diluted to 1 mM in PBS, and the myelin debris pellet was resuspended in 200 μl of 1 mM F-NHS. The reaction was allowed to proceed for 30 min under gentle agitation while protected from light. After 30 min, myelin debris was collected via centrifugation (15 000 g for 10 min) and resuspended in 300 μl 1x PBS containing 100 mM lysine to quench unreacted F-NHS. Fluorescent myelin was washed two more times with PBS, resuspended in 100 μl PBS containing 1% P/S, and frozen at −80 °C until use.
2.5. Fabrication of AdipoRon-loaded microparticles
To form DS:Fb microparticles, 175 μl DS solution (4 mg ml−1 in acetate buffer) was mixed with an equal volume of AdipoRon solution (Cayman Chemical; 0.343 mg ml−1 in 0.9% NaCl solution containing 2.86% w/v polyethylene glycol). PEG was added to prevent particle aggregation and sticking. To the resulting mixture, which remained clear, 350 μl of Fb (12.5 or 15 mg ml−1 Fb corresponding to DS:Fb mass ratios of 1:6.25 or 1:7.5, respectively, dissolved in 0.9% NaCl) was rapidly added, resulting in microparticle self-assembly. Each stepwise addition was carried out under constant magnetic stirring at the maximum speed to ensure rapid mixing. Particles were collected via centrifugation, washed thoroughly, and finally resuspended in a 5% mannitol solution and lyophilized overnight. The supernatant was reserved for evaluation of drug loading. Upon rehydration, approximately 1.25 or 1.5 mg of adiporon-loaded microparticles (corresponding to DS:Fb ratios of 1:6.25 and 1:7.5, respectively) were suspended in 15 μl of 2% w/v agarose solution and transferred to 4 °C for 15 min, resulting in hydrogel replicates with a final volume of approximately 25 μl each.
2.6. In vitro AdipoRon release
To perform release studies, agarose hydrogels loaded with AdipoRon-containing microparticles (as described in section 2.5) were incubated with 100 μl of artificial cerebrospinal fluid (150 mM Na, 3 mM K, 1.4 mM Ca, 0.8 mM Mg, 1.0 mM P, 155 mM Cl, and 5 mM HEPES, pH 7.3) at 37 °C. Release media was removed, reserved, and replaced with fresh media every 24 h. Release media were then stored at −80 °C until measurement via high performance liquid chromatography (HPLC).
2.7. HPLC
AdipoRon concentrations in supernatants and in release samples were determined via HPLC. Samples were analyzed using a Waters 1525 binary HPLC pump equipped with a Model 1500 Column Heater (Waters), 9725i manual injector (Waters), and Waters 2489 UV-Vis detector set to 283 nm. 20 μl of each sample was injected onto an XBridge BEH C18 column (Waters, 130 Å, 3.5 μm sorbent particles, 4.6 mm × 50 mm) maintained at 30 °C and protected by an XBridge BEH C18 VanGuard guard column system (Waters, 130 Å, 3.5 μm sorbent particles, 3.9 mm × 5 mm). Gradient mobile phase was employed consisting of 25 mM acetic acid with pH adjusted to 3 and methanol containing 25 mM acetic acid. Elution started at 100% acetate buffer/0% methanol with 25 mM acetic acid (v/v), and the concentration of methanol with acetic acid was linearly increased over 5 min until 0% acetate buffer/100% methanol with acetic acid was achieved. After holding at 100% methanol with acetic acid for 1 min, the concentration was decreased over the remaining 4 min until starting conditions were obtained again. Throughout the entire gradient, flow rate was held constant at 1.5 ml min−1. By this method, AdipoRon consistently eluted as a sharp peak at a retention time of 3.4 s.
2.8. Statistical analysis
All experiments were repeated at least three times. All data are presented as mean ± standard deviation. To identify differences between groups, one-way analysis of variance (ANOVA) followed by Tukey or Games-Howell post hoc tests were conducted, depending on whether variances were homogenous or not. Significance was evaluated at a cutoff of p < 0.05. All data were analyzed using SPSS software (IBM).
3. Results and discussion
3.1. AdipoRon attenuates myelin debris uptake by rat macrophages
When macrophages phagocytose myelin debris, ingested lipids are processed and packaged into lipid droplets which accumulate and give foam cells their characteristic foamy appearance under the microscope.
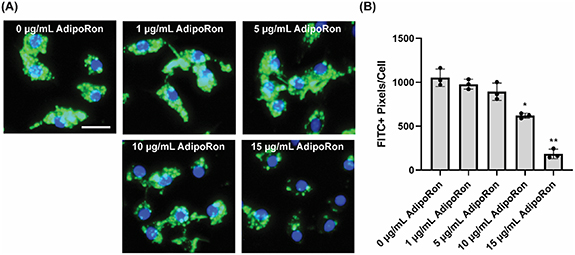
First, we investigated the effect of AdipoRon treatment on myelin lipid uptake. In this study, rat bone-marrow derived macrophages (BMDMs) were first incubated with 1, 5, 10, and 15 μg ml−1 AdipoRon for 24 h, and then exposed to 0.25 mg ml−1 fluorescein-labeled myelin debris for 2 h. By only allowing cells to interact with myelin debris for 2 h, lipid uptake can be studied before the cells have sufficient time to thoroughly metabolize and efflux ingested myelin lipid [31]. After washing detached cells and non-phagocytosed myelin debris, cells were fixed, and nuclei were labeled with DAPI (blue). Fluorescein-labeled myelin debris (green) could be readily visualized with a fluorescent microscope. Rat BMDMs exhibited rapid uptake of myelin debris, resulting in the appearance of brightly fluorescent myelin debris inside the cells (figure 1(A)). Phagocytosed debris were roughly circular, but exhibited obvious heterogeneity in both size and shape. In addition, internalized lipid could only be very dimly labeled with ORO (data not shown). Collectively, these data indicate that after 2 h, little processing of ingested lipid had occurred. As the AdipoRon concentration increased, myelin debris uptake decreased (figure 1). 10 and 15 μg ml−1 AdipoRon treatment significantly inhibited uptake of debris in a dose-dependent manner, with 15 μg ml−1 -treated cells exhibiting significantly reduced uptake as compared with 10 μg ml−1 treatment.
Figure 1. Rat BMDMs were treated with varying concentrations of AdipoRon for 24 h, and then exposed to fluorescein-labeled (FITC+) myelin debris (green) (n= 3) for 2 h. AdipoRon inhibits macrophage uptake of myelin debris in a dose-dependent manner. (A) Representative fluorescent images and (B) quantification of fluorescein-labeled myelin debris. *P < 0.01 and **P < 0.001 as compared to all other groups; one-way ANOVA followed by Tukey post-hoc; data presented as mean ± standard deviation. Scale bar = 15 μm.
Download figure:
Standard image High-resolution image3.2. AdipoRon stimulates lipid efflux from rat macrophage foam cells
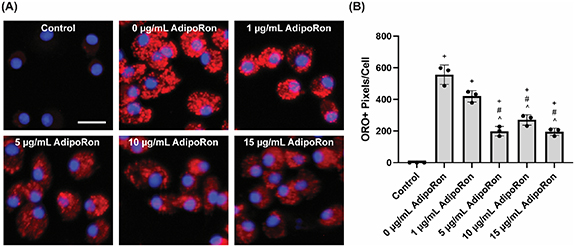
To study lipid efflux, rat BMDMs were treated with myelin debris for 24 h in the absence of AdipoRon to induce lipid accumulation, then the cells were thoroughly washed, and exposed to various concentrations of AdipoRon for an additional 24 h. After fixation, accumulated lipid droplet-associated neutral lipids and cholesterol esters were stained with the lypophilic dye ORO [12, 32]. Very little ORO staining could be detected in untreated control, but following addition of myelin debris (0 μg ml−1 AdipoRon group), bright red lipid droplets were observed throughout the cells. Inclusion of AdipoRon during efflux resulted in significantly reduced droplet accumulation in the cells, even at just 1 μg ml−1 (figure 2). 5 μg ml−1 AdipoRon resulted in further reduction, while increasing beyond 5 μg ml−1 led to a plateau effect, indicating maximal activation had already occurred.
Figure 2. Rat BMDMs were exposed to myelin debris alone for 24 h, and then allowed to metabolize and efflux phagocytosed material for 24 h in the presence of varying concentrations of AdipoRon (n= 3). AdipoRon stimulates lipid efflux in a dose-dependent manner. (A) Representative fluorescent images and (B) quantification of oil red o (ORO)+ lipid droplets. +P < 0.05 as compared to untreated control, #P < 0.05 as compared to 0 μg ml−1 AdipoRon, ^P < 0.05 as compared to 1 μg ml−1 AdipoRon treated groups; one-way ANOVA followed by Games-Howell post-hoc; data presented as mean ± standard deviation. Scale bar = 15 μm.
Download figure:
Standard image High-resolution image3.3. AdipoRon treatment during both myelin lipid uptake and efflux stages dramatically decreases lipid droplet accumulation in rat macrophages
Finally, we studied the effect of AdipoRon treatment during both myelin lipid uptake and efflux stages on lipid droplet accumulation in macrophages. BMDMs were treated with 0.25 mg ml−1 myelin debris for 24 h to allow for myelin lipid uptake. Subsequently, the cells were cultured for an additional 24 h without myelin debris to allow for lipid efflux. Meanwhile, cells were incubated with varying concentrations of AdipoRon throughout the 48 h study, spanning 24 h of myelin uptake followed by 24 h of lipid efflux. As shown in figure 3, addition of AdipoRon at both lipid uptake and efflux stages resulted in dose-dependent, significantly reduced lipid droplet accumulation, with 5–15 μg ml−1 AdipoRon dramatically reducing lipid droplet accumulation nearly to untreated control levels (figure 3).
Figure 3. 48 h AdipoRon treatment spanning 24 h of myelin uptake and 24 h of lipid efflux results in dramatically reduced lipid droplet accumulation in rat macrophages (n= 3). (A) Representative fluorescent images and (B) quantification of oil red o (ORO)+ lipid droplets. ++P < 0.04 as compared to control; one-way ANOVA followed by Games-Howell post hoc; data presented as mean ± standard deviation. Scale bar = 15 μm.
Download figure:
Standard image High-resolution imageZhou et al recently showed that AdipoRon treatment induced lipid efflux in myelin-derived foam cells using mouse macrophages [21]. Notably, in that study AdipoRon treatment during the lipid efflux stage resulted in 15%–40% reductions in myelin lipid accumulation. In our study, however, inclusion of AdipoRon during both lipid uptake and efflux stages resulted in 63%–95% reduction in lipid accumulation, suggesting a potential role for AdipoRon in inhibiting uptake of myelin lipid by rat macrophages, in addition to the lipid efflux effect.
Adiponectin has been shown to stimulate cholesterol efflux from murine macrophage cell line RAW264.7-derived foam cells [33], while Zhou et al showed AdipoRon to have the same activity in primary mouse macrophages [21]. In both cases, enhanced efflux was attributed to upregulation of the transporter protein ABCA1. Although we did not study gene expression, it is likely that AdipoRon stimulates lipid efflux from myelin-laden rat BMDMs through the same pathway. The inhibitory effect of AdipoRon on lipid uptake by macrophages has not previously been studied. On the other hand, adiponectin was found to decrease uptake of oxidized low-density lipoprotein through downregulation of scavenger receptor AI in THP-1 cells [16]. The same study also identified adiponectin-associated upregulation of hormone sensitive lipase, which helps to catabolize triglycerides, as well as scavenger receptor BI, which can also participate in cholesterol efflux. It is possible that AdipoRon similarly regulates multiple lipid metabolism processes, which could explain why AdipoRon treatment targeting both uptake and efflux yields such a dramatic reduction in lipid accumulation in myelin-treated rat BMDMs.
3.4. AdipoRon treatment modestly attenuates myelin-induced potentiation of inflammation
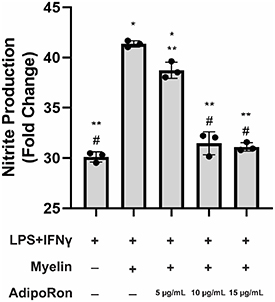
As an inflammatory mediator, myelin debris can produce a diverse set of behaviors ranging from inhibition to potentiation of macrophage responses to pro-inflammatory stimuli, depending on the context in which myelin debris are presented [1]. Within the context of SCI, however, myelin phagocytosis and lipid accumulation appears to exert pro-inflammatory effects, as pro-regenerative (M2) to pro-inflammatory (M1) macrophage phenotype switching is correlated with foam cell accumulation at the lesion site [12], and strategies aimed at reducing lipid accumulation have been shown to be therapeutic [21, 34]. To evaluate the effect of AdipoRon on lipid accumulation-induced inflammation, we developed an in vitro model of pro-inflammatory foam cells. Upregulation of inducible nitric oxide synthase (a marker for M1 macrophages) leads to increased production of nitric oxide (NO), a key inflammatory mediator and a neurotoxic molecule [35]. Nitrite levels in the culture medium, as an indicator of NO production, was measured using Griess reagent to evaluate inflammation. While myelin treatment alone had no significant effect on nitrite production (supplementary figure 1), BMDMs primed with classic pro-inflammatory stimuli LPS and interferon gamma (IFNγ) exhibited a robust response to myelin debris as compared to LPS/IFNγ alone, indicating that myelin debris can potentiate the BMDM response to pro-inflammatory stimuli (figure 4). While AdipoRon had no effect on cells treated with LPS/IFNγ only (supplementary figure 2), inclusion of AdipoRon at 5 μg ml−1 resulted in a modest but significant nitrite reduction from LPS/IFNγ/myelin-treated pro-inflammatory foam cells (figure 4). Further increasing AdipoRon dose to 10–15 μg ml−1 resulted in a return to nitrite production statistically similar to LPS/IFNγ alone, suggesting that AdipoRon can ameliorate the inflammation potentiation effect of myelin debris in BMDMs.
Figure 4. AdipoRon treatment significantly attenuates myelin-induced potentiation of macrophage response to inflammatory stimuli (n= 3). *P < 0.001 as compared to LPS/IFNγ alone, **P < 0.01 as compared to LPS/IFNγ + myelin, # P < 0.001 as compared to LPS/IFNγ + myelin + 5 μg ml−1 AdipoRon; one-way ANOVA followed by Tukey post-hoc; data expressed as fold change from untreated control and presented as mean ± standard deviation.
Download figure:
Standard image High-resolution imagePrevious work has identified myelin debris alone as pro-inflammatory, in contrast to our findings [21]. This is likely due to the detection methods employed- while the Greiss assay used in our study is time- and cost-efficient, detection of NO production in culture media is far less sensitive than gene expression analysis. Because the debris-rich injury epicenter is also a hotbed of chronic pro-inflammatory stimuli production, however, studying macrophage responses to myelin debris in the presence of inflammatory stimuli is more relevant than studying that to myelin debris in isolation. While AdipoRon mildly attenuated myelin-induced potentiation of BMDM nitrite production in just 24 h, longer-term treatment yielding more complete efflux of accumulated lipids may provide additional anti-inflammatory benefit. In our model of pro-inflammatory foam cells, however, BMDMs could only be maintained for 48 h before viability rapidly decreased. In the injured spinal cord, these pro-inflammatory BMDMs persist, possibly due to the presence of macrophage survival factors such as macrophage-colony stimulating factor (M-CSF), which has been shown to be upregulated by dorsal root ganglion following tibial and peroneal nerve transection [36] and brain microglia following facial nerve injury [37]. In our study, M-CSF was included during expansion and differentiation of BMDMs, but removed from culture media for all experiments in order to avoid the potentially confounding effects of cell proliferation.
3.5. Dextran sulfate:fibrinogen microparticles was developed to deliver bioactive AdipoRon
Zhou et al recently administered AdipoRon to contused mice via tail vein injection every three days for four weeks, resulting in reduced lipid accumulation in spinal cord lesions and improved functional recovery [21]. Although AdipoRon is relatively small and hydrophobic, suggesting it can cross the blood-spinal cord barrier, systemic exposure is likely to exert a wide range of off-target effects as adiponectin receptors are also expressed by macrophages remaining in the periphery, cardiomyocytes [38], adipose tissue, skeletal muscle, and liver [39], and likely regulate a huge range of functions in various cell types and tissues. To provide localized delivery of AdipoRon without eliciting potentially deleterious side effects, we loaded AdipoRon into microparticles composed of DS, a negatively charged polysaccharide, and Fb, a clotting protein readily found in serum. Under mildly acidic conditions, Fb takes on a net positive charge, while DS remains highly negatively charged. When mixed in the presence of acetate buffer (pH 3), DS and Fb readily self-assemble into microparticles. AdipoRon contains secondary and tertiary amines that act as weak bases and accept protons under acidic conditions, resulting in a net positive charge on the drug. To load AdipoRon into DS:Fb particles, DS and AdipoRon were first mixed together in acidic conditions, allowing them to interact with each other. Upon addition of Fb, particles rapidly formed.
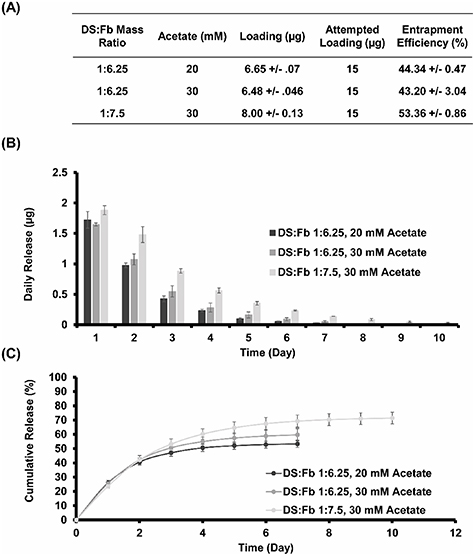
AdipoRon-loaded DS:Fb particles were thoroughly washed to remove the acid. Lyophilized particles can be resuspended agarose hydrogels to localize the particles into the intrathecal space between the dura and injured cord. This route of drug delivery is preferred because it bypasses the dura mater as a diffusion barrier and does not damage the spinal cord tissue [40]. To control the release rate from the delivery system, three particle formulations were investigated. DS:Fb mass ratio was varied from 1:6.25–1:7.5, and acetate concentration was varied from 20 to 30 mM (figure 5(A)). The highest loading, highest daily dose, and longest release duration resulted from particles formed with a 1:7.5 mass ratio in the presence of 30 mM acetate (figure 5). Notably, daily release can provide physiologically relevant doses of ∼0.25–2 μg/day.
Figure 5. (A) AdipoRon loading, (B) daily release, and (C) cumulative release from DS:Fb microparticles.
Download figure:
Standard image High-resolution imageIf particle-loaded hydrogels are injected into the intrathecal space, released drug can distribute throughout adjacent spinal cord tissue, with some loss to circulating CSF. If the total volume of distribution in rats is roughly estimated to be 200 μl (∼100 μl CSF [39], ∼100 μl surrounding tissue), 0.25–2 μg release can be roughly estimated to yield concentrations of 1.25–10 μg ml−1, which is similar to the concentrations used in our in vitro studies.
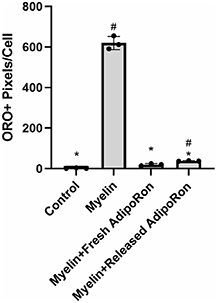
To assess bioactivity of released AdipoRon, rat BMDMs were treated with myelin for 24 h and then allowed to efflux for another 24 h, all in the presence of 5 μg ml−1 fresh AdipoRon or released AdipoRon that was diluted to 5 μg m−1 . To obtain released AdipoRon, release media from each 25 μl microparticle-loaded agarose gel replicate was diluted in cell culture media. While myelin treatment resulted in significant lipid accumulation, both fresh and released AdipoRon significantly reduced lipid accumulation (figure 6). Fresh and released AdipoRon-treated groups were not significantly different from each other, indicating that released drug retained the ability to promote foam cell resolution in vitro. Furthermore, AdipoRon measured at all days of release exhibited nearly identical retention times and peak shapes on HPLC. Because these peak features are highly dependent on structure-based affinities between analyte and column, this suggests that AdipoRon did not undergo degradation throughout the duration of release.
Figure 6. AdipoRon released from DS:Fb microparticles and diluted to 5 μg ml−1 in cell culture media retains the ability to stimulate foam cell resolution (n= 3); #P < 0.01 as compared to control, *P < 0.01 as compared to myelin; one-way ANOVA followed by Games-Howell post-hoc; data presented as means ± standard deviation.
Download figure:
Standard image High-resolution image4. Conclusion
In this study, we found that activation of rat BMDM adiponectin receptors with AdipoRon both inhibited myelin lipid uptake and promoted lipid efflux from myelin-laden rat BMDM foam cells in a dose-dependent manner. We also found that AdipoRon treatment significantly attenuated myelin-induced aggravation of BMDM inflammatory responses to classic inflammatory stimuli LPS/IFNγ, establishing AdipoRon as a promising drug candidate for the treatment of SCI. Next, we developed an injectable drug delivery system capable of sustained local delivery of bioactive AdipoRon at physiologically relevant doses.
Notably, because peripheral macrophages do not invade the spinal cord for 3–7 d after injury [8], AdipoRon treatment targeting both myelin debris uptake and efflux can be initiated days after the initial trauma without compromising therapeutic benefit. This makes our treatment strategy a highly attractive candidate for clinical translation. Future studies are thus necessitated to test the efficacy of this drug delivery system in animal models of SCI. Such studies should focus on fine-tuning parameters of the drug delivery system formulations to yield physiological concentrations of drug in injured spinal cord tissue. These parameters may not perfectly match our previous estimate due to factors such as AdipoRon pharmacokinetics, which currently have not been closely studied. Upon optimization of in vivo release, further studies are required to evaluate the effects of local delivery of AdipoRon on lipid accumulation in macrophages, chronic inflammation, tissue pathology and functional outcomes after SCI.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Funding statement
RBS was funded by the USA Department of Education's Graduate Assistance in Areas of National Need (GAANN) Program.
Conflict of interest
The authors declare no conflicts of interest.
Supplementary data (0.1 MB PDF)