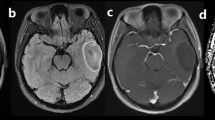
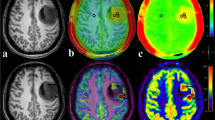
Abstract
Purpose
Currently, there remains a scarcity of established preoperative tests to accurately predict the isocitrate dehydrogenase (IDH) mutation status in clinical scenarios, with limited research has explored the potential synergistic diagnostic performance among metabolite, perfusion, and diffusion parameters. To address this issue, we aimed to develop an imaging protocol that integrated 2-hydroxyglutarate (2HG) magnetic resonance spectroscopy (MRS) and intravoxel incoherent motion (IVIM) by comprehensively assessing metabolic, cellular, and angiogenic changes caused by IDH mutations, and explored the diagnostic efficiency of this imaging protocol for predicting IDH mutation status in clinical scenarios.
Methods
Patients who met the inclusion criteria were categorized into two groups: IDH-wild type (IDH-WT) group and IDH-mutant (IDH-MT) group. Subsequently, we quantified the 2HG concentration, the relative apparent diffusion coefficient (rADC), the relative true diffusion coefficient value (rD), the relative pseudo-diffusion coefficient (rD*) and the relative perfusion fraction value (rf). Intergroup differences were estimated using t-test and Mann–Whitney U test. Finally, we performed receiver operating characteristic (ROC) curve and DeLong’s test to evaluate and compare the diagnostic performance of individual parameters and their combinations.
Results
64 patients (female, 21; male, 43; age, 47.0 ± 13.7 years) were enrolled. Compared with IDH-WT gliomas, IDH-MT gliomas had higher 2HG concentration, rADC and rD (P < 0.001), and lower rD* (P = 0.013). The ROC curve demonstrated that 2HG + rD + rD* exhibited the highest areas under curve (AUC) value (0.967, 95%CI 0.889–0.996) for discriminating IDH mutation status. Compared with each individual parameter, the predictive efficiency of 2HG + rADC + rD* and 2HG + rD + rD* shows a statistically significant enhancement (DeLong’s test: P < 0.05).
Conclusions
The integration of 2HG MRS and IVIM significantly improves the diagnostic efficiency for predicting IDH mutation status in clinical scenarios.



Similar content being viewed by others
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Miller JJ, Gonzalez Castro LN, McBrayer S et al (2022) Isocitrate dehydrogenase (IDH) mutant gliomas: a Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro Oncol 25:4. https://doi.org/10.1093/neuonc/noac207
Cahill DP (2021) Extent of resection of glioblastoma: a critical evaluation in the molecular era. Neurosurg Clin N Am 32:23–29. https://doi.org/10.1016/j.nec.2020.09.006
Suh CH, Kim HS, Jung SC et al (2018) 2-hydroxyglutarate MR spectroscopy for prediction of isocitrate dehydrogenase mutant glioma: a systemic review and meta-analysis using individual patient data. Neuro Oncol 20:1573–1583. https://doi.org/10.1093/neuonc/noy113
Jain R, Johnson DR, Patel SH et al (2020) “Real world” use of a highly reliable imaging sign: “T2-FLAIR mismatch” for identification of IDH mutant astrocytomas. Neuro Oncol 22:936–943. https://doi.org/10.1093/neuonc/noaa041
Cindil E, Sendur HN, Cerit MN et al (2022) Prediction of IDH mutation status in high-grade gliomas using DWI and high T1-weight DSC-MRI. Acad Radiol 29(Suppl 3):S52–S62. https://doi.org/10.1016/j.acra.2021.02.002
Nuessle NC, Behling F, Tabatabai G et al (2021) ADC-based stratification of molecular glioma subtypes using high b-value diffusion-weighted imaging. J Clin Med 10:3541. https://doi.org/10.3390/jcm10163451
Li S, Zheng Y, Sun W et al (2021) Glioma grading, molecular feature classification, and microstructural characterization using MR diffusional variance decomposition (DIVIDE) imaging. Eur Radiol 31:8197–8207. https://doi.org/10.1007/s00330-021-07959-x
van Santwijk L, Kouwenberg V, Meijer F et al (2022) A systematic review and meta-analysis on the differentiation of glioma grade and mutational status by use of perfusion-based magnetic resonance imaging. Insights Imaging 13:102. https://doi.org/10.1186/s13244-022-01230-7
Alvarez-Torres MDM, Fuster-Garcia E, Juan-Albarracin J et al (2022) Local detection of microvessels in IDH-wildtype glioblastoma using relative cerebral blood volume: an imaging marker useful for astrocytoma grade 4 classification. BMC Cancer 22:40. https://doi.org/10.1186/s12885-021-09117-4
Federau C, Meuli R, O’Brien K et al (2014) Perfusion measurement in brain gliomas with intravoxel incoherent motion MRI. AJNR Am J Neuroradiol 35:256–62. https://doi.org/10.3174/ajnr.A3686
Togao O, Hiwatashi A, Yamashita K et al (2016) Differentiation of high-grade and low-grade diffuse gliomas by intravoxel incoherent motion MR imaging. Neuro Oncol 18:132–41. https://doi.org/10.1093/neuonc/nov147
Lu J, Li X, Li H (2021) Perfusion parameters derived from MRI for preoperative prediction of IDH mutation and MGMT promoter methylation status in glioblastomas. Magn Reson Imaging 83:189–195. https://doi.org/10.1016/j.mri.2021.09.005
Bhandari A, Sharma C, Ibrahim M et al (2021) The role of 2-hydroxyglutarate magnetic resonance spectroscopy for the determination of isocitrate dehydrogenase status in lower grade gliomas versus glioblastoma: a systematic review and meta-analysis of diagnostic test accuracy. Neuroradiology 63:1823–1830. https://doi.org/10.1007/s00234-021-02702-1
Cuccarini V, Antelmi L, Pollo B et al (2019) In vivo 2-hydroxyglutarate-proton magnetic resonance spectroscopy (3 T, PRESS technique) in treatment-naive suspect lower-grade gliomas: feasibility and accuracy in a clinical setting. Neurol Sci 41:347. https://doi.org/10.1007/s10072-019-04087-9
Andronesi OC, Rapalino O, Gerstner E et al (2013) Detection of oncogenic IDH1 mutations using magnetic resonance spectroscopy of 2-hydroxyglutarate. J Clin Invest 123:3659–63. https://doi.org/10.1172/JCI67229
de la Fuente MI, Young RJ, Rubel J et al (2016) Integration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro Oncol 18:283–90. https://doi.org/10.1093/neuonc/nov307
Suh CH, Kim HS, Paik W et al (2019) False-positive measurement at 2-hydroxyglutarate MR spectroscopy in isocitrate dehydrogenase wild-type glioblastoma: a multifactorial analysis. Radiology 291:752–762. https://doi.org/10.1148/radiol.2019182200
Gatenby R, Grove O, Gillies R (2013) Quantitative imaging in cancer evolution and ecology. Radiology 269:8–15. https://doi.org/10.1148/radiol.13122697
Tatekawa H, Hagiwara A, Uetani H et al (2020) Multiparametric MR-PET measurements in hypermetabolic regions reflect differences in molecular status and tumor grade in treatment-naive diffuse gliomas. J Neurooncol 149:337–346. https://doi.org/10.1007/s11060-020-03613-6
Bumes E, Wirtz FP, Fellner C et al (2020) Non-invasive prediction of IDH mutation in patients with glioma WHO II/III/IV based on F-18-FET PET-guided in vivo (1)H-magnetic resonance spectroscopy and machine learning. Cancers (Basel) 12:3406. https://doi.org/10.3390/cancers12113406
Jezek P (2020) 2-hydroxyglutarate in cancer cells. Antioxid Redox Signal 33:903–926. https://doi.org/10.1089/ars.2019.7902
Kayabolen A, Yilmaz E, Bagci-Onder T (2021) IDH mutations in glioma: double-edged sword in clinical applications? Biomedicines 9:799. https://doi.org/10.3390/biomedicines9070799
Suh CH, Kim HS, Jung SC et al (2018) Imaging prediction of isocitrate dehydrogenase (IDH) mutation in patients with glioma: a systemic review and meta-analysis. European Radiology 29:745–758. https://doi.org/10.1007/s00330-018-5608-7
Choi C, Ganji S, Hulsey K et al (2013) A comparative study of short- and long-TE (1)H MRS at 3 T for in vivo detection of 2-hydroxyglutarate in brain tumors. NMR Biomed 26:1242–50. https://doi.org/10.1002/nbm.2943
Suh CH, Kim HS, Park JE et al (2020) Comparative value of 2-hydroxyglutarate-to-lipid and lactate ratio versus 2-hydroxyglutarate concentration on MR spectroscopic images for predicting isocitrate dehydrogenase mutation status in gliomas. Radiol Imaging Cancer 2:e190083. https://doi.org/10.1148/rycan.2020190083
Pope WB, Prins RM, Albert Thomas M et al (2012) Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol 107:197–205. https://doi.org/10.1007/s11060-011-0737-8
Bindra RS, Glazer PM (2005) Genetic instability and the tumor microenvironment: towards the concept of microenvironment-induced mutagenesis. Mutat Res 569:75–85. https://doi.org/10.1016/j.mrfmmm.2004.03.013
Lee S, Choi SH, Ryoo I et al (2015) Evaluation of the microenvironmental heterogeneity in high-grade gliomas with IDH1/2 gene mutation using histogram analysis of diffusion-weighted imaging and dynamic-susceptibility contrast perfusion imaging. Journal of Neuro-Oncology 121:141–150. https://doi.org/10.1007/s11060-014-1614-z
Choi C, Raisanen JM, Ganji SK et al (2016) Prospective longitudinal analysis of 2-hydroxyglutarate magnetic resonance spectroscopy identifies broad clinical utility for the Management of Patients with IDH-mutant glioma. J Clin Oncol 34:4030–4039. https://doi.org/10.1200/jco.2016.67.1222
Nagashima H, Tanaka K, Sasayama T et al (2016) Diagnostic value of glutamate with 2-hydroxyglutarate in magnetic resonance spectroscopy for IDH1 mutant glioma. Neuro Oncol 18:1559–1568. https://doi.org/10.1093/neuonc/now090
Tietze A, Choi C, Mickey B et al (2018) Noninvasive assessment of isocitrate dehydrogenase mutation status in cerebral gliomas by magnetic resonance spectroscopy in a clinical setting. J Neurosurg 128:391–398. https://doi.org/10.3171/2016.10.JNS161793
Bertolino N, Marchionni C, Ghielmetti F et al (2014) Accuracy of 2-hydroxyglutarate quantification by short-echo proton-MRS at 3 T: a phantom study. Phys Med 30:702–7. https://doi.org/10.1016/j.ejmp.2014.03.002
Askari P, Dimitrov IE, Ganji SK et al (2021) Spectral fitting strategy to overcome the overlap between 2-hydroxyglutarate and lipid resonances at 2.25 ppm. Magn Reson Med 86:1818–1828. https://doi.org/10.1002/mrm.28829
Li X, Strasser B, Jafari-Khouzani K et al (2020) Super-resolution whole-brain 3D MR spectroscopic imaging for mapping D-2-hydroxyglutarate and tumor metabolism in isocitrate dehydrogenase 1-mutated human gliomas. Radiology 294:589–597. https://doi.org/10.1148/radiol.2020191529
Tiwari V, Mashimo T, An Z et al (2020) In vivo MRS measurement of 2-hydroxyglutarate in patient-derived IDH-mutant xenograft mouse models versus glioma patients. Magn Reson Med 84:1152–1160. https://doi.org/10.1002/mrm.28183
Zhang J, Peng H, Wang YL et al (2021) Predictive role of the apparent diffusion coefficient and MRI morphologic features on IDH status in patients with diffuse glioma: a retrospective cross-sectional study. Front Oncol 11:640738. https://doi.org/10.3389/fonc.2021.640738
Du N, Zhou X, Mao R et al (2022) Preoperative and noninvasive prediction of gliomas histopathological grades and IDH molecular types using multiple MRI characteristics. Frontiers in Oncology 12:873839. https://doi.org/10.3389/fonc.2022.873839
Liu D, Gao S-X, Liao H-F et al (2020) A comparative study of 2 different segmentation methods of ADC histogram for differentiation genetic subtypes in lower-grade diffuse gliomas. BioMed Res Int 2020:1–13. https://doi.org/10.1155/2020/9549361
Koivunen P, Lee S, Duncan CG et al (2012) Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 483:484–8. https://doi.org/10.1038/nature10898
Le Bihan D (2019) What can we see with IVIM MRI? Neuroimage 187:56–67. https://doi.org/10.1016/j.neuroimage.2017.12.062
van der Voort SR, Incekara F, Wijnenga MMJ et al (2022) Combined molecular subtyping, grading, and segmentation of glioma using multi-task deep learning. Neuro Oncol. https://doi.org/10.1093/neuonc/noac166
Szczepankiewicz F, van Westen D, Englund E et al (2016) The link between diffusion MRI and tumor heterogeneity: mapping cell eccentricity and density by diffusional variance decomposition (DIVIDE). Neuroimage 142:522–532. https://doi.org/10.1016/j.neuroimage.2016.07.038
Watkins S, Robel S, Kimbrough IF et al (2014) Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun 5:4196. https://doi.org/10.1038/ncomms5196
Moteki T, Horikoshi H (2011) Evaluation of noncirrhotic hepatic parenchyma with and without significant portal vein stenosis using diffusion-weighted echo-planar MR on the basis of multiple-perfusion-components theory. Magn Reson Imaging 29:64–73. https://doi.org/10.1016/j.mri.2010.07.008
Funding
This work was supported by National Natural Science Foundation of China (21927808, 81702451, 81930048).
Author information
Authors and Affiliations
Contributions
Huicong Shen contributed to the study conception and design.
All authors contributed to material preparation, data collection.
Meimei Yu and Ying Ge contributed to data analysis and interpretation.
The first draft of the manuscript was written by Meimei Yu and Ying Ge, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This is an observational study. The Beijing Tiantan Hospital Research Ethics Committee has confirmed that no ethical approval is required.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 1.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, M., Ge, Y., Wang, Z. et al. The diagnostic efficiency of integration of 2HG MRS and IVIM versus individual parameters for predicting IDH mutation status in gliomas in clinical scenarios: A retrospective study. J Neurooncol 167, 305–313 (2024). https://doi.org/10.1007/s11060-024-04609-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-024-04609-2