Abstract
This brief review article was conducted to summarize the findings regarding correlation and agreement between different methods to assess muscle stiffness (shear wave elastography (SWE), myotonometry, and passive joint stiffness measurements). Muscle stiffness, an important biomechanical characteristic, influences joint flexibility, postural stability, injury risk, and athletic performance. SWE provides insights into tissue elasticity by measuring the propagation speed of shear waves, while myotonometry assesses stiffness through induced muscle oscillations. Passive joint stiffness measurements offer a holistic perspective, capturing the resistance of the entire joint to movement. However, distinguishing the contributions of muscular and non-muscular tissues remains a challenge in this method. The article highlights the variability in the correlation between these methodologies, influenced by factors such as muscle length, age, and examiner technique. While some studies report good agreement between SWE and myotonometry, others note discrepancies, underscoring the need for careful method selection based on the research or clinical context. This review highlights the complexity of assessing muscle stiffness and the necessity of a nuanced approach in interpreting data from different measurement techniques, aiming to guide researchers and clinicians in their choice of method for a precise and accurate evaluation of muscle stiffness.
Similar content being viewed by others
Introduction
In this brief review, we focus on muscle stiffness, a key biomechanical characteristic that influences musculoskeletal function. We critically examine and compare the methodologies and findings of shear-wave elastography (SWE), myotonometry, and passive joint stiffness measurements. This comparison aims to provide insights into the correlation and agreement between these different assessment methods, highlighting their implications in both research and clinical settings. Muscle stiffness, a pivotal biomechanical characteristic, plays an integral role in bodily functions ranging from joint flexibility [1] and postural balance and is possibly linked to musculoskeletal injuries [2, 3] and athletic performance [4]. Its assessment is crucial in both clinical diagnostics and rehabilitation, as well as in sports science research. The concept of stiffness is based on Hooke’s law, which states that the force required to deform an object is directly related to a constant value (spring constant) and the amount of deformation [5, 6]. Simply put, stiffness describes how an object responds to an applied force by measuring its deformation [7]. In relation to the human body, stiffness refers to the biomechanical characteristic of the tissue and explains its resistance to contraction or an external force that deforms its original shape [8]. Muscle stiffness, referred to as passive or active, is the muscle’s ability to maintain its initial structure against external forces to prevent deformities [9]. Passive muscle stiffness (i.e., muscle stiffness at rest) is influenced by three main factors: the predominant type of muscle fibers, muscle length, and the quantity of collagenous tissue in the muscle [10,11,12,13]. These are different from the factors that determine active muscle stiffness (i.e., muscle stiffness during contraction), which depends on the level of motor unit recruitment, length and force, and movement history [14, 15]. Muscle stiffness is known to influence joint flexibility, postural balance, and the risk of sustaining injuries [16,17,18]. Furthermore, an appropriate level of lower extremity stiffness is required for achieving optimal performance in activities like running, jumping, and hopping [19].
Muscle or tendon stiffness measurement serves several valuable purposes: it can help detect pathological changes, monitor rehabilitation program efficacy, indirectly evaluate athletic performance, and can be also used for research purposes to compare the effectiveness of different interventions. For example, decreased stiffness of the Achilles tendon may indicate an increased risk for Achilles tendinopathy [20]. Furthermore, stiffness of the tensor fascia latae may be useful to track the progression of rehabilitation of iliotibial band syndrome [21]. Regarding athletic performance, sufficient leg stiffness can help an athlete store more elastic energy at landing and generate more concentric force output at push of, which is beneficial for both, rapid stretch–shortening cycle activities, as well as for actions involving high speed of movement [7, 22]. For instance, resting stiffness of the medial gastrocnemius (MG) muscle can be considered as one of the factors that influence drop jump performance [14]. In summary, the optimal level of stiffness seems to depend on the muscle group, population, and context.
Various non-invasive methods can be used to assess muscle or tendon stiffness, including ultrasound SWE [23, 24], myotonometry [25], and passive joint torque measurements [26]. Briefly, SWE quantifies shear modulus (an estimate of soft tissue elasticity or stiffness) by measuring the propagation velocity of ultrasound-induced shear-waves in tissue [23, 24]. An alternative method is myotonometry, which is less expensive than SWE and requires less technical expertise for the assessment of muscle mechanical properties [27, 28]. Myotonometry measurements can be taken with the MyotonPRO, a non-invasive handheld device which generates an oscillation in soft tissues resulting in a calculation of the mechanical properties of the tissues [20]. In addition, muscle/tendon stiffness can be assessed by measuring torque–angle relationships of joints, because passive joint torque is influenced by musculotendinous structures around the joint [29, 30]. Nevertheless, it remains uncertain whether these methods can be used interchangeably. A recent investigation using phantom models reported a good correlation (r = 0.75 – 0.98) among various methods for stiffness assessment [31]. However, in vivo human studies produced mixed results; some studies reported good correlation between SWE and myotonometry measurements [32, 33], while others did not [34]. A recent study by Voglar et al. [35] compared SWE with passive joint torque measurement and found SWE to be more sensitive to intra-muscular changes in hamstring muscles, induced by eccentric exercise.
In summary, SWE enables direct measurements of muscle stiffness in selected regions of interest. Myotonometry, a more accessible and less technical approach, measures muscle oscillations to determine stiffness but is limited to superficial tissues. Passive joint stiffness measurements offer a holistic perspective, integrating the resistance of the entire joint complex. Each method has distinct advantages and applications, making their comparison essential for selecting the most appropriate technique based on specific research or clinical requirements. Our paper aims to provide an overview of the studies that explored the correlation between the results of SWE, myotonometry, and passive joint torque measurements and whether they respond similarly to different interventions. Understanding the agreement and correlation between different methods for assessing biomechanical properties is imperative for enhancing the precision and reliability of clinical evaluations and interventions.
Methodology
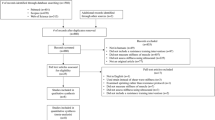
A comprehensive literature search was conducted to identify studies that focused on the agreement and correlation between SWE, myotonometry, and passive joint stiffness measurements. Such review of existing research will help to better understand and compare the different methods used to assess muscle stiffness. Databases including PubMed, Scopus, and Google Scholar were searched up to July 2023. The search terms used were a combination of keywords and MeSH terms such as “shear-wave elastography,” “myotonometry,” “passive joint stiffness,” “correlation,” and “agreement.” In addition, reference lists of included articles were searched. Studies were included based on the following eligibility criteria: (a) original research articles published in peer-reviewed journals; (b) studies that compared at least two of the measurement methods: SWE, myotonometry, and passive joint stiffness; and (c) studies published in English. Data extracted from each study included author names, year of publication, measurement methods compared, key findings, and limitations. Given the heterogeneity of the studies, only a narrative synthesis was conducted to summarize the findings.
Relationship Between Shear-Wave Elastography and Passive Joint Stiffness Measurements
The relationship between muscle stiffness measured via SWE and passive joint stiffness has yielded inconsistent results across studies. Several factors seem to influence this correlation, including the specific joint measured, its position, age, and normalization of joint stiffness by body mass.
Most studies we reviewed investigated the shear modulus of the triceps surae muscle in relation to passive ankle stiffness. Chino et al. [36] found no significant correlation between the shear modulus of the MG (muscle located at the back part of the lower leg) and passive ankle stiffness. However, in their earlier study, a positive correlation was observed when the ankle was in dorsal flexion (r = 0.40), but not in plantar flexion [37]. This was confirmed by Hirata et al. [38] who found a positive correlation between ankle stiffness and shear modulus of MG (r = 0.45) and soleus (SO) (r = 0.51) in a dorsiflexed position. This correlation was not present in a neutral position of the ankle, suggesting that shear modulus can reflect ankle joint stiffness when substantially stretched, while its shear modulus in a more slacked position is not related to passive joint stiffness in a corresponding region of range of motion. Furthermore, the correlation was not observed in older adults, which can be a consequence of aging. With aging the muscle mass decreases and the quantity and quality of non-muscular tissues change. This could be the reason that the material properties of the muscles themselves contribute less to the joint stiffness. It is important to add that the authors also calculated the body-mass-normalized passive ankle stiffness and found that it correlated well with the shear modulus of the triceps surae muscles in dorsal flexion (r = 0.46–0.56) as well in the neutral position (r = 0.47–0.51), both in younger and older adults [38]. This may be due to the normalization of body mass, as the absolute passive joint stiffness is a size-dependent measure, while the shear modulus represents a size-independent material property. Along with MG and Achilles tendon (AT) elasticity expressed with Young’s modulus, Chino et al. [39] found a significant correlation between stiffness indices (estimated from muscle belly and muscle–tendon unit changes during passive DF) and passive ankle stiffness. However, no correlation was observed with Young’s modulus of MG or AT. The lack of this correlation may have resulted from a narrower range of values (reflected in lower coefficient of variation) for tissue elasticity as compared with those of joint stiffness. Maisetti et al. [40] reported a strong correlation between muscle shear modulus-length and force–length relationships, suggesting a parallel change in shear modulus and passive muscle force during stretching.
Other studies explored the shear modulus of hamstrings in relation to passive hip stiffness. While Miyamoto et al. [41] found a positive correlation between shear modulus of each hamstring muscle and both absolute and normalized hip stiffness (r = 0.71–0.75), Voglar et al. [35] found contradicting results. They explored the effects of eccentric exercise on passive hamstring muscle stiffness and found that the changes in shear modulus of neither of the hamstrings were in correlation with the changes in passive hip torque or passive joint stiffens. One possible explanation is that the relationship between passive torque measures and shear modulus could be angle-specific. This inconsistency might stem from angle-specific relationships between passive torque and shear modulus, as suggested by Xu et al. [42].
Overall, the research underscores that numerous factors influence the correlation between shear modulus and passive joint stiffness. This relationship seems to be different in older adults and is influenced by muscle length and normalization by body mass. Further research is warranted to understand these dynamics across different muscle groups and joints, especially beyond the triceps surae. From the practical standpoint, the research suggests that shear modulus changes in parallel with passive muscle force during a stretch, and that shear modulus assessed at longer muscle length is associated with passive joint stiffness in the corresponding range of motion. However, shear modulus scores obtained at neutral or slack muscle lengths seem to be related to passive joint stiffness.
Relationship Between Myotonometry and Passive Joint Stiffness
Only three studies have assessed the relationship between myotonometry and passive joint stiffness assessment, with two conducted in patient populations. Rydahl et al. [43] included 23 stroke survivors (67.5 ± 10.9 years) and 24 control subjects (71.2 ± 9.0 years) for gastrocnemius stiffness. The stroke patients exhibited higher passive stiffness and dynamic stiffness (myotonometry). The methodology allowed the authors to conclude that myotonometric measurements were particularly reflective of intrinsic (but not reflexive) muscle stiffness. Li et al. [44] included 14 subjects with hemiplegia (61 ± 10 years) and assessed biceps brachii dynamic stiffness (myotonometry), as well as passive elbow flexor torque. Statistically significant correlations were observed between passive stiffness and dynamic stiffness (r = 0.55), indicating high sensitivity of the myotonometry to the detection of spasticity and provided a validation of the technique. Finally, Tennant et al. [45] reported no relationship between passive mechanical stiffness of the lumbar spine and myotonometry-based outcomes (neither for erector spinae muscle and supraspinous ligament). In addition, neither passive mechanical stiffness nor the lumbar spine and myotonomy-based outcomes correlated with clinical measures of stiffness, such as “passive intervertebral motion test” and “posteroanterior spring test”.
In summary, recent research efforts that combined myotonometry and passive joint stiffness assessment have produced mixed outcomes. While some studies have found correlations between passive joint stiffness and dynamic stiffness, highlighting the potential of myotonometry in detecting intrinsic muscle stiffness and spasticity in plantar flexors and elbow flexors, others reported no clear relationship in the lumbar spine region. These discrepancies suggest that the applicability of myotonometry might vary based on the specific anatomical context and population in question.
Relationship Between Shear-Wave Elastography and Myotonometry
Previous research has probed the relationship between dynamic stiffness (measured with a myotonometer) and shear modulus both at rest and during muscle contraction. Although various muscle groups have been assessed, the majority of studies concentrate on the lower leg. Feng et al. [46] reported moderate correlations between stiffness indices from MyotonPRO and Young’s modulus for MG (r = 0.46), lateral gastrocnemius (LG) (r = 0.54), and AT (r = 0.54). These correlations were confirmed by Lee et al. [33] with similar correlation coefficients for MG (r = 0.669 and 0.551) and tibialis anterior (TA) (r = 0.56; 0.540) while resting and during contraction, respectively. Furthermore, Kelly et al. [47] found significant correlations between shear modulus and dynamic stiffness for gastrocnemius at 40% maximal voluntary contraction (MVC) (r = 0.54) and 80% MVC (r = 0.55). Comparing young adults to elderly, Do et al. [48] found moderate to strong correlations for both groups at rest (MG, r = 0.41–0.63; TA, r = 0.48–0.56) and during contraction (MG, r = 0.46–0.47; TA, r = 0.636), with an exception of TA in elderly. Khowailed et al. [32] assessed the differences in shear modulus and dynamic stiffness during the menstrual cycle in young women. Shear modulus of MG and TA correlated well with myotonometer measurement in the follicular phase, both while resting (TA, r = 0.56; MG, r = 0.78) as well as during muscle contractions (TA, r = 0.42; MG, r = 0.56). During ovulation, there was a significant correlation between MG shear modulus and dynamic stiffness in resting position (r = 0.48); however, this correlation was not significant for the TA. Furthermore, no relationships between SWE and myotonometry outcomes were found during contraction in this phase.
Researching the relationship of the shear modulus and dynamic stiffness of vastus lateralis (VL), Bravo-Sanchez et al. [27] found no correlation, suggesting that SWE assesses a different type of stiffness from the MyotonPRO. Contradicting results were observed by Lee et al. [33] who reported small to moderate correlations between the shear modulus of rectus femoris and its dynamic stiffness, both at rest and during contraction (r = 0.39–0.42). The same authors reported even stronger correlations between the shear modulus and dynamic stiffness for biceps femoris, both at rest and during contraction (r = 0.59–0.65).
Furthermore, studies have assessed the relationship between shear modulus and myotonometry in various muscles of the back and rotator cuff. While Kelly et al. [47] found a small to moderate association between the two methods in the infraspinatus at 80% MVC (r = 0.37) and erector spinae at 80% MVC (r = 0.54), Pimentel-Santos et al. [34] did not report statistically significant correlations for the lumbar muscles. Furthermore, the changes in shear modulus of the upper trapezius did not correlate with the changes in muscle dynamic stiffness after eccentric exercise [28]. Lastly, Tantipoon et al. [49] found significant correlations between elasticity and dynamic stiffness for the flexor digitorum superficialis muscle at rest (r = 0.489) and 40% MVC (r = 0.479), but not at 60 and 80% of MVC. The authors pointed out that certain ultrasound systems face challenges in detecting shear waves in muscles with higher stiffness, particularly near maximal voluntary contraction levels. This limitation highlights the importance of carefully selecting the ultrasound system for such evaluations.
Studies focused on the lower leg muscles consistently indicate that both SWE and myotonometry are effective tools for evaluating muscle stiffness at rest and during contraction. Notably, the correlation between these methods appears to diminish during the ovulation phase. For other muscle groups, conclusive evidence regarding the correlation between the two methods is still needed. Additionally, future investigations should prioritize identifying the most suitable ultrasound systems for SWE measurements, especially since some systems might struggle to detect shear waves in highly stiffened muscles.
Discussion
The relationship between SWE, myotonometry, and passive joint stiffness has been explored in various studies, yielding a mix of consistent and inconsistent results. SWE measures muscle stiffness and has shown a correlation with passive joint stiffness, particularly in the triceps surae muscle and during ankle dorsiflexion. However, this correlation varies with joint position, age, and body mass normalization. Older adults and slack muscle positions show less correlation, underscoring the influence of aging and muscle length. Myotonometry, another tool for assessing muscle stiffness, has produced mixed outcomes in relation to passive joint stiffness. While it has shown potential in detecting intrinsic muscle stiffness and spasticity in specific muscle groups, its applicability seems to vary across different anatomical regions and populations. Comparatively, studies have also investigated the relationship between SWE and myotonometry, predominantly focusing on the lower leg muscles. Consistent correlations have been found between these two methods, especially at rest and during muscle contraction. However, the correlation appears to diminish during the ovulation phase and in highly stiffened muscles, indicating the need for careful selection of ultrasound systems for SWE measurements. Overall, while these tools offer valuable insights into muscle stiffness and joint stiffness, the interplay between them is complex and influenced by various factors. Future research is essential to further unravel these dynamics across different muscle groups, joints, and populations, and to identify the most suitable tools and techniques for specific assessments.
When it comes to assessing muscle-specific stiffness, SWE and myotonometry stand out as prominent techniques, each with its unique approach and underlying principles. SWE measures the elasticity of tissues by generating shear waves within the muscle and then capturing the speed at which these waves travel [23, 24, 50]. The speed of shear waves is directly related to the stiffness of the tissue (i.e., faster waves indicate stiffer tissues). However, SWE is influenced by the anisotropy of muscle tissue (how the muscle properties differ along the fibers compared to across them [51]). In myotonometry, the device administers a controlled tap to the muscle, instigating oscillations or vibrations. The speed at which the muscle returns to its resting state after this mechanical impulse provides a measure of its stiffness. The discrepancy between SWE and myotonometry may stem from several different factors. Notably, SWE typically involves an assessment of muscle stiffness in a broader region of interests [52], while myotonometry results reflect primarily the stiffness of the superficial portion of the muscle. Despite this difference, promising results showing agreement between the two methods have emerged for lower limb muscles [33, 47]. Several factors should be considered when interpreting the agreement between the methods. For instance, skin and subcutaneous fat content and their mechanical properties may largely influence the validity of myotonometry [53]. That being said, a recent study indicated that SWE outcomes are also potentially influenced by subcutaneous fat [54]. Additionally, the technique employed by the examiner, including the applied pressure on the probe or myotonometry device, could variably influence the stiffness outcomes in each method. In SWE, placing the probe in direct contact with the skin using minimal pressure results in optimal reliability [55]. In addition, clinicians must be aware of a large influence of muscle length on stiffness measurement. Following the logic of length-tension relationship, a clear increase in shear modulus is observed as the muscle is passively lengthened [56]. Furthermore, measurement of shear modulus at different muscle lengths shows different sensitivity to muscle damage caused by eccentric exercise [57]—for instance, knee extensors showed no response in shear modulus after eccentric exercise if assessed at short muscle length (30° knee angle), while a large increase was when the assessment was done with the muscles stretched (110° knee angle) [58]. Therefore, great caution in needed to standardize muscle length during clinical assessment of muscle stiffness with SWE. Future studies are needed to explore the effect of the abovementioned factors on agreement between SWE and myotonometry.
Passive joint stiffness measurements present an alternative avenue for inferring muscle stiffness. This method assesses the resistance of a joint to passive movement, capturing the combined stiffness of all tissues spanning the joint, including muscles, tendons, ligaments, and joint capsules [59]. While this approach provides a more holistic view of joint stiffness, elucidating the specific contributions of muscular versus non-muscular tissues presents a significant challenge. The complexity of joint anatomy means that multiple tissues contribute to the overall stiffness measured, and isolating the muscle component is too complicated for everyday practice. Nevertheless, muscles play a crucial role in joint stiffness, with their passive and active properties influencing the joint's resistance to movement. It seems that muscular contributions to passive joint torque increase in the range of motion where muscles are stretched [40]. Therefore, end-range passive joint stiffness seems to be a good proxy measure for muscle stiffness. However, understanding the muscle-specific changes in stiffness in the context of overall joint stiffness is paramount for accurate assessment and effective intervention strategies (e.g., targeting a particular muscle within rehabilitation and athletic training settings). To illustrate, SWE studies have shown that different muscles spanning the same joint and performing the same action show different responses to interventions such as eccentric exercise. For instance, biceps femoris, and semitendinosus but not semimembranosus stiffness was elevated after eccentric exercise for hamstrings [35]. In their study, Green et al. [30] observed an immediate elevation in the shear modulus of the soleus following a 15-min session of backward walking. However, this change was not sustained, as the shear modulus returned to baseline levels 48 h post-exercise. On the other hand, the shear modulus of the gastrocnemius exhibited a different pattern. While there was only a marginal increase noted immediately after the exercise, a statistically significant rise in the shear modulus was documented at the 48-h mark following the activity. Further studies have even shown divergent responses to eccentric exercise within different regions of the same muscle [60]. Ultimately, integrating insights from both passive joint stiffness measurements and advanced imaging techniques like SWE is crucial for a comprehensive assessment of stiffness.
Practical Application
The variability in correlation between SWE, myotonometry, and passive joint stiffness measurements has significant practical implications for both clinical decision-making and research design. Clinicians must recognize that each method may yield different stiffness values for the same muscle or joint. This can lead to variations in diagnosing conditions related to muscle stiffness and in formulating treatment plans. For instance, decisions regarding physiotherapy techniques, exercise prescriptions, and monitoring of recovery progress could be influenced by the chosen assessment method. Given the variability, it becomes crucial to tailor the stiffness assessment method to the specific clinical scenario. For example, SWE might be preferable in cases where deep muscle assessment is essential, while myotonometry could be more practical for routine monitoring of superficial muscle changes.
Researchers should carefully choose the stiffness assessment method based on the study’s objectives. Studies aiming to compare muscle stiffness across populations or interventions should consider using multiple methods to account for the variability in correlations. Researchers must be cautious when interpreting stiffness data, especially when comparing results across studies that use different methods. Acknowledging the methodological variability is essential in drawing accurate conclusions.
The choice of method should align with the specific objective of the assessment. For instance, SWE could be more suitable for detailed biomechanical studies, while myotonometry might suffice for preliminary clinical assessments. Factors such as age, muscle condition, and presence of pathology can influence the appropriateness of a method. SWE might be more sensitive in detecting subtle changes in muscle tissue properties, which could be crucial in elderly or pathological populations. The availability of resources, including equipment and technical expertise, can also dictate the choice of method. SWE requires specialized equipment and trained personnel, while myotonometry is more accessible and easier to use.
In summary, understanding the variability in correlations between different muscle stiffness assessment methodologies is crucial for informed clinical and research decisions. This understanding guides the selection of the most appropriate method, ensuring accurate assessment, diagnosis, and intervention in clinical settings, and robust design and interpretation in research studies.
Conclusion
In summarizing this short review, it becomes evident that the measurement of muscle stiffness necessitates a careful approach. SWE, myotonometry, and passive joint stiffness measurements each offer unique insights, yet they are not without their distinct challenges and limitations. The key takeaways of the paper are as follows: (1) SWE provides a robust quantification of tissue elasticity, yet is susceptible to the anisotropic nature of muscle tissue and the influence of subcutaneous fat; (2) Myotonometry, though user-friendly and less expensive, primarily reflects the stiffness of superficial muscle regions and can be affected by skin and subcutaneous tissue properties; (3) Passive joint stiffness measurements, while offering a more comprehensive view of joint resistance, fail to isolate muscular contributions from other tissues; and (4) the discrepancies among these methods highlighted in this review emphasize the necessity for careful selection and interpretation of measurement techniques.
Future research should focus on refining these methodologies, exploring their applicability across diverse populations and settings, and striving for a standardized approach to enhance the reliability and validity of muscle stiffness assessment. Such efforts will not only expand the current body of knowledge but also pave the way for technological innovations in the biomedical engineering landscape. When selecting a method for clinical muscle stiffness assessment, consider the specific context and the muscle or joint of interest. SWE is recommended for a detailed analysis of muscle tissue properties, particularly in cases where deep muscle assessment is crucial. Myotonometry is suitable for quick assessments and monitoring superficial muscle changes, especially in rehabilitation settings. Passive joint stiffness measurements are ideal for a holistic assessment of joint health, particularly in conditions affecting multiple joint structures.
Data Availability
No original data was generated for this manuscript.
Code Availability
Not applicable.
Abbreviations
- AT :
-
Achilles tendon
- LG :
-
Lateral gastrocnemius
- MG :
-
Medial gastrocnemius
- MVC :
-
Maximal voluntary contraction
- SWE :
-
Shear wave elastography
- SO :
-
Soleus
- TA :
-
Tibialis anterior
- VL :
-
Vastus lateralis
References
Morales-Artacho AJ, Lacourpaille L, Guilhem G. Effects of warm-up on hamstring muscles stiffness: cycling vs foam rolling. Scand J Med Sci Sport. 2017;27:1959–69. https://doi.org/10.1111/sms.12832.
Taş S, Korkusuz F, Erden Z. Neck muscle stiffness in participants with and without chronic neck pain: a shear-wave elastography study. J Manipulative Physiol Ther. 2018;41:580–8. https://doi.org/10.1016/j.jmpt.2018.01.007.
Vatovec R, Voglar M. Changes of trunk muscle stiffness in individuals with low back pain: a systematic review with meta-analysis. BMC Musculoskelet Disord. 2024;25:155. https://doi.org/10.1186/s12891-024-07241-3.
Burgess KE, Connick MJ, Graham-Smith P, Pearson SJ. Plyometric vs. isometric training influences on tendon properties and muscle output. J Strength Cond Res. 2007;21:986–9. https://doi.org/10.1519/R-20235.1.
Brughelli M, Cronin J. Influence of running velocity on vertical, leg and joint stiffness. Sport Med. 2008;38:647–57. https://doi.org/10.2165/00007256-200838080-00003.
Serpell BG, Ball NB, Scarvell JM, Smith PN. A review of models of vertical, leg, and knee stiffness in adults for running, jumping or hopping tasks. J Sports Sci. 2012;30:1347–63. https://doi.org/10.1080/02640414.2012.710755.
Brazier J, Bishop C, Simons C, Antrobus M, Read PJ, Turner AN. Lower extremity stiffness. Strength Cond J. 2014;36:103–12. https://doi.org/10.1519/SSC.0000000000000094.
Gavronski G, Veraksitš A, Vasar E, Maaroos J. Evaluation of viscoelastic parameters of the skeletal muscles in junior triathletes. Physiol Meas. 2007;28:625–37. https://doi.org/10.1088/0967-3334/28/6/002.
Stamenkovic A, Clark BC, Pidcoe PE, van der Veen SM, France CR, Russ DW, Kinser PA, Thomas JS. Distinguishing chronic low back pain in young adults with mild to moderate pain and disability using trunk compliance. Sci Rep. 2021;11:7592. https://doi.org/10.1038/s41598-021-87138-6.
Ducomps C, Mauriège P, Darche B, Combes S, Lebas F, Doutreloux JP. Effects of jump training on passive mechanical stress and stiffness in rabbit skeletal muscle: role of collagen. Acta Physiol Scand. 2003;178:215–24. https://doi.org/10.1046/j.1365-201X.2003.01109.x.
Hirata K, Yamadera R, Akagi R. Can static stretching reduce stiffness of the triceps surae in older men? Med Sci Sport Exerc. 2020;52:673–9. https://doi.org/10.1249/MSS.0000000000002186.
Kovanen V, Suominen H, Heikkinen E. Mechanical properties of fast and slow skeletal muscle with special reference to collagen and endurance training. J Biomech. 1984;17:725–35. https://doi.org/10.1016/0021-9290(84)90103-9.
Mutungi G, Ranatunga KW. The viscous, viscoelastic and elastic characteristics of resting fast and slow mammalian (rat) muscle fibres. J Physiol. 1996;496:827–36. https://doi.org/10.1113/jphysiol.1996.sp021730.
Ando R, Sato S, Hirata N, Tanimoto H, Imaizumi N, Suzuki Y, Hirata K, Akagi R. Relationship between drop jump training–induced changes in passive plantar flexor stiffness and explosive performance. Front Physiol. 2021;12. https://doi.org/10.3389/fphys.2021.777268.
Nichols TR, Huyghues-Despointes CMJI. Muscular stiffness. In Encyclopedia of Neuroscience; Springer Berlin Heidelberg: Berlin, Heidelberg, 2009;2515–2519.
Kumagai H, Miyamoto-Mikami E, Hirata K, Kikuchi N, Kamiya N, Hoshikawa S, Zempo H, Naito H, Miyamoto N, Fuku N. ESR1 Rs2234693 Polymorphism is associated with muscle injury and muscle stiffness. Med Sci Sport Exerc. 2019;51:19–26. https://doi.org/10.1249/MSS.0000000000001750.
Miyamoto N, Hirata K, Miyamoto-Mikami E, Yasuda O, Kanehisa H. Associations of passive muscle stiffness, muscle stretch tolerance, and muscle slack angle with range of motion: individual and sex differences. Sci Rep. 2018;8:8274. https://doi.org/10.1038/s41598-018-26574-3.
Nelson AG, Kokkonen J, Arnall DA, Li L. Acute stretching increases postural stability in nonbalance trained individuals. J Strength Cond Res. 2012;26:3095–100. https://doi.org/10.1519/JSC.0b013e3182430185.
Kuitunen S, Komi PV, Kyröläinen H. Knee and ankle joint stiffness in sprint running. Med Sci Sport Exerc. 2002;34:166–73. https://doi.org/10.1097/00005768-200201000-00025.
Morgan GE, Martin R, Williams L, Pearce O, Morris K. Objective assessment of stiffness in achilles tendinopathy: a novel approach using the MyotonPRO. BMJ Open Sport Exerc Med. 2018;4:e000446. https://doi.org/10.1136/bmjsem-2018-000446.
Friede MC, Klauser A, Fink C, Csapo R. Stiffness of the iliotibial band and associated muscles in runner’s knee: assessing the effects of physiotherapy through ultrasound shear wave elastography. Phys Ther Sport. 2020;45:126–34. https://doi.org/10.1016/j.ptsp.2020.06.015.
Ramírez-delaCruz M, Bravo-Sánchez A, Esteban-García P, Jiménez F, Abián-Vicén J. Effects of plyometric training on lower body muscle architecture, tendon structure, stiffness and physical performance: a systematic review and meta-analysis. Sport Med - Open. 2022;8:40. https://doi.org/10.1186/s40798-022-00431-0.
Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM, Gao L, Witte RS. Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics. 2017;37:855–70. https://doi.org/10.1148/rg.2017160116.
Creze M, Nordez A, Soubeyrand M, Rocher L, Maître X, Bellin MF. Shear wave sonoelastography of skeletal muscle: basic principles, biomechanical concepts, clinical applications, and future perspectives. Skeletal Radiol. 2018;47:457–71. https://doi.org/10.1007/s00256-017-2843-y.
Kong PW, Chua YH, Kawabata M, Burns SF, Cai C. Effect of post-exercise massage on passive muscle stiffness measured using myotonometry – a double-blind study. J Sport Sci Med. 2018;17:599–606.
Raastad T, Owe SG, Paulsen Gø, Enns D, Overgaard K, Crameri R, Kiil S, Belcastro A, Bergersen L, Hallén J. Changes in calpain activity, muscle structure, and function after eccentric exercise. Med Sci Sports Exerc. 2010;42:86–95. https://doi.org/10.1249/MSS.0b013e3181ac7afa.
Bravo-Sánchez A, Abián P, Sánchez-Infante J, Esteban-Gacía P, Jiménez F, Abián-Vicén J. Objective assessment of regional stiffness in vastus lateralis with different measurement methods: a reliability study. Sensors 2021;21. https://doi.org/10.3390/s21093213.
Kisilewicz A, Madeleine P, Ignasiak Z, Ciszek B, Kawczynski A, Larsen RG. Eccentric exercise reduces upper trapezius muscle stiffness assessed by shear wave elastography and myotonometry. Front Bioeng Biotechnol. 2020;8. https://doi.org/10.3389/fbioe.2020.00928.
Kawakami Y, Kanehisa H, Fukunaga T. The relationship between passive ankle plantar flexion joint torque and gastrocnemius muscle and achilles tendon stiffness: implications for flexibility. J Orthop Sport Phys Ther. 2008;38:269–76. https://doi.org/10.2519/jospt.2008.2632.
Green MA, Sinkus R, Gandevia SC, Herbert RD, Bilston LE. Measuring changes in muscle stiffness after eccentric exercise using elastography. NMR Biomed. 2012;25:852–8. https://doi.org/10.1002/nbm.1801.
Bartsch K, Brandl A, Weber P, Wilke J, Bensamoun SF, Bauermeister W, Klingler W, Schleip R. Assessing reliability and validity of different stiffness measurement tools on a multi-layered phantom tissue model. Sci Rep. 2023;13. https://doi.org/10.1038/s41598-023-27742-w.
Khowailed IA, Lee Y, Lee H. Assessing the differences in muscle stiffness measured with shear wave elastography and myotonometer during the menstrual cycle in young women. Clin Physiol Funct Imaging. 2022;42:320–6. https://doi.org/10.1111/cpf.12763.
Lee Y, Kim M, Lee H. The measurement of stiffness for major muscles with shear wave elastography and myoton: a quantitative analysis study. Diagnostics. 2021;11. https://doi.org/10.3390/diagnostics11030524.
Pimentel-Santos FM, Manica SR, Alfonse TM, Lagoas-Gomes J, Santos MB, Ramiro S, Sepriano A, Nair K, Costa J, Gomes-Alves P, et al. Lumbar myofascial physical properties in healthy adults: myotonometry vs. shear wave elastography measurements. Acta Reumatol Port. 2021;46:110–9.
Voglar M, Vatovec R, Kozinc Ž, Šarabon N. The effects of eccentric exercise on passive hamstring muscle stiffness: comparison of shear-wave elastography and passive knee torque outcomes. Eur J Transl Myol. 2022. https://doi.org/10.4081/ejtm.2022.10567.
Chino K, Takahashi H. Association of gastrocnemius muscle stiffness with passive ankle joint stiffness and sex-related difference in the joint stiffness. J Appl Biomech. 2018;34:169–74. https://doi.org/10.1123/jab.2017-0121.
Chino K, Takahashi H. Measurement of gastrocnemius muscle elasticity by shear wave elastography: association with passive ankle joint stiffness and sex differences. Eur J Appl Physiol. 2016;116:823–30. https://doi.org/10.1007/s00421-016-3339-5.
Hirata K, Akagi R. Contribution of muscle stiffness of the triceps surae to passive ankle joint stiffness in young and older adults. Front Physiol. 2022;13. https://doi.org/10.3389/fphys.2022.972755.
Chino K, Takahashi H. The association of muscle and tendon elasticity with passive joint stiffness: in vivo measurements using ultrasound shear wave elastography. Clin Biomech. 2015;30:1230–5. https://doi.org/10.1016/j.clinbiomech.2015.07.014.
Maïsetti O, Hug F, Bouillard K, Nordez A. Characterization of passive elastic properties of the human medial gastrocnemius muscle belly using supersonic shear imaging. J Biomech. 2012;45:978–84. https://doi.org/10.1016/j.jbiomech.2012.01.009.
Miyamoto N, Hirata K. Moderate associations of muscle elasticity of the hamstring with hip joint flexibility. Int J Sports Med. 2019;40:717–24. https://doi.org/10.1055/a-0981-7282.
Xu J, Hug F, Fu SN. Stiffness of individual quadriceps muscle assessed using ultrasound shear wave elastography during passive stretching. J Sport Heal Sci. 2018.
Rydahl SJ, Brouwer BJ. Ankle stiffness and tissue compliance in stroke survivors: a validation of myotonometer measurements. Arch Phys Med Rehabil. 2004;85:1631–7. https://doi.org/10.1016/j.apmr.2004.01.026.
Li X, Shin H, Li S, Zhou P. Assessing muscle spasticity with myotonometric and passive stretch measurements: validity of the myotonometer. Sci Rep. 2017;7. https://doi.org/10.1038/srep44022.
Tennant LM, Nelson-Wong E, Kuest J, Lawrence G, Levesque K, Owens D, Prisby J, Spivey S, Albin SR, Jagger K, et al. A comparison of clinical spinal mobility measures to experimentally derived lumbar spine passive stiffness. J Appl Biomech. 2020;36:397–407. https://doi.org/10.1123/JAB.2020-0030.
Feng YN, Li YP, Liu CL, Zhang ZJ. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Sci Rep. 2018;8. https://doi.org/10.1038/s41598-018-34719-7.
Kelly JP, Koppenhaver SL, Michener LA, Proulx L, Bisagni F, Cleland JA. Characterization of tissue stiffness of the infraspinatus, erector spinae, and gastrocnemius muscle using ultrasound shear wave elastography and superficial mechanical deformation. J Electromyogr Kinesiol. 2018;38:73–80. https://doi.org/10.1016/j.jelekin.2017.11.001.
Do Y, Lall PS, Lee H. Assessing the effects of aging on muscle stiffness using shear wave elastography and myotonometer. Healthc. 2021;9. https://doi.org/10.3390/healthcare9121733.
Tantipoon P, Praditpod N, Pakleppa M, Li C, Huang Z. Characterization of flexor digitorum superficialis muscle stiffness using ultrasound shear wave elastography and MyotonPRO: a cross-sectional study investigating the correlation between different approaches. Appl Sci. 2023;13. https://doi.org/10.3390/app13116384.
Snoj Ž, Wu CH, Taljanovic MS, Dumić-Čule I, Drakonaki EE, Klauser AS. Ultrasound elastography in musculoskeletal radiology: past, present, and future. Semin Musculoskelet Radiol. 2020;24:156–66. https://doi.org/10.1055/s-0039-3402746.
Gennisson J-L, Catheline S, Chaffaı̈ S, Fink M. Transient elastography in anisotropic medium: application to the measurement of slow and fast shear wave speeds in muscles. J Acoust Soc Am. 2003;114:536–41. https://doi.org/10.1121/1.1579008.
Ewertsen C, Carlsen JF, Christiansen IR, Jensen JA, Nielsen MB. Evaluation of healthy muscle tissue by strain and shear wave elastography - dependency on depth and ROI position in relation to underlying bone. Ultrasonics. 2016;71:127–33. https://doi.org/10.1016/j.ultras.2016.06.007.
Mencel J, Jaskólska A, Marusiak J, Kisiel-Sajewicz K, Siemiatycka M, Kaminski L, Jaskólski A. Effect of gender, muscle type and skinfold thickness on myometric parameters in young people. PeerJ. 2021;9. https://doi.org/10.7717/peerj.12367.
Yoshiko A, Ando R, Akima H. Passive muscle stiffness is correlated with the intramuscular adipose tissue in young individuals. Eur J Appl Physiol. 2023;In Print.
Alfuraih AM, O’Connor P, Hensor E, Tan AL, Emery P, Wakefield RJ. The effect of unit, depth, and probe load on the reliability of muscle shear wave elastography: variables affecting reliability of SWE. J Clin Ultrasound. 2018;46:108–15. https://doi.org/10.1002/jcu.22534.
Koo TK, Hug F. Factors that influence muscle shear modulus during passive stretch. J Biomech. 2015;48:3539–42. https://doi.org/10.1016/j.jbiomech.2015.05.038.
Ličen U, Kozinc Ž. Using shear-wave elastography to assess exercise-induced muscle damage: a review. Sensors. 2022;22. https://doi.org/10.3390/s22197574.
Lacourpaille L, Nordez A, Hug F, Doguet V, Andrade R, Guilhem G. Early Detection of exercise-induced muscle damage using elastography. Eur J Appl Physiol. 2017;117:2047–56. https://doi.org/10.1007/s00421-017-3695-9.
Amiri S, Cooke D, Kim IY, Wyss U. Mechanics of the passive knee joint. Part 2: interaction between the ligaments and the articular surfaces in guiding the joint motion. Proc Inst Mech Eng Part H J Eng Med. 2007;221:821–32.
Lacourpaille L, Nordez A, Hug F, Couturier A, Dibie C, Guilhem G. Time-course effect of exercise-induced muscle damage on localized muscle mechanical properties assessed using elastography. Acta Physiol. 2014;211:135–46. https://doi.org/10.1111/apha.12272.
Funding
The study was supported by the Slovenian Research Agency through the research program KINSPO—Kinesiology for the effectiveness and prevention of musculoskeletal injuries in sports (P5-0443).
Author information
Authors and Affiliations
Contributions
UL and ZK conceptualized the paper. UL and MO performed the literature review. UL and ZK constructed the basic outline of the manuscript and chapters. UL and MO wrote the first manuscript draft. All authors worked on finalizing the paper.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Declaration of originality: The authors declare that this article has not been published elsewhere and that it has not been simultaneously submitted for publication elsewhere. All materials are original work by the authors and no permissions are required.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ličen, U., Opara, M. & Kozinc, Ž. The Agreement and Correlation Between Shear-Wave Elastography, Myotonometry, and Passive Joint Stiffness Measurements: A Brief Review. SN Compr. Clin. Med. 6, 27 (2024). https://doi.org/10.1007/s42399-024-01658-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-024-01658-6