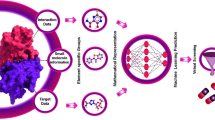
Abstract
Inflammation is a biological resistant response to harmful stimuli, such as infection, damaged cells, toxic chemicals, or tissue injuries. Inflammation eradicates pathogenic microorganisms or irritants and facilitates tissue repair. Prolonged inflammation can result in chronic inflammatory diseases. In the last decade, peptide therapeutics have gained significant attention due to their high specificity in targeting affected cells without affecting healthy cells. Motivated by the significance of peptide-based therapies, Anti-inflammatory peptides (AIPs) related models have become increasingly popular due to their significant characteristics. The systematic experimental identifying methods for AIPs deals with various problems of the existing in-vitro methods. Hence, to develop an accurate and reliable prediction of AIPs, numerous computational models have been developed. These models demonstrate high diversity in their datasets, feature representation, feature selection, model training, and evaluation methods. In this study, we conducted an extensive survey of the existing models for discriminating AIPs and point out the differences between these approaches. We evaluate the prediction results of the models based on independent tests and AUC values. Moreover, we emphasize the challenges and prospects in this field, paving the way for developing more effective prediction models that can enhance the predictive outcomes in predicting AIPs.






Similar content being viewed by others
References
Ferrero-Miliani L et al (2007) Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol 147(2):227–235
Watson J et al (2020) Raised inflammatory markers as a predictor of one-year mortality: a cohort study in primary care in the UK using electronic health record data. BMJ Open 10(10):e036027
Tsai D-H et al (2019) Effects of short- and long-term exposures to particulate matter on inflammatory marker levels in the general population. Environ Sci Pollut Res 26:19697–19704
Deepak P, Axelrad JE, Ananthakrishnan AN (2019) The role of the radiologist in determining disease severity in inflammatory bowel diseases. Gastrointest Endosc Clin 29(3):447–470
de Barcelos IP, Troxell RM, Graves JS (2019) Mitochondrial dysfunction and multiple sclerosis. Biology 8(2):37
Zouki C, Ouellet S, Filep JG (2000) The anti-inflammatory peptides, antiflammins, regulate the expression of adhesion molecules on human leukocytes and prevent neutrophil adhesion to endothelial cells. FASEB J 14(3):572–580
Wiebe N, Stenvinkel P, Tonelli M (2019) Associations of chronic inflammation, insulin resistance, and severe obesity with mortality, myocardial infarction, cancer, and chronic pulmonary disease. JAMA Netw Open 2(8):e1910456
Liu CH et al (2017) Biomarkers of chronic inflammation in disease development and prevention: challenges and opportunities. Nat Immunol 18(11):1175–1180
Pahwa R, Goyal A, Jialal I (2021) Chronic inflammation. StatPearls, Treasure Island
Weissman S et al (2020) Atherosclerotic cardiovascular disease in inflammatory bowel disease: the role of chronic inflammation. World J Gastrointest Pathophysiol 11(5):104
Wang B et al (2021) Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct Target Ther 6(1):94
Chen L et al (2018) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9(6):7204
Germolec DR et al (2018) Markers of inflammation. In: Immunotoxicity testing: methods and protocols. Humana Press, New York, p 57–79
Corrigan M et al (2015) Autoimmune hepatitis: an approach to disease understanding and management. Br Med Bull 114(1):181–191
Fosgerau K, Hoffmann T (2015) Peptide therapeutics: current status and future directions. Drug Discov Today 20(1):122–128
Vázquez-Prieto S et al (2016) QSPR-perturbation models for the prediction of B-epitopes from immune epitope database: a potentially valuable route for predicting “in silico” new optimal peptide sequences and/or boundary conditions for vaccine development. Int J Pept Res Ther 22:445–450
Rastogi S et al (2019) Peptide-based therapeutics: quality specifications, regulatory considerations, and prospects. Drug Discov Today 24(1):148–162
Craik DJ et al (2013) The future of peptide-based drugs. Chem Biol Drug Des 81(1):136–147
Liu W et al (2021) Peptide-based therapeutic cancer vaccine: current trends in clinical application. Cell Prolif 54(5):e13025
Miele L et al (1988) Novel anti-inflammatory peptides from the region of highest similarity between uteroglobin and lipocortin I. Nature 335(6192):726–730
Gonzalez-Rey E, Anderson P, Delgado M (2007) Emerging roles of vasoactive intestinal peptide: a new approach for autoimmune therapy. Ann Rheum Dis 66(Suppl 3):iii70–iii76
Delgado M, Ganea D (2008) Anti-inflammatory neuropeptides: a new class of endogenous immunoregulatory agents. Brain Behav Immun 22(8):1146–1151
Banchereau J, Pascual V, O’Garra A (2012) From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol 13(10):925–931
Steinman L et al (2012) Optimization of current and future therapy for autoimmune diseases. Nat Med 18(1):59–65
Odegaard JI, Chawla A (2013) Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 339(6116):172–177
Patterson H et al (2014) Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin Exp Immunol 176(1):1–10
Hernández-Flórez D, Valor L (2016) Los inhibidores de las proteínas-cinasas en enfermedades autoinmunes e inflamatorias: presente y futuro de nuevas dianas terapéuticas. Reumatol clín 12(2):91–99
Zhao L et al (2016) Purification and identification of anti-inflammatory peptides derived from simulated gastrointestinal digests of velvet antler protein (Cervus elaphus Linnaeus). J Food Drug Anal 24(2):376–384
Tabas I, Glass CK (2013) Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 339(6116):166–172
Gupta S et al (2017) Prediction of anti-inflammatory proteins/peptides: an in silico approach. J Transl Med 15(1):1–11
Manavalan B et al (2018) AIPpred: sequence-based prediction of anti-inflammatory peptides using random forest. Front Pharmacol 9:276
Khatun M, Hasan M, Kurata H (2019) PreAIP: computational prediction of anti-inflammatory peptides by integrating multiple complementary features. Front Genet 10:129
Zhang J et al (2020) AIEpred: an ensemble predictive model of classifier chain to identify anti-inflammatory peptides. IEEE/ACM Trans Comput Biol Bioinform 18(5):1831–1840
Wei L et al (2019) PEPred-Suite: improved and robust prediction of therapeutic peptides using adaptive feature representation learning. Bioinformatics 35(21):4272–4280
Zhao D et al (2021) iAIPs: identifying anti-inflammatory peptides using random forest. Front Genet 12:773202
Guo Y et al (2021) PreTP-EL: prediction of therapeutic peptides based on ensemble learning. Brief Bioinform 22(6):bbab358
Yan K et al (2022) PreTP-Stack: prediction of therapeutic peptide based on the stacked ensemble learning. IEEE/ACM Trans Comput Biol Bioinform 20(2):1337–1344
Yan K et al (2022) TPpred-ATMV: therapeutic peptide prediction by adaptive multi-view tensor learning model. Bioinformatics 38(10):2712–2718
Deng H et al (2022) Prediction of anti-inflammatory peptides by a sequence-based stacking ensemble model named AIPStack. Iscience 25(9):104967
Gaffar S et al (2023) IF-AIP: a machine learning method for the identification of anti-inflammatory peptides using multi-feature fusion strategy. Comput Biol Med 168:107724
Kim Y et al (2012) Immune epitope database analysis resource. Nucleic Acids Res 40(W1):W525–W530
Vita R et al (2015) The immune epitope database (IEDB) 3.0. Nucleic Acids Res 43(D1):D405–D412
Huang Y, Niu B, Gao Y, Fu L, Li W (20l0) CDHTT Suite: a web server for clustering and comparing biological sequences. Bioinformatics 26:680–682
Nakashima H, Nishikawa K, Ooi T (1986) The folding type of a protein is relevant to the amino acid composition. J Biochem 99(1):153–162
Ali F, Hayat M (2015) Classification of membrane protein types using Voting Feature Interval in combination with Chou’s Pseudo Amino Acid Composition. J Theor Biol 384:78–83
Ghulam A et al (2022) ACP-2DCNN: deep learning-based model for improving prediction of anticancer peptides using two-dimensional convolutional neural network. Chemom Intell Lab Syst 226:104589
Ahmad A et al (2022) Identification of antioxidant proteins using a discriminative intelligent model of k-space amino acid pairs based descriptors incorporating with ensemble feature selection. Biocybern Biomed Eng 42(2):727–735
Zayas JF (1997) Functionality of proteins in food. Springer, Berlin
Kawashima S, Kanehisa M (2000) AAIndex: amino acid index database. Nucleic Acids Res 28(1):374–374
Saha I et al (2012) Fuzzy clustering of physicochemical and biochemical properties of amino acids. Amino Acids 43:583–594
Saravanan V, Gautham N (2015) Harnessing computational biology for exact linear B-cell epitope prediction: a novel amino acid composition-based feature descriptor. Omics J Integr Biol 19(10):648–658
Ali F, Hayat M (2016) Machine learning approaches for discrimination of Extracellular Matrix proteins using hybrid feature space. J Theor Biol 403:30–37
Ahmad A et al (2021) Deep-AntiFP: prediction of antifungal peptides using distant multi-informative features incorporating with deep neural networks. Chemom Intell Lab Syst 208:104214
Bhasin M, Raghava GP (2004) Classification of nuclear receptors based on amino acid composition and dipeptide composition. J Biol Chem 279(22):23262–23266
Sun J-N et al (2020) Prediction of cyclin protein using two-step feature selection technique. IEEE Access 8:109535–109542
Liu Y, Wang X, Liu B (2019) A comprehensive review and comparison of existing computational methods for intrinsically disordered protein and region prediction. Brief Bioinform 20(1):330–346
Ahmad A et al (2022) iAFPs-EnC-GA: identifying antifungal peptides using sequential and evolutionary descriptors based multi-information fusion and ensemble learning approach. Chemom Intell Lab Syst 222:104516
Banjar A et al (2022) iDBP-PBMD: a machine learning model for detection of DNA-binding proteins by extending compression techniques into evolutionary profile. Chemom Intell Lab Syst 231:104697
Ali F et al (2022) Deep-PCL: a deep learning model for prediction of cancer lectins and non cancer lectins using optimized integrated features. Chemom Intell Lab Syst 221:104484
Kabir M et al (2018) Improving prediction of extracellular matrix proteins using evolutionary information via a grey system model and asymmetric under-sampling technique. Chemom Intell Lab Syst 174:22–32
Akbar S et al (2021) iAtbP-Hyb-EnC: prediction of antitubercular peptides via heterogeneous feature representation and genetic algorithm based ensemble learning model. Comput Biol Med 137:104778
Ali F et al (2021) AFP-CMBPred: computational identification of antifreeze proteins by extending consensus sequences into multi-blocks evolutionary information. Comput Biol Med 139:105006
Akbar S et al (2020) iHBP-DeepPSSM: identifying hormone binding proteins using PsePSSM based evolutionary features and deep learning approach. Chemom Intell Lab Syst 204:104103
Akbar S et al (2022) Prediction of Antiviral peptides using transform evolutionary and SHAP analysis based descriptors by incorporation with ensemble learning strategy. Chemom Intell Lab Syst 230:104682
Rangwala H, Karypis G (2005) Profile-based direct kernels for remote homology detection and fold recognition. Bioinformatics 21(23):4239–4247
Liu B, Long R, Chou K-C (2016) iDHS-EL: identifying DNase I hypersensitive sites by fusing three different modes of pseudo nucleotide composition into an ensemble learning framework. Bioinformatics 32(16):2411–2418
Gao X et al (2019) iRBP-motif-PSSM: identification of RNA-binding proteins based on collaborative learning. IEEE Access 7:168956–168962
Zhang J, Liu B (2017) PSFM-DBT: identifying DNA-binding proteins by combing position specific frequency matrix and distance-bigram transformation. Int J Mol Sci 18(9):1856
Liu B et al (2012) Using amino acid physicochemical distance transformation for fast protein remote homology detection. PLoS ONE 7(9):e46633
Liu B et al (2014) Using distances between Top-n-gram and residue pairs for protein remote homology detection. BMC Bioinform 15:S3
Wang N, Zhang J, Liu B (2021) IDRBP-PPCT: identifying nucleic acid-binding proteins based on position-specific score matrix and position-specific frequency matrix cross transformation. IEEE/ACM Trans Comput Biol Bioinform 19(4):2284–2293
Liu B et al (2008) A discriminative method for protein remote homology detection and fold recognition combining Top-n-grams and latent semantic analysis. BMC Bioinform 9:1–16
Carugo O (2013) Frequency of dipeptides and antidipeptides. Comput Struct Biotechnol J 8(11):e201308001
Hasan MM, Khatun MS, Kurata H (2018) A comprehensive review of in silico analysis for protein S-sulfenylation sites. Protein Pept Lett 25(9):815–821
Hasan MM et al (2015) Computational identification of protein pupylation sites by using profile-based composition of k-spaced amino acid pairs. PLoS ONE 10(6):e0129635
Xu R et al (2015) Identifying DNA-binding proteins by combining support vector machine and PSSM distance transformation. BMC Syst Biol. https://doi.org/10.1186/1752-0509-9-s1-s10
Liu S et al (2022) Ensemble learning-based feature selection for phage protein prediction. Front Microbiol 13:932661
Abbasi Mesrabadi H, Faez K, Pirgazi J (2023) Drug–target interaction prediction based on protein features, using wrapper feature selection. Sci Rep 13(1):3594
Frank E et al (2004) Data mining in bioinformatics using WEKA. Bioinformatics 20(15):2479–2481
Azhagusundari B, Thanamani AS (2013) Feature selection based on information gain. Int J Innov Technol Explor Eng 2(2):18–21
Huang SH (2015) Supervised feature selection: a tutorial. Artif Intell Res 4(2):22–37
Ding H et al (2014) Identification of bacteriophage virion proteins by the ANOVA feature selection and analysis. Mol BioSyst 10(8):2229–2235
Boser BE, Guyon IM, Vapnik VN (1992) A training algorithm for optimal margin classifiers. In: Proceedings of the fifth annual workshop on computational learning theory, 1992
Ali F et al (2018) DBPPred-PDSD: machine learning approach for prediction of DNA-binding proteins using Discrete Wavelet Transform and optimized integrated features space. Chemom Intell Lab Syst 182:21–30
Chou K-C, Shen H-B (2007) MemType-2L: a web server for predicting membrane proteins and their types by incorporating evolution information through Pse-PSSM. Biochem Biophys Res Commun 360(2):339–345
Khan ZU et al (2019) iRSpot-SPI: deep learning-based recombination spots prediction by incorporating secondary sequence information coupled with physio-chemical properties via Chou’s 5-step rule and pseudo components. Chemom Intell Lab Syst 189:169–180
Schapire RE (2003) The boosting approach to machine learning: an overview. In: Nonlinear estimation and classification. Springer, New York, p 149–171
Friedman JH (2001) Greedy function approximation: a gradient boosting machine. Ann Stat 29(5):1189–1232
Basith S et al (2018) iGHBP: computational identification of growth hormone binding proteins from sequences using extremely randomised tree. Comput Struct Biotechnol J 16:412–420
Ali F et al (2019) DP-BINDER: machine learning model for prediction of DNA-binding proteins by fusing evolutionary and physicochemical information. J Comput Aided Mol Des 33(7):645–658
Akbar S et al (2017) iACP-GAEnsC: evolutionary genetic algorithm based ensemble classification of anticancer peptides by utilizing hybrid feature space. Artif Intell Med 79:62–70
Liu B et al (2018) iEnhancer-EL: identifying enhancers and their strength with ensemble learning approach. Bioinformatics 34(22):3835–3842
Akbar S et al (2019) iAFP-gap-SMOTE: an efficient feature extraction scheme gapped dipeptide composition is coupled with an oversampling technique for identification of antifreeze proteins. Lett Org Chem 16(4):294–302
Akbar S et al (2023) pAtbP-EnC: identifying anti-tubercular peptides using multi-feature representation and genetic algorithm based deep ensemble model. IEEE Access 11:137099–137114
Akbar S et al (2023) Identifying neuropeptides via evolutionary and sequential based multi-perspective descriptors by incorporation with ensemble classification strategy. IEEE Access 11:49024–49034
Akbar S et al (2023) Prediction of amyloid proteins using embedded evolutionary and ensemble feature selection based descriptors with eXtreme gradient boosting model. IEEE Access 11:39024–39036
Zhang YP, Zou Q (2020) PPTPP: a novel therapeutic peptide prediction method using physicochemical property encoding and adaptive feature representation learning. Bioinformatics 36(13):3982–3987
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raza, A., Uddin, J., Akbar, S. et al. Comprehensive Analysis of Computational Methods for Predicting Anti-inflammatory Peptides. Arch Computat Methods Eng (2024). https://doi.org/10.1007/s11831-024-10078-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11831-024-10078-7