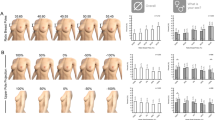
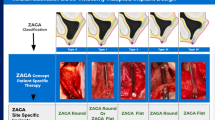
Abstract
Purpose
New deep learning and statistical shape modelling approaches aim to automate the design process for patient-specific cranial implants, as highlighted by the MICCAI AutoImplant Challenges. To ensure applicability, it is important to determine if the training data used in developing these algorithms represent the geometry of implants designed for clinical use.
Methods
Calavera Surgical Design provided a dataset of 206 post-craniectomy skull geometries and their clinically used implants. The MUG500+ dataset includes 29 post-craniectomy skull geometries and implants designed for automating design. For both implant and skull shapes, the inner and outer cortical surfaces were segmented, and the thickness between them was measured. For the implants, a ‘rim’ was defined that transitions from the repaired defect to the surrounding skull. For unilateral defect cases, skull implants were mirrored to the contra-lateral side and thickness differences were quantified.
Results
The average thickness of the clinically used implants was 6.0 ± 0.5 mm, which approximates the thickness on the contra-lateral side of the skull (relative difference of −0.3 ± 1.4 mm). The average thickness of the MUG500+ implants was 2.9 ± 1.0 mm, significantly thinner than the intact skull thickness (relative difference of 2.9 ± 1.2 mm). Rim transitions in the clinical implants (average width of 8.3 ± 3.4 mm) were used to cap and create a smooth boundary with the skull.
Conclusions
For implant modelers or manufacturers, this shape analysis quantified differences of cranial implants (thickness, rim width, surface area, and volume) to help guide future automated design algorithms. After skull completion, a thicker implant can be more versatile for cases involving muscle hollowing or thin skulls, and wider rims can smooth over the defect margins to provide more stability. For clinicians, the differing measurements and implant designs can help inform the options available for their patient specific treatment.







Similar content being viewed by others
Abbreviations
- SSM:
-
Statistical shape modelling
- DL:
-
Deep learning
References
Brichacek M, Antonyshyn O, Edwards G, Mainprize JG, Da Costa L (2021) Decision-making in adult cranial vault reconstruction. Plast Reconstr Surg 148:109E-121E. https://doi.org/10.1097/PRS.0000000000008058
Pinheiro M, Ma X, Fagan MJ, McIntyre GT, Lin P, Sivamurthy G, Mossey PA (2019) A 3D cephalometric protocol for the accurate quantification of the craniofacial symmetry and facial growth. J Biol Eng 13:1–11. https://doi.org/10.1186/s13036-019-0171-6
Morales-Gómez JA, Garcia-Estrada E, Leos-Bortoni JE, Delgado-Brito M, Flores-Huerta LE, De La Cruz-Arriaga AA, Torres-Díaz LJ, Martínez-Ponce de León ÁR (2019) Cranioplasty with a low-cost customized polymethylmethacrylate implant using a desktop 3D printer. J Neurosurg 130:1721–1727. https://doi.org/10.3171/2017.12.JNS172574
Li J, Egger J (2020) Towards the automatization of cranial implant design in cranioplasty. In: Li J, Egger J (eds) First challenge, AutoImplant 2020, Held in conjunction with MICCAI 2020, Lima, Peru, October 8, 2020, Proceedings. Springer International Publishing
Li J, Egger J (2021) Towards the automatization of cranial implant design in cranioplasty II. In: Li J, Egger J (eds) Second challenge, AutoImplant 2021, Held in conjunction with MICCAI 2021, Strasbourg, France, October 1, 2021, Proceedings. Springer International Publishing
Mainprize JG, Fishman Z, Hardisty MR (2020) Shape completion by U-Net: an approach to the autoimplant MICCAI cranial implant design challenge. In: Li J, Egger J (eds) Towards the automatization of cranial implant design in cranioplasty. AutoImplant 2020. Springer International Publishing, Berlin, pp 65–76
Mahdi H, Clement A, Kim E, Fishman Z, Whyne CMM, Mainprize JG, Hardisty MR (2021) A U-Net based system for cranial implant design with pre-processing and learned implant filtering. In: Li J, Egger J (eds) Towards the automatization of cranial implant design in cranioplasty II. Springer International Publishing, Berlin, pp 63–79
Li J, Pimentel P, Szengel A, Ehlke M, Lamecker H, Zachow S, Estacio L, Doenitz C, Ramm H, Shi H, Chen X, Matzkin F, Newcombe V, Ferrante E, Jin Y, Ellis DG, Aizenberg MR, Kodym O, Spanel M, Herout A, Mainprize JG, Fishman Z, Hardisty MR, Bayat A, Shit S, Wang B, Liu Z, Eder M, Pepe A, Gsaxner C, Alves V, Zefferer U, Von Campe G, Pistracher K, Schafer U, Schmalstieg D, Menze BH, Glocker B, Egger J (2021) AutoImplant 2020-first MICCAI challenge on automatic cranial implant design. IEEE Trans Med Imag 40:2329–2342. https://doi.org/10.1109/TMI.2021.3077047
Li J, Ellis DG, Kodym O, Rauschenbach L, Ries C, Sure U, Wrede KH, Alvarez CM, Wodzinski M, Daniol M, Hemmerling D, Mahdi H, Clement A, Kim E, Fishman Z, Whyne CM, Mainprize JG, Hardisty MR, Pathak S, Sindhura C, Gorth RKSS, Kiran DV, Gorthi S, Yang B, Fang K, Li X, Kroviakov A, Yu L, Pepe A, Gsaxner C, Herout A, Alves V, Spanel M, Aizenberg MR, Kleesiek J, Egger J (2022) Towards clinical applicability and computation efficiency in automatic cranial implant design: an overview of the AutoImplant 2021 cranial implant design challenge. Med Image Anal. In Submission
Kodym O, Li J, Pepe A, Gsaxner C, Chilamkurthy S, Egger J, Španěl M (2021) SkullBreak/SkullFix–dataset for automatic cranial implant design and a benchmark for volumetric shape learning tasks. Data Br 35:106902. https://doi.org/10.1016/j.dib.2021.106902
Ellis DG, Alvarez CM, Aizenberg MR (2021) Qualitative criteria for feasible cranial implant designs. Lecture notes in computer science (including subseries lecture notes in artificial intelligence and lecture notes in bioinformatics). Springer International Publishing, Cham, pp 8–18
Li J, Krall M, Trummer F, Memon AR, Pepe A, Gsaxner C, Jin Y, Chen X, Deutschmann H, Zefferer U, Schäfer U, von Campe G, Egger J (2021) MUG500+: database of 500 high-resolution healthy human skulls and 29 craniotomy skulls and implants. Data Br 39:0–5. https://doi.org/10.1016/j.dib.2021.107524
Kodym O, Španěl M, Herout A (2021) Deep learning for cranioplasty in clinical practice: Going from synthetic to real patient data. Comput Biol Med 137:104766. https://doi.org/10.1016/j.compbiomed.2021.104766
Jonkergouw J, van de Vijfeijken SECM, Nout E, Theys T, Van de Casteele E, Folkersma H, Depauw PRAM, Becking AG (2016) Outcome in patient-specific PEEK cranioplasty: a two-center cohort study of 40 implants. J Cranio-Maxillofacial Surg 44:1266–1272. https://doi.org/10.1016/j.jcms.2016.07.005
Lillie EM, Urban JE, Lynch SK, Weaver AA, Stitzel JD (2016) Evaluation of skull cortical thickness changes with age and sex from computed tomography scans. J Bone Miner Res 31:299–307. https://doi.org/10.1002/jbmr.2613
Wodzinski M, Daniol M, Socha M, Hemmerling D, Stanuch M, Skalski A (2022) Deep learning-based framework for automatic cranial defect reconstruction and implant modeling. Comput Methods Programs Biomed 226:107173. https://doi.org/10.1016/j.cmpb.2022.107173
Delye H, Clijmans T, Mommaerts MY, Vander SJ, Goffin J (2015) Creating a normative database of age-specific 3D geometrical data, bone density, and bone thickness of the developing skull: a pilot study. J Neurosurg Pediatr 16:687–702. https://doi.org/10.3171/2015.4.PEDS1493
Park SE, Park EK, Shim KW, Kim DS (2019) Modified cranioplasty technique using 3-dimensional printed implants in preventing temporalis muscle hollowing. World Neurosurg 126:e1160–e1168. https://doi.org/10.1016/j.wneu.2019.02.221
Zhong S, Huang GJ, Susarla SM, Swanson EW, Huang J, Gordon CR (2015) Quantitative analysis of dual-purpose, patient-specific craniofacial implants for correction of temporal deformity. Oper Neurosurg 11:220–229. https://doi.org/10.1227/NEU.0000000000000679
Persson J, Helgason B, Engqvist H, Ferguson SJ, Persson C (2018) Stiffness and strength of cranioplastic implant systems in comparison to cranial bone. J Cranio-Maxillofacial Surg 46:418–423. https://doi.org/10.1016/j.jcms.2017.11.025
Marcián P, Narra N, Borák L, Chamrad J, Wolff J (2019) Biomechanical performance of cranial implants with different thicknesses and material properties: a finite element study. Comput Biol Med 109:43–52. https://doi.org/10.1016/j.compbiomed.2019.04.016
Murphy MJ, Sunderland I, Edwards G, Mainprize J, Whyne C, Antonyshyn O (2016) Comparative impact resistance of titanium mesh (Ti), polymethyl methacrylate (PMMA) and polyether ether ketone (PEEK) in an in vitro cranioplasty model. Plast Reconstr Surg Glob Open 4:6–7. https://doi.org/10.1097/01.gox.0000502880.18813.5d
Antonyshyn OM, Edwards G, Mainprize J (2012) Patent US 2012/0010711 A1: method of forming patient-specific implant
van de Vijfeijken SECM, Münker TJAG, Spijker R, Karssemakers LHE, Vandertop WP, Becking AG, Ubbink DT (2018) Autologous bone is inferior to alloplastic cranioplasties: safety of autograft and allograft materials for cranioplasties, a systematic review. World Neurosurg 117:443-452.e8. https://doi.org/10.1016/j.wneu.2018.05.193
Acknowledgements
The authors would like to thank the AutoImplant Challenge community. Financial support was received from a Catalyst grant (FRN: 169989) from the Canadian Institutes of Health Research (CIHR) and a CREATE grant (543378-2020) from the Natural Sciences and Engineering Research Council of Canada (NSERC), and an INOVAIT grant (2020-1003).
Funding
This study was funded by the Canadian Institutes of Health Research (CIHR) (Catalyst grant FRN: 169989), the Natural Sciences and Engineering Research Council of Canada (NSERC) (CREATE grant 543378-2020), and by an INOVAIT grant through the Government of Canada’s Strategic Innovation Fund (2020-1003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Calavera Surgical Design Inc specializes in the design of craniofacial implants and surgical planning for reconstruction. For research grants shared between the Orthopaedic Biomechanics Lab and Calavera Surgical Design, Calavera provided in-kind consulting time and industry-matched funding. ZF has received research grants with Calavera Surgical Design Inc. JG Mainprize owns stock in Calavera Surgical Design Inc. GE owns stock in Calavera Surgical Design Inc. OA owns stock in Calavera Surgical Design Inc. MH has received research grants with Calavera Surgical Design Inc. CMW has received research grants with Calavera Surgical Design Inc.
Ethical appoval
Retrospective analysis of the skull data provided by Calavera Surgical Design was approved by the Sunnybrook Research Institute’s ethics board (Sun-4824). Skull geometries provided by the MUG500+ dataset are publicly available for research.
Human or animal rights
For this retrospective study, formal consent is not required. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fishman, Z., Mainprize, J.G., Edwards, G. et al. Thickness and design features of clinical cranial implants—what should automated methods strive to replicate?. Int J CARS 19, 747–756 (2024). https://doi.org/10.1007/s11548-024-03068-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-024-03068-4