Abstract
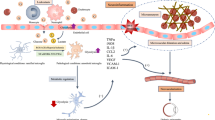
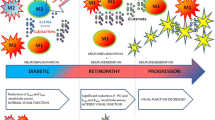
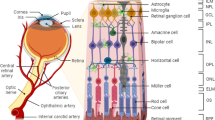
Diabetic retinopathy (DR) is recognized as a neurovascular complication of diabetes, and emerging evidence underscores the pivotal role of inflammation in its pathophysiology. Macrophage activation is increasingly acknowledged as a key contributor to the onset and progression of DR. Different populations of macrophages originating from distinct sources contribute to DR-associated inflammation. Retinal macrophages can be broadly categorized into two main groups based on their origin: intrinsic macrophages situated within the retina and vitreoretinal interface and macrophages derived from infiltrating monocytes. The former comprises microglia (MG), perivascular macrophages, and macrophage-like hyalocytes. Retinal MG, as the principal population of tissue-resident population of mononuclear phagocytes, exhibits high heterogeneity and plasticity while serving as a crucial connector between retinal capillaries and synapses. This makes MG actively involved in the pathological processes across various stages of DR. Activated hyalocytes also contribute to the pathological progression of advanced DR. Additionally, recruited monocytes, displaying rapid turnover in circulation, augment the population of retinal macrophages during DR pathogenesis, exerting pathogenic or protective effect based on different subtypes. In this review, we examine novel perspectives on macrophage biology based on recent studies elucidating the diversity of macrophage identity and function, as well as the mechanisms influencing macrophage behavior. These insights may pave the way for innovative therapeutic strategies in the management of DR.

Similar content being viewed by others
Availability of data and material
Not applicable.
References
Nentwich MM, Ulbig MW (2015) Diabetic retinopathy - ocular complications of diabetes mellitus. World J Diabetes 6:489–499. https://doi.org/10.4239/wjd.v6.i3.489
Rajesh A, Droho S, Lavine JA (2022) Macrophages in close proximity to the vitreoretinal interface are potential biomarkers of inflammation during retinal vascular disease. J Neuroinflammation 19:203. https://doi.org/10.1186/s12974-022-02562-3
Bressler NM, Beaulieu WT, Glassman AR, Blinder KJ, Bressler SB, Jampol LM, Melia M, Wells JA (2018) Persistent macular thickening following intravitreous Aflibercept, Bevacizumab, or Ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol 136:257–269. https://doi.org/10.1001/jamaophthalmol.2017.6565
Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL, Friedman SM, Glassman AR, Miller KM et al (2010) Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 117:1064–1077. https://doi.org/10.1016/j.ophtha.2010.02.031
Nian S, Lo ACY, Mi Y, Ren K, Yang D (2021) Neurovascular unit in diabetic retinopathy: pathophysiological roles and potential therapeutical targets. Eye Vis (Lond) 8:15. https://doi.org/10.1186/s40662-021-00239-1
Ibrahim AS, El-Remessy AB, Matragoon S, Zhang W, Patel Y, Khan S, Al-Gayyar MM, El-Shishtawy MM, Liou GI (2011) Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes 60:1122–1133. https://doi.org/10.2337/db10-1160
Gardner TW, Davila JR (2017) The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 255:1–6. https://doi.org/10.1007/s00417-016-3548-y
Zong H, Ward M, Stitt AW (2011) AGEs, RAGE, and diabetic retinopathy. Curr Diab Rep 11:244–252. https://doi.org/10.1007/s11892-011-0198-7
Rübsam A, Parikh S, Fort PE (2018) Role of inflammation in diabetic retinopathy. Int J Mol Sci 19:942. https://doi.org/10.3390/ijms19040942
Abcouwer SF, Gardner TW (2014) Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann N Y Acad Sci 1311:174–190. https://doi.org/10.1111/nyas.12412
Pan WW, Lin F, Fort PE (2021) The innate immune system in diabetic retinopathy. Prog Retin Eye Res 84:100940. https://doi.org/10.1016/j.preteyeres.2021.100940
Obasanmi G, Lois N, Armstrong D, Hombrebueno J, Lynch A, Chen M, Xu H (2023) Peripheral blood mononuclear cells from patients with type 1 diabetes and diabetic retinopathy produce higher levels of IL-17A, IL-10 and IL-6 and lower levels of IFN-γ-a pilot study. Cells 12:467. https://doi.org/10.3390/cells12030467
Kovoor E, Chauhan S, Hajrasouliha A (2022) Role of inflammatory cells in pathophysiology and management of diabetic retinopathy. Surv Ophthalmol 67:1563–1573. https://doi.org/10.1016/j.survophthal.2022.07.008
Elbeyli A, Kurtul B, Ozcan S, Ozarslan Ozcan D (2022) The diagnostic value of systemic immune-inflammation index in diabetic macular oedema. Clin Exp Optom 105:831–835. https://doi.org/10.1080/08164622.2021.1994337
Huang J, Zhou Q (2022) Identification of the relationship between hub genes and immune cell infiltration in vascular endothelial cells of proliferative diabetic retinopathy using bioinformatics methods 2022:7231046. https://doi.org/10.1155/2022/7231046
Zeng HY, Green WR, Tso MO (2008) Microglial activation in human diabetic retinopathy. Arch Ophthalmol 126:227–232. https://doi.org/10.1001/archophthalmol.2007.65
Blot G, Karadayi R, Przegralek L, Sartoris TM, Charles-Messance H, Augustin S, Negrier P, Blond F, Muñiz-Ruvalcaba FP, Rivera-de la Parra D et al (2023) Perilipin 2-positive mononuclear phagocytes accumulate in the diabetic retina and promote PPARγ-dependent vasodegeneration. J Clin Invest 133:e161348. https://doi.org/10.1172/jci161348
Mills SA, Jobling AI, Dixon MA, Bui BV, Vessey KA, Phipps JA, Greferath U, Venables G, Wong VHY, Wong CHY et al (2021) Fractalkine-induced microglial vasoregulation occurs within the retina and is altered early in diabetic retinopathy. Proc Natl Acad Sci USA 118:e2112561118. https://doi.org/10.1073/pnas.2112561118
Chen M, Luo C, Zhao J, Devarajan G, Xu H (2019) Immune regulation in the aging retina. Prog Retin Eye Res 69:159–172. https://doi.org/10.1016/j.preteyeres.2018.10.003
Altmann C, Schmidt MHH (2018) The role of microglia in diabetic retinopathy: inflammation, microvasculature defects and neurodegeneration. Int J Mol Sci 19:110. https://doi.org/10.3390/ijms19010110
Ong JX, Nesper PL, Fawzi AA, Wang JM, Lavine JA (2021) Macrophage-like cell density is increased in proliferative diabetic retinopathy characterized by optical coherence tomography angiography. Invest Ophthalmol Vis Sci 62:2. https://doi.org/10.1167/iovs.62.10.2
Wang X, Zhao L, Zhang J, Fariss RN, Ma W, Kretschmer F, Wang M, Qian HH, Badea TC, Diamond JS et al (2016) Requirement for microglia for the maintenance of synaptic function and integrity in the mature retina. J Neurosci 36:2827–2842. https://doi.org/10.1523/jneurosci.3575-15.2016
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER et al (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841–845. https://doi.org/10.1126/science.1194637
Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW et al (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336:86–90. https://doi.org/10.1126/science.1219179
Wolf J, Boneva S, Rosmus DD, Agostini H, Schlunck G, Wieghofer P, Schlecht A, Lange C (2022) In-depth molecular profiling specifies human retinal microglia identity. Front Immunol 13:863158. https://doi.org/10.3389/fimmu.2022.863158
Schafer ST, Mansour AA, Schlachetzki JCM, Pena M, Ghassemzadeh S, Mitchell L, Mar A, Quang D, Stumpf S, Ortiz IS et al (2023) An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell 186:2111–2126. https://doi.org/10.1016/j.cell.2023.04.022
Chen L, Yang P, Kijlstra A (2002) Distribution, markers, and functions of retinal microglia. Ocul Immunol Inflamm 10:27–39. https://doi.org/10.1076/ocii.10.1.27.10328
Singaravelu J, Zhao L, Fariss RN, Nork TM, Wong WT (2017) Microglia in the primate macula: specializations in microglial distribution and morphology with retinal position and with aging. Brain Struct Funct 222:2759–2771. https://doi.org/10.1007/s00429-017-1370-x
Li L, Eter N, Heiduschka P (2018) The microglia in healthy and diseased retina. Exp Eye Res 136:116–130. https://doi.org/10.1016/j.exer.2015.04.020
Silverman SM, Wong WT (2018) Microglia in the retina: roles in development, maturity, and disease. Annu Rev Vis Sci 4:45–77. https://doi.org/10.1146/annurev-vision-091517-034425
Lukowski SW, Lo CY, Sharov AA, Nguyen Q, Fang L, Hung SS, Zhu L, Zhang T, Grünert U, Nguyen T (2019) A single-cell transcriptome atlas of the adult human retina. Embo j 38:100811. https://doi.org/10.15252/embj.2018100811
Lee JE, Liang KJ, Fariss RN, Wong WT (2008) Ex vivo dynamic imaging of retinal microglia using time-lapse confocal microscopy. Invest Ophthalmol Vis Sci 49:4169–4176. https://doi.org/10.1167/iovs.08-2076
Zhang Y, Zhao L, Wang X, Ma W, Lazere A, Qian HH, Zhang J, Abu-Asab M, Fariss RN, Roger JE et al (2018) Repopulating retinal microglia restore endogenous organization and function under CX3CL1-CX3CR1 regulation. Sci Adv 4:eaap8492. https://doi.org/10.1126/sciadv.aap8492
Wang X, Wang T, Lam E, Alvarez D, Sun Y (2023) Ocular vascular diseases: from retinal immune privilege to inflammation. Int J Mol Sci 24:12090. https://doi.org/10.3390/ijms241512090
Taylor AW, Ng TF (2018) Negative regulators that mediate ocular immune privilege. J Leukoc Biol 103:1179–1187. https://doi.org/10.1002/jlb.3mir0817-337r
Reyes NJ, O’Koren EG, Saban DR (2017) New insights into mononuclear phagocyte biology from the visual system. Nat Rev Immunol 17:322–332. https://doi.org/10.1038/nri.2017.13
Cherry JD, Olschowka JA, O’Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98. https://doi.org/10.1186/1742-2094-11-98
Kaur C, Rathnasamy G, Ling EA (2012) Roles of activated microglia in hypoxia induced neuroinflammation in the developing brain and the retina. J Neuroimmune Pharmacol 8:66–78. https://doi.org/10.1007/s11481-012-9347-2
Klotzsche-von Ameln A, Sprott D (2022) Harnessing retinal phagocytes to combat pathological neovascularization in ischemic retinopathies? Pflugers Arch 474:575–590. https://doi.org/10.1007/s00424-022-02695-7
Su F, Yi H, Xu L, Zhang Z (2015) Fluoxetine and S-citalopram inhibit M1 activation and promote M2 activation of microglia in vitro. Neuroscience 294:60–68. https://doi.org/10.1016/j.neuroscience.2015.02.028
Arroba AI, Alcalde-Estevez E, García-Ramírez M, Cazzoni D, de la Villa P, Sánchez-Fernández EM, Mellet CO, García Fernández JM, Hernández C, Simó R et al (2016) Modulation of microglia polarization dynamics during diabetic retinopathy in db/db mice. Biochim Biophys Acta 1862:1663–1674. https://doi.org/10.1016/j.bbadis.2016.05.024
Martinez FO, Helming L, Gordon S (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27:451–483. https://doi.org/10.1146/annurev.immunol.021908.132532
Zhou YD, Yoshida S, Peng YQ, Kobayashi Y, Zhang LS, Tang LS (2017) Diverse roles of macrophages in intraocular neovascular diseases: a review. Int J Ophthalmol 10:1902–1908
Kinuthia UM, Wolf A, Langmann T (2020) Microglia and inflammatory responses in diabetic retinopathy. Front Immunol 11:564077. https://doi.org/10.3389/fimmu.2020.564077
Chen T, Zhu W, Wang C, Dong X, Yu F, Su Y, Huang J, Huo L, Wan P (2022) ALKBH5-mediated mA modification of A20 regulates microglia polarization in diabetic retinopathy. Front Immunol 13:813979. https://doi.org/10.3389/fimmu.2022.813979
Lampron A, Elali A, Rivest S (2013) Innate immunity in the CNS: redefining the relationship between the CNS and Its environment. Neuron 78:214–232. https://doi.org/10.1016/j.neuron.2013.04.005
Wang Z, Koenig AL, Lavine KJ, Apte RS (2019) Macrophage plasticity and function in the eye and heart. Trends Immunol 40:825–841. https://doi.org/10.1016/j.it.2019.07.002
Graeber MB, Li W, Rodriguez ML (2011) Role of microglia in CNS inflammation. FEBS Lett 585:3798–3805. https://doi.org/10.1016/j.febslet.2011.08.033
Stratoulias V, Venero JL, Tremblay M, Joseph B (2019) Microglial subtypes: diversity within the microglial community. Embo J 38:e101997. https://doi.org/10.15252/embj.2019101997
O’Koren EG, Mathew R, Saban DR (2016) Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Sci Rep 6:20636. https://doi.org/10.1038/srep20636
Rosmus DD, Wieghofer P (2022) Guardians of the eye: new tales about retinal microglia and other ocular macrophages. Neural Regen Res 17:1275–1277. https://doi.org/10.4103/1673-5374.327335
Wieghofer P, Hagemeyer N, Sankowski R, Schlecht A, Staszewski O, Amann L, Gruber M, Koch J, Hausmann A, Zhang P et al (2021) Mapping the origin and fate of myeloid cells in distinct compartments of the eye by single-cell profiling. Embo j 40:e105123. https://doi.org/10.15252/embj.2020105123
Li Q, Cheng Z, Zhou L, Darmanis S, Neff NF, Okamoto J, Gulati G, Bennett ML, Sun LO, Clarke LE et al (2019) Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101:207–223. https://doi.org/10.1016/j.neuron.2018.12.006
Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J et al (2019) Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50:253–271. https://doi.org/10.1016/j.immuni.2018.11.004
O’Koren E, Yu C, Klingeborn M, Wong A, Prigge C, Mathew R, Kalnitsky J, Msallam R, Silvin A, Kay J et al (2019) Microglial function is distinct in different anatomical locations during retinal homeostasis and degeneration. Immunity 50:723–737. https://doi.org/10.1016/j.immuni.2019.02.007
Hu Z, Mao X, Chen M, Wu X, Zhu T, Liu Y, Zhang Z, Fan W, Xie P, Yuan S et al (2022) Single cell transcriptomics reveals novel role of microglia in fibrovascular membrane of proliferative diabetic retinopathy. Diabetes 71:762–773. https://doi.org/10.2337/db21-0551
Corano Scheri K, Lavine JA, Tedeschi T, Thomson BR, Fawzi AA (2023) Single-cell transcriptomics analysis of proliferative diabetic retinopathy fibrovascular membranes reveals AEBP1 as fibrogenesis modulator. JCI Insight 17:84. https://doi.org/10.1172/jci.insight.172062
Gosselin D (2020) Epigenomic and transcriptional determinants of microglial cell identity. Glia 68(8):1643–1654. https://doi.org/10.1002/glia.23787. Epub 2020/01/30.
Chen Y, Colonna M (2021) Microglia in Alzheimer’s disease at single-cell level. Are there common patterns in humans and mice? J Exp Med 218:e20202717. https://doi.org/10.1084/jem.20202717
Jordão MJC, Sankowski R, Brendecke SM, Sagar Locatelli G, Tai YH, Tay TL, Schramm E, Armbruster S, Hagemeyer N et al (2019) Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363:eaat7554. https://doi.org/10.1126/science.aat7554
Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Sagar Scheiwe C, Nessler S, Kunz P, van Loo G et al (2019) Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566:388–392. https://doi.org/10.1038/s41586-019-0924-x
Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E, Xu Y, Fanek Z et al (2017) The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47:566–581. https://doi.org/10.1016/j.immuni.2017.08.008
Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, Lassmann H (2017) Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140:1900–1913. https://doi.org/10.1093/brain/awx113
DePaula-Silva AB, Gorbea C, Doty DJ, Libbey JE, Sanchez JMS, Hanak TJ, Cazalla D, Fujinami RS (2019) Differential transcriptional profiles identify microglial- and macrophage-specific gene markers expressed during virus-induced neuroinflammation. J Neuroinflammation 16:152. eng. https://doi.org/10.1186/s12974-019-1545-x
Masuda T, Amann L, Sankowski R, Staszewski O, Lenz M, d´ Errico P, Snaidero N, Costa Jordão MJ, Böttcher C, Kierdorf K, Jung S et al (2020) Novel Hexb-based tools for studying microglia in the CNS. Nat Immunol 21:802–815. https://doi.org/10.1038/s41590-020-0707-4
Lier J, Streit WJ, Bechmann I (2021) Beyond activation: characterizing microglial functional phenotypes. Cells 10:2236. https://doi.org/10.3390/cells10092236
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B et al (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169:1276–1290. https://doi.org/10.1016/j.cell.2017.05.018
Hou J, Chen Y, Grajales-Reyes G, Colonna M (2022) TREM2 dependent and independent functions of microglia in Alzheimer’s disease. Mol Neurodegener 17:84. https://doi.org/10.1186/s13024-022-00588-y
Liu Z, Shi H, Xu J, Yang Q, Ma Q, Mao X, Xu Z, Zhou Y, Da Q, Cai Y et al (2022) Single-cell transcriptome analyses reveal microglia types associated with proliferative retinopathy. JCI Insight 7:e160940. https://doi.org/10.1172/jci.insight.160940
Li X, Yu Z, Li H, Yuan Y, Gao X, Kuang H (2021) Retinal microglia polarization in diabetic retinopathy. Vis Neurosci 38:E006. https://doi.org/10.1017/s0952523821000031
Aires ID, Madeira MH, Boia R, Rodrigues-Neves AC, Martins JM, Ambrósio AF, Santiago AR (2019) Intravitreal injection of adenosine A(2A) receptor antagonist reduces neuroinflammation, vascular leakage and cell death in the retina of diabetic mice. Sci Rep 9:17207. https://doi.org/10.1038/s41598-019-53627-y
Scott IU, Jackson GR, Quillen DA, Klein R, Liao J, Gardner TW (2014) Effect of doxycycline vs placebo on retinal function and diabetic retinopathy progression in mild to moderate nonproliferative diabetic retinopathy: a randomized proof-of-concept clinical trial. JAMA Ophthalmol 132:1137–1142. https://doi.org/10.1001/jamaophthalmol.2014.1422
Church KA, Rodriguez D, Mendiola AS, Vanegas D, Gutierrez IL, Tamayo I, Amadu A, Velazquez P, Cardona SM, Gyoneva S et al (2023) Pharmacological depletion of microglia alleviates neuronal and vascular damage in the diabetic CX3CR1-WT retina but not in CX3CR1-KO or hCX3CR1(I249/M280)-expressing retina. Front Immunol 14:1130735. https://doi.org/10.3389/fimmu.2023.1130735
Wang SK, Lapan SW, Hong CM, Krause TB, Cepko CL (2020) In situ detection of adeno-associated viral vector genomes with SABER-FISH. Mol Ther Methods Clin Dev 19:376–386. https://doi.org/10.1016/j.omtm.2020.10.003
Rosario AM, Cruz PE, Ceballos-Diaz C, Strickland MR, Siemienski Z, Pardo M, Schob KL, Li A, Aslanidi GV, Srivastava A et al (2016) Microglia-specific targeting by novel capsid-modified AAV6 vectors. Mol Ther Methods Clin Dev 3:16026. https://doi.org/10.1038/mtm.2016.26
Maes ME, Colombo G, Schulz R, Siegert S (2019) Targeting microglia with lentivirus and AAV: recent advances and remaining challenges. Neurosci Lett 707:134310. https://doi.org/10.1016/j.neulet.2019.134310
Xiong W, Wu DM, Xue Y, Wang SK, Chung MJ, Ji X, Rana P, Zhao SR, Mai S, Cepko CL (2019) AAV cis-regulatory sequences are correlated with ocular toxicity. Proc Natl Acad Sci U S A 116:5785–5794. https://doi.org/10.1073/pnas.1821000116
Chan YK, Wang SK, Chu CJ, Copland DA, Letizia AJ, Costa Verdera H, Chiang JJ, Sethi M, Wang MK, Neidermyer WJ et al (2021) Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci Transl Med 13:eabd3438. https://doi.org/10.1126/scitranslmed.abd3438
Shin TH, Lee DY, Manavalan B, Basith S, Na YC, Yoon C, Lee HS, Paik MJ, Lee G (2021) Silica-coated magnetic nanoparticles activate microglia and induce neurotoxic D-serine secretion. Part Fibre Toxicol 18:30. https://doi.org/10.1186/s12989-021-00420-3
Kaneko H, Nishiguchi KM, Nakamura M, Kachi S, Terasaki H (2008) Characteristics of bone marrow-derived microglia in the normal and injured retina. Invest Ophthalmol Vis Sci 49:4162–4168. https://doi.org/10.1167/iovs.08-1738
Kataoka K, Nishiguchi KM, Kaneko H, van Rooijen N, Kachi S, Terasaki H (2010) The roles of vitreal macrophages and circulating leukocytes in retinal neovascularization. Invest Ophthalmol Vis Sci 52:1431–1438. https://doi.org/10.1167/iovs.10-5798
Chinnery HR, McMenamin PG, Dando SJ (2017) Macrophage physiology in the eye. Pflugers Arch 469:501–515. https://doi.org/10.1007/s00424-017-1947-5
McLeod DS, Lefer DJ, Merges C, Lutty GA (1995) Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol 147(3):642–53. PMID 7545873
Valle A, Giamporcaro GM, Scavini M, Stabilini A, Grogan P, Bianconi E, Sebastiani G, Masini M, Maugeri N, Porretti L et al (2013) Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes 62:2072–2077. https://doi.org/10.2337/db12-1345
Tang L, Xu GT, Zhang JF (2022) Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy. Neural Regen Res 18:976–982. https://doi.org/10.4103/1673-5374.355743
Geissmann F, Jung S, Littman DR (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71–82. https://doi.org/10.1016/s1074-7613(03)00174-2
Hazra S, Jarajapu YP, Stepps V, Caballero S, Thinschmidt JS, Sautina L, Bengtsson N, Licalzi S, Dominguez J, Kern TS et al (2013) Long-term type 1 diabetes influences haematopoietic stem cells by reducing vascular repair potential and increasing inflammatory monocyte generation in a murine model. Diabetologia 56:644–53. https://doi.org/10.1007/s00125-012-2781-0
Saadane A, Veenstra AA, Minns MS, Tang J, Du Y, Abubakr Elghazali F, Lessieur EM, Pearlman E, Kern TS (2023) CCR2-positive monocytes contribute to the pathogenesis of early diabetic retinopathy in mice. Diabetologia 66:590–602. https://doi.org/10.1007/s00125-022-05860-w
Ma W, Zhang Y, Gao C, Fariss RN, Tam J, Wong WT (2017) Monocyte infiltration and proliferation reestablish myeloid cell homeostasis in the mouse retina following retinal pigment epithelial cell injury. Sci Rep 7:8433. https://doi.org/10.1038/s41598-017-08702-7
Tecilazich F, Phan TA, Simeoni F, Scotti GM, Dagher Z, Lorenzi M (2020) Patrolling monocytes are recruited and activated by diabetes to protect retinal microvessels. Diabetes 69:2709–2719. https://doi.org/10.2337/db19-1043
Beli E, Dominguez J, Hu P, Thinschmidt J, Caballero S, Li Calzi S, Luo D, Shanmugam S, Salazar T, Duan Y et al (2016) CX3CR1 deficiency accelerates the development of retinopathy in a rodent model of type 1 diabetes. J Mol Med (Berl) 94:1255–1265. https://doi.org/10.1007/s00109-016-1433-0
Castanos MV, Zhou DB, Linderman RE, Allison R, Milman T, Carroll J, Migacz J, Rosen RB, Chui TYP (2020) Imaging of macrophage-like cells in living human retina using clinical OCT. Invest Ophthalmol Vis Sci 61:48. https://doi.org/10.1167/iovs.61.6.48
Vagaja NN, Chinnery HR, Binz N, Kezic JM, Rakoczy EP, McMenamin PG (2012) Changes in murine hyalocytes are valuable early indicators of ocular disease. Invest Ophthalmol Vis Sci 53:1445–1451. https://doi.org/10.1167/iovs.11-8601
Lazarus HS, Hageman GS (1994) In situ characterization of the human hyalocyte. Arch Ophthalmol 112:1356–1362. https://doi.org/10.1001/archopht.1994.01090220106031
Zhu M, Penfold PL, Madigan MC, Billson FA (1997) Effect of human vitreous and hyalocyte-derived factors on vascular endothelial cell growth. Aust N Z J Ophthalmol 1:S57-60. https://doi.org/10.1111/j.1442-9071.1997.tb01758.x
Boneva SK, Wolf J, Rosmus DD, Schlecht A, Prinz G, Laich Y, Boeck M, Zhang P, Hilgendorf I, Stahl A et al (2020) Transcriptional profiling uncovers human hyalocytes as a unique innate immune cell population. Front Immunol 11:567274. https://doi.org/10.3389/fimmu.2020.567274
Mendes-Jorge L, Ramos D, Luppo M, Llombart C, Alexandre-Pires G, Nacher V, Melgarejo V, Correia M, Navarro M, Carretero A et al (2009) Scavenger function of resident autofluorescent perivascular macrophages and their contribution to the maintenance of the blood-retinal barrier. Invest Ophthalmol Vis Sci 50:5997–6005. https://doi.org/10.1167/iovs.09-3515
Koizumi T, Kerkhofs D, Mizuno T, Steinbusch HWM, Foulquier S (2019) Vessel-associated immune cells in cerebrovascular diseases: from perivascular macrophages to vessel-associated microglia. Front Neurosci 13:1291. https://doi.org/10.3389/fnins.2019.01291
Zhang NT, Nesper PL, Ong JX, Wang JM, Fawzi AA, Lavine JA (2022) Macrophage-like cells are increased in patients with vision-threatening diabetic retinopathy and correlate with macular edema. Diagnostics (Basel, Switzerland) 12:2793. https://doi.org/10.3390/diagnostics12112793
Yamaguchi M, Nakao S, Wada I, Matoba T, Arima M, Kaizu Y, Shirane M, Ishikawa K, Nakama T, Murakami Y et al (2022) Identifying hyperreflective foci in diabetic retinopathy via VEGF-induced local self-renewal of CX3CR1+ vitreous resident macrophages. Diabetes 71:2685–2701. https://doi.org/10.2337/db21-0247
Wang Z, An H, Tang J, Jin E, Li S, Zhang L, Huang L, Qu J (2023) Elevated number and density of macrophage-like cell as a novel inflammation biomarker in diabetic macular edema. Sci Rep 13:5320. https://doi.org/10.1038/s41598-023-32455-1
Wang W, Sun G, Xu A, Chen C (2023) Proliferative diabetic retinopathy and diabetic macular edema are two factors that increase macrophage-like cell density characterized by en face optical coherence tomography. BMC Ophthalmol 23:46. https://doi.org/10.1186/s12886-023-02794-8
Wolf J, Boneva S, Rosmus DD, Agostini H, Schlunck G, Wieghofer P, Schlecht A, Lange C (2022) Deciphering the molecular signature of human hyalocytes in relation to other innate immune cell populations. Invest Ophthalmol Vis Sci 63:9. https://doi.org/10.1167/iovs.63.3.9
Boneva SK, Wolf J, Hajdú RI, Prinz G, Salié H, Schlecht A, Killmer S, Laich Y, Faatz H, Lommatzsch A et al (2021) In-depth molecular characterization of neovascular membranes suggests a role for hyalocyte-to-myofibroblast transdifferentiation in proliferative diabetic retinopathy. Front Immunol 12:757607. https://doi.org/10.3389/fimmu.2021.757607
Bisen JB, Heisel CJ, Duffy BV, Decker NL, Fukuyama H, Ghazi O et al (2023) Association between macrophage-like cell density and ischemia metrics in diabetic eyes. Exp Eye Res 237:109703. https://doi.org/10.1016/j.exer.2023.109703
Fumagalli S, Perego C, Pischiutta F, Zanier ER, De Simoni MG (2015) The ischemic environment drives microglia and macrophage function. Front Neurol 6:81. https://doi.org/10.3389/fneur.2015.00081
Park YG, Lee JY, Kim C, Park YH (2021) Early microglial changes associated with diabetic retinopathy in rats with streptozotocin-induced diabetes. J Diabetes Res 2021:492093. https://doi.org/10.1155/2021/4920937
Jiang M, Xie H, Zhang C, Wang T, Tian H, Lu L, Xu JY, Xu GT, Liu L, Zhang J (2022) Enhancing fractalkine/CX3CR1 signalling pathway can reduce neuroinflammation by attenuating microglia activation in experimental diabetic retinopathy. J Cell Mol Med 26:1229–1244. https://doi.org/10.1111/jcmm.17179
Lv K, Ying H, Hu G, Hu J, Jian Q, Zhang F (2022) Integrated multi-omics reveals the activated retinal microglia with intracellular metabolic reprogramming contributes to inflammation in STZ-induced early diabetic retinopathy. Front Immunol 13:942768. https://doi.org/10.3389/fimmu.2022.942768
Sun L, Wang R, Hu G, Liu H, Lv K, Duan Y, Shen N, Wu J, Hu J, Liu Y et al (2021) Single cell RNA sequencing (scRNA-Seq) deciphering pathological alterations in streptozotocin-induced diabetic retinas. Exp Eye Res 210:108718. https://doi.org/10.1016/j.exer.2021.108718
Zhang R, Huang C, Chen Y, Li T, Pang L (2022) Single-cell transcriptomic analysis revealing changes in retinal cell subpopulation levels and the pathways involved in diabetic retinopathy. Ann Transl Med 10:562. https://doi.org/10.21037/atm-22-1546
Lee H, Jang H, Choi YA, Kim HC, Chung H (2018) Association between soluble CD14 in the aqueous humor and hyperreflective foci on optical coherence tomography in patients with diabetic macular edema. Invest Ophthalmol Vis Sci 59:715–721. https://doi.org/10.1167/iovs.17-23042
Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T (2015) Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res 45:30–57. https://doi.org/10.1016/j.preteyeres.2014.11.004
Murenu E, Gerhardt MJ, Biel M, Michalakis S (2022) More than meets the eye: the role of microglia in healthy and diseased retina. Front Immunol 13:1006897. https://doi.org/10.3389/fimmu.2022.1006897
Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW (2005) Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes 54:1559–1565. https://doi.org/10.2337/diabetes.54.5.1559
Zeng XX, Ng YK, Ling EA (2000) Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci 17:463–471. https://doi.org/10.1017/s0952523800173122
Rungger-Brändle E, Dosso AA, Leuenberger PM (2000) Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci 41:1971–1980. https://doi.org/10.1002/jcb.30378
Xiao Y, Hu X, Fan S, Zhong J, Mo X, Liu X, Hu Y (2021) Single-cell transcriptome profiling reveals the suppressive role of retinal neurons in microglia activation under diabetes mellitus. Front Cell Dev Bio 9:680947. https://doi.org/10.3389/fcell.2021.680947
Chang KC, Shieh B, Petrash JM (2019) Role of aldose reductase in diabetes-induced retinal microglia activation. Chem Biol Interact 302:46–52. https://doi.org/10.1016/j.cbi.2019.01.020
Schlotterer A, Kolibabka M, Lin J, Acunman K, Dietrich N, Sticht C, Fleming T, Nawroth P, Hammes HP (2019) Methylglyoxal induces retinopathy-type lesions in the absence of hyperglycemia: studies in a rat model. Faseb j 33:4141–4153. https://doi.org/10.1096/fj.201801146RR
Torres-Castro I, Arroyo-Camarena ÚD, Martínez-Reyes CP, Gómez-Arauz AY, Dueñas-Andrade Y, Hernández-Ruiz J, Béjar YL, Zaga-Clavellina V, Morales-Montor J, Terrazas LI et al (2016) Human monocytes and macrophages undergo M1-type inflammatory polarization in response to high levels of glucose. Immunol Lett 176:81–89. https://doi.org/10.1016/j.imlet.2016.06.001
Cheng CI, Chen PH, Lin YC, Kao YH (2015) High glucose activates Raw264.7 macrophages through RhoA kinase-mediated signaling pathway. Cell Signal 27:283–292. https://doi.org/10.1016/j.cellsig.2014.11.012
Al-Rashed F, Sindhu S, Arefanian H, Al Madhoun A, Kochumon S, Thomas R, Al-Kandari S, Alghaith A, Jacob T, Al-Mulla F et al (2020) Repetitive intermittent hyperglycemia drives the M1 polarization and inflammatory responses in THP-1 macrophages through the mechanism involving the TLR4-IRF5 pathway. Cells 9:1892. https://doi.org/10.3390/cells9081892
Jia Y, Zhou Y (2020) Involvement of lncRNAs and macrophages: potential regulatory link to angiogenesis. J Immunol Res 2020:1704631. https://doi.org/10.1155/2020/1704631
Pavlou S, Lindsay J, Ingram R, Xu H, Chen M (2018) Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immuno 19:24. https://doi.org/10.1186/s12865-018-0261-0
Santiago AR, Boia R, Aires ID, Ambrósio AF, Fernandes R (2018) Sweet stress: coping with vascular dysfunction in diabetic retinopathy. Front Physiol 9:820. https://doi.org/10.3389/fphys.2018.00820
Hwang SJ, Ahn BJ, Shin MW, Song YS, Choi Y, Oh GT, Kim KW, Lee HJ (2022) miR-125a-5p attenuates macrophage-mediated vascular dysfunction by targeting Ninjurin1. Cell Death Differ 29:1199–1210. https://doi.org/10.1038/s41418-021-00911-y
Wen Y, Chen X, Feng H, Wang X, Kang X, Zhao P, Zhao C, Wei Y (2022) Kdm6a deficiency in microglia/macrophages epigenetically silences Lcn2 expression and reduces photoreceptor dysfunction in diabetic retinopathy. Metabolism 136:155293. https://doi.org/10.1016/j.metabol.2022.155293
Inagaki Y, Yamagishi S, Okamoto T, Takeuchi M, Amano S (2003) Pigment epithelium-derived factor prevents advanced glycation end products-induced monocyte chemoattractant protein-1 production in microvascular endothelial cells by suppressing intracellular reactive oxygen species generation. Diabetologia 46:284–287. https://doi.org/10.1007/s00125-002-1013-4
Wieghofer P, Engelbert M, Chui T, Rosen R, Sakamoto T, Sebag J (2022) Hyalocyte origin, structure, and imaging. Expert review of ophthalmology 17:233–248. https://doi.org/10.1080/17469899.2022.2100762
Acknowledgements
The figure in this review was created with Biorender.
Funding
This research was funded by the Key Research and Development Program of Shaanxi Province (2023-YBSF-585) and the Natural Science Basic Research Program of Shaanxi Province (2022JQ-857).
Author information
Authors and Affiliations
Contributions
Conceptualization, AZ; methodology, YZ; writing—original draft preparation, YZ; writing—review and editing, AZ; visualization, YZ.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Zhou, A. Macrophage activation contributes to diabetic retinopathy. J Mol Med 102, 585–597 (2024). https://doi.org/10.1007/s00109-024-02437-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-024-02437-5