Abstract
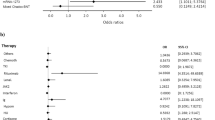
Monoclonal antibodies, as tixagevimab/cilgavimab, have been introduced as prophylaxis against COVID-19 infections in high-risk populations. However, data on efficacy are limited. This study investigates efficacy and tolerability of tixagevimab/cilgavimab in hematological patients under real-life conditions. Tixagevimab/cilgavimab was administered to 155 hematological patients (March-August 2022) at two Austrian centres. S/RBD-antibody assessments were performed before (T0), four weeks (T1), and six months (T2) after application. Side effects, the occurrence of COVID-19 infections, and the course of S/RBD-antibody titres were analysed retrospectively in relation to clinical variables. 155 hematological patients, who refused tixagevimab/cilgavimab, were included as a control group to compare the frequency of COVID-19 infections. Of all immunised patients (52.3% males; 91% triple vaccinated), 25.8% had a COVID-19 breakthrough infection (76% mild) compared to 43.9% in the control group. Patients with chronic lymphocytic leukaemia (CLL)/lymphoma were at highest risk of a COVID-19 infection (OR = 2.21; 95% CI 1.05–4.65; p = 0.037). After immunisation, a steep increase in median antibody levels (1193.4BAU/ml, IQR 0–2318.94) was observed in 67.8%, followed by a rapid decrease between T1 and T2 (465.95BAU/ml, IQR 0–1900.65.3) with the greatest declines in CLL/lymphoma (848.7BAU/ml, IQR 0–1949.6, p = 0.026). Side-effects occurred in 21.2% (CTCAE I/II). These real-world data indicate that S/RBD antibodies respond rapidly after passive immunisation in all hematological patients without safety concerns. Given the rapid decline in S/RBD antibodies, early booster immunisations should be considered for future scenarios in this vulnerable group.





Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
WHO. WHO Coronavirus (COVID-19) dashboard. Available from: https://covid19.who.int/. Accessed 13.09.2023
Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B et al (2020) Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood 136(25):2881–2892
Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A et al (2020) Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov 10(7):935–941
Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G et al (2020) Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med 8(9):853–862
Benda M, Mutschlechner B, Ulmer H, Grabher C, Severgnini L, Volgger A et al (2021) Serological SARS-CoV-2 antibody response, potential predictive markers and safety of BNT162b2 mRNA COVID-19 vaccine in haematological and oncological patients. Br J Haematol. 195(4):523–531
Malard F, Gaugler B, Gozlan J, Bouquet L, Fofana D, Siblany L et al (2021) Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J 11(8):142
Pimpinelli F, Marchesi F, Piaggio G, Giannarelli D, Papa E, Falcucci P et al (2021) Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol 14(1):81
Shen Y, Freeman JA, Holland J, Naidu K, Solterbeck A, Van Bilsen N et al (2022) Multiple COVID-19 vaccine doses in CLL and MBL improve immune responses with progressive and high seroconversion. Blood 140(25):2709–2721
Lee HK, Hoechstetter MA, Buchner M, Pham TT, Huh JW, Müller K et al. Analysis of immune responses in patients with CLL after heterologous COVID-19 vaccination. Blood Adv 7(10):2214-2227. https://doi.org/10.1182/bloodadvances.2022008445
Belda-Iniesta C (2021) Optimising SARS-CoV-2 vaccination schedules. Lancet. 398(10303):819–821
Reimann P, Ulmer H, Mutschlechner B, Benda M, Severgnini L, Volgger A et al (2022) Efficacy and safety of heterologous booster vaccination with Ad26.COV2.S after BNT162b2 mRNA COVID-19 vaccine in haemato-oncological patients with no antibody response. Br J Haematol. 196(3):577–584
Al Hajji Y, Taylor H, Starkey T, Lee LYW, Tilby M (2022) Antibody response to a third booster dose of SARS-CoV-2 vaccination in adults with haematological and solid cancer: a systematic review. Br J Cancer 1–10
Patel R, Kaki M, Potluri VS, Kahar P, Khanna D (2022) A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum Vaccin Immunother 18(1):2002083
Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E et al (2021) Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 373:n1088
Langerbeins P, Hallek M (2022) COVID-19 in patients with hematologic malignancy. Blood 140(3):236–252
Pagano L, Salmanton-García J, Marchesi F, Busca A, Corradini P, Hoenigl M et al (2021) COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA). J Hematol Oncol 14(1):168
Mink S, List W, Hoefle G, Frick M, Suessenbacher A, Winder T et al (2023) Evaluation of SARS-CoV-2 antibody levels on hospital admission as a correlate of protection against mortality. J Intern Med 293(6):694–703
Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A et al (2022) Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19. N Engl J Med 386(23):2188–2200
Nguyen Y, Flahault A, Chavarot N, Melenotte C, Cheminant M, Deschamps P et al (2022) Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Clin Microbiol Infect 28(12):1654.e1651-1654
Benotmane I, Velay A, Vargas GG, Olagne J, Cognard N, Heibel F et al (2022) A rapid decline in the anti-receptor-binding domain of the SARS-CoV-2 spike protein IgG titer in kidney transplant recipients after tixagevimab-cilgavimab administration. Kidney Int 102(5):1188–1190
Roche Deutschland Holding GmbH. Elecsys Anti-SARS-CoV-2-S 2021. Available from: https://diagnostics.roche.com/us/en/products/params/elecsys-anti-sars-cov-2-s.html#productInfo. Accessed 11.03.2023
Abbott. SARS-COV-2 IMMUNOASSAYS 2021. Available from: https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2-. Accessed 11.03.2023
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S et al (2020) Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 383(27):2603–2615
United States Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed 25.06.2023
Petzer V, Steiner N, Angelova-Unterberger O, Hetzenauer G, Philipp-Abbrederis K, Willenbacher E et al (2022) Serologic Responses to COVID-19 Vaccines in Hematological Patients Are Predominantly Impaired in Lymphoid but not in Myeloid Malignancies. Hemasphere 6(3):e686
Davis JA, Granger K, Roubal K, Smith D, Gaffney KJ, McGann M et al (2023) Efficacy of tixagevimab-cilgavimab in preventing SARS-CoV-2 for patients with B-cell malignancies. Blood 141(2):200–203
Austrian Agency for Health and Food Safety. Coronavirus - AGES [Available from: https://www.ages.at/en/human/disease/pathogens-from-a-to-z/coronavirus. Accessed 04.04.2023
Mauro FR, Visentin A, Giannarelli D, Molinari MC, Proietti G, Petrella M et al (2023) Pre-exposure prophylaxis with tixagevimab/cilgavimab in patients with chronic lymphocytic leukaemia treated with targeted agents. Br J Haematol. 201(3):564–567
Focosi D, Casadevall A (2022) A critical analysis of the use of Cilgavimab plus Tixagevimab Monoclonal Antibody Cocktail (Evusheld™) for COVID-19 prophylaxis and treatment. Viruses. 14(9):1999
Mellinghoff SC, Cornely OA (2022) SARS-CoV-2 vaccination in CLL: how often is enough? Blood 140(25):2655–2657
Mikolajewska A, Kling K, Piechotta V, Koch J, Burchard G, Garbe E et al (2023) Wissenschaftliche Begründung der STIKO zur Aktualisierung der Empfehlung zur SARS-CoV-2-Prä-Expositionsprophylaxe mit Tixagevimab/Cilgavimab (Evusheld). Epid Bull 8:39–44
AstraZeneca. Update on US Food and Drug Administration Emergency Use Authorisation of Evusheld. Available from: https://www.astrazeneca.com/media-centre/press-releases/2023/update-on-evusheld-us-eua.html. Accessed 25.06.2023
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors of this research paper have directly participated in the planning, execution, or analysis of the study.
MB, PR, BH and TW contributed to the study conception and design. MB, PR, VP, AM, BH, LS, AW, TL, MH, KG, JR, MA, SM, PF, SS, NS, AG, EG, DN, WW, DW and TW performed data collection and analysis. MB and PR wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Medical University of Innsbruck (EC No: 1088/2021 and 1355/2022).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Patrick Reimann and Verena Petzer contributed equally to this work as co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reimann, P., Petzer, V., Mündlein, A. et al. Efficacy and safety of tixagevimab/cilgavimab as passive immunisation against COVID-19 infections in patients with hematological malignancies. Ann Hematol (2024). https://doi.org/10.1007/s00277-024-05671-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00277-024-05671-6