Abstract
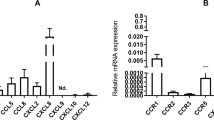
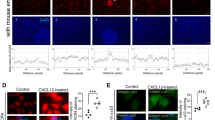
C-C motif chemokine ligand 2 (CCL2) has been reported to be expressed in the bovine endometrium during pregnancy. However, the details of its functions involved in the implantation mechanism are still not clear. The purpose of this study is to analyze the functional properties of CCL2 in the bovine endometrium and embryos. The expression of CCR2 was not different between the luteal phase and implantation phase of their endometrial tissues, but was significantly high in IFNa treated bovine endometrial stromal (BES) cells in vitro. The expressions of PGES1, PGES2, AKR1C4, and AKR1C4 were high at the implantation stage compared with the luteal stage. On the other hand, PGES2 and AKR1B1 in BEE and PGES3 and AKR1A1 in BES were significantly increased by CCL2 treatment, respectively. The expressions of PCNA and IFNt were found significantly high in the bovine trophoblastic cells (BT) treated with CCL2 compared to the control. CCL2 significantly increased the attachment rate of BT vesicles to BEE in in vitro co-culture system. The expression of OPN and ICAM-1 increased in BEE, and ICAM-1 increased in BT by CCL2 treatment, respectively. The present results indicate that CCL2 has the potential to regulate the synthesis of PGs in the endometrium and the embryo growth. In addition, CCL2 has the possibility to regulate the process of bovine embryo attachment to the endometrium by modulation of binding molecules expression.







Similar content being viewed by others
Data availability
All data included in this study are available upon request by contact with the corresponding author.
References
Andoh K, Nishimori A, Sakumoto R, Hayashi K-G, Hatama S (2020) The chemokines CCL2 and CXCL10 produced by bovine endometrial epithelial cells induce migration of bovine B lymphocytes, contributing to transuterine transmission of BLV infection. Vet Microbiol 242:108598
Asselin E, Johnson GA, Spencer TE, Bazer FW (2001) Monocyte chemotactic protein-1 and-2 messenger ribonucleic acids in the ovine uterus: regulation by pregnancy, progesterone, and interferon-τ. Biol Reprod 64:992–1000
Attar R, Agachan B, Kuran SB, Cacina C, Sozen S, Yurdum LM, Attar E, Isbir T (2010) Association of CCL2 and CCR2 gene variants with endometrial cancer in Turkish women. In Vivo 24:243–248
Bose S, Cho J (2013) Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch Pharmacal Res 36:1039–1050
Burns MJ, Nixon GJ, Foy CA, Harris N (2005) Standardisation of data from real-time quantitative PCR methods – evaluation of outliers and comparison of calibration curves. BMC Biotechnol 5:31. https://doi.org/10.1186/1472-6750-5-31
Du M-R, Wang S-C, Li D-J (2014) The integrative roles of chemokines at the maternal–fetal interface in early pregnancy. Cell Mol Immunol 11:438–448
Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA (1986) Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol 137:245–254
Ezashi T, Ealy AD, Ostrowski MC, Roberts RM (1998) Control of interferon-τ gene expression by Ets-2. Proc Natl Acad Sci 95:7882–7887
Gao W, Tang X, Chen Z, Guo Y, Wang L, Zhang M, Huang G (2013) Effects of acupuncture on CCL2 and CXCL8 expression and the subset of uNK cells in rats with embryo implantation failure. Evid Based Complement Altern Med 2013:678390. https://doi.org/10.1155/2013/678390
Gschwandtner M, Derler R, Midwood KS (2019) More than just attractive: how CCL2 influences myeloid cell behavior beyond chemotaxis. Front Immunol 10:2759
Hannan NJ, Salamonsen LA (2007) Role of chemokines in the endometrium and in embryo implantation. Curr Opin Obstet Gynecol 19:266–272
Idelevich A, Vilella F (2020) Mother and Embryo Cross-Communication Genes 11:376
Imakawa K, Carlson K, McGuire W, Christenson R, Taylor A (1997) Enhancement of ovine trophoblast interferon by granulocyte macrophage-colony stimulating factor: possible involvement of protein kinase C. J Mol Endocrinol 19:121–130
Imakawa K, Sato D, Sakurai T, Godkin JD (2009) Molecular mechanisms associated with conceptus-endometrium interactions during the peri-implantation period in ruminants. J Mamm Ova Res 26:98–110
Ireland JJ, Murphee R, Coulson P (1980) Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum1. J Dairy Sci 63:155–160
Ishikura H, Takahashi C, Kanagawa K, Hirata H, Imai K, Yoshiki T (1991) Cytokine regulation of ICAM-1 expression on human renal tubular epithelial cells in vitro. Transplantation 51:1272–1275
Johnson GA, Burghardt RC, Bazer FW, Spencer TE (2003) Osteopontin: roles in implantation and placentation. Biol Reprod 69:1458–1471. https://doi.org/10.1095/biolreprod.103.020651
Koenen A, Babendreyer A, Schumacher J, Pasqualon T, Schwarz N, Seifert A, Deupi X, Ludwig A, Dreymueller D (2017) The DRF motif of CXCR6 as chemokine receptor adaptation to adhesion. PLoS ONE 12:e0173486
Koshi K, Suzuki Y, Nakaya Y, Imai K, Hosoe M, Takahashi T, Kizaki K, Miyazawa T, Hashizume K (2012) Bovine trophoblastic cell differentiation and binucleation involves enhanced endogenous retrovirus element expression. Reprod Biol Endocrinol 10:1–11
Lacroix-Pépin N, Danyod G, Krishnaswamy N, Mondal S, Rong P-M, Chapdelaine P, Fortier MA (2011) The multidrug resistance-associated protein 4 (MRP4) appears as a functional carrier of prostaglandins regulated by oxytocin in the bovine endometrium. Endocrinology 152:4993–5004
Lecce L, Kaneko Y, Madawala RJ, Murphy CR (2011) ICAM1 and fibrinogen-γ are increased in uterine epithelial cells at the time of implantation in rats. Mol Reprod Dev 78:318–327
Lee J, McCracken JA, Stanley JA, Nithy TK, Banu SK, Arosh JA (2012) Intraluteal prostaglandin biosynthesis and signaling are selectively directed towards PGF2alpha during luteolysis but towards PGE2 during the establishment of pregnancy in sheep. Biol Reprod 87(97):1–14
Li M-Q, Li H-P, Meng Y-H, Wang X-Q, Zhu X-Y, Mei J, Li D-J (2012) Chemokine CCL2 enhances survival and invasiveness of endometrial stromal cells in an autocrine manner by activating Akt and MAPK/Erk1/2 signal pathway. Fertil Steril 97(919–29):e1
Lim W, Bae H, Bazer FW, Kim SM, Song G (2018) C—C motif chemokine ligand 2 regulates lps-induced inflammation and ER stress to enhance proliferation of bovine endometrial epithelial cells. J Cell Physiol 233:3141–3151
Lin Y-M, Hsu C-J, Liao Y-Y, Chou M-C, Tang C-H (2012) The CCL2/CCR2 axis enhances vascular cell adhesion molecule-1 expression in human synovial fibroblasts. PLoS ONE 7:e49999
Mamo S, Mehta JP, McGettigan P, Fair T, Spencer TE, Bazer FW, Lonergan P (2011) RNA sequencing reveals novel gene clusters in bovine conceptuses associated with maternal recognition of pregnancy and implantation. Biol Reprod 85:1143–1151
Mansouri-Attia N, Oliveira LJ, Forde N, Fahey AG, Browne JA, Roche JF, Sandra O, Reinaud P, Lonergan P, Fair T (2012) Pivotal role for monocytes/macrophages and dendritic cells in maternal immune response to the developing embryo in cattle. Biol Reprod 87(123):1–12
Masuko-Hongo K, Sato T, Nishioka K (2005) Chemokines differentially induce matrix metalloproteinase-3 and prostaglandin E. Clin Exp Rheumatol 23:57–62
Moore K, Thatcher W (2006) Major advances associated with reproduction in dairy cattle. J Dairy Sci 89:1254–1266
Moraes JG, Behura SK, Geary TW, Hansen PJ, Neibergs HL, Spencer TE (2018) Uterine influences on conceptus development in fertility-classified animals. Proc Natl Acad Sci 115:E1749–E1758
Nishino D, Kotake A, Yun CS, Rahman A-NMI, El-Sharawy M, Yamanaka K-i, Khandoker MY, Yamauchi N (2021) Gene expression of bovine endometrial epithelial cells cultured in matrigel. Cell Tissue Res 385(1):265–275
Parent J, Chapdelaine P, Sirois J, Fortier MA (2002) Expression of microsomal prostaglandin E synthase in bovine endometrium: coexpression with cyclooxygenase type 2 and regulation by interferon-τ. Endocrinology 143:2936–2943
Sakumoto R, Hayashi K-G, Fujii S, Kanahara H, Hosoe M, Furusawa T, Kizaki K (2017) Possible roles of CC-and CXC-chemokines in regulating bovine endometrial function during early pregnancy. Int J Mol Sci 18:742
Sakurai T, Bai H, Bai R, Arai M, Iwazawa M, Zhang J, Konno T, Godkin JD, Okuda K, Imakawa K (2012) Coculture system that mimics in vivo attachment processes in bovine trophoblast cells. Biol Reprod 87(60):1–11
Schiffmacher AT, Keefer CL (2013) CDX2 regulates multiple trophoblast genes in bovine trophectoderm CT-1 cells. Mol Reprod Dev 80:826–839
Shao J, He Y-Y, Li D-J, Li M-Q (2017) CCL2 enhances the viability of human chorionic trophoblast cell line HTR-8/SVneo Cells by inhibiting Interleukin-24 Expression. Reprod Dev Med 1:198
Sökeland G, Schumacher U (2019) The functional role of integrins during intra-and extravasation within the metastatic cascade. Mol Cancer 18:1–19
Spencer TE, Bazer FW (2004) Conceptus signals for establishment and maintenance of pregnancy. Reprod Biol Endocrinol 2:1–15
Suzuki T, Sakumoto R, Hayashi K-G, Ogiso T, Kunii H, Shirozu T, Kim S-W, Bai H, Kawahara M, Kimura K (2018) Involvement of interferon-tau in the induction of apoptotic, pyroptotic, and autophagic cell death-related signaling pathways in the bovine uterine endometrium during early pregnancy. J Reprod Dev 64:495–502
Ulbrich S, Schulke K, Groebner A, Reichenbach H, Angioni C, Geisslinger G, Meyer H (2009) Quantitative characterization of prostaglandins in the uterus of early pregnant cattle. Reproduction (cambridge, England) 138:371–382
Viney JM, Andrew DP, Phillips RM, Meiser A, Patel P, Lennartz-Walker M, Cousins DJ, Barton NP, Hall DA, Pease JE (2014) Distinct conformations of the chemokine receptor CCR4 with implications for its targeting in allergy. J Immunol 192:3419–3427
Woclawek-Potocka I, Komiyama J, Saulnier-Blache JS, Brzezicka E, Bah MM, Okuda K, Skarzynski DJ (2008) Lysophosphatic acid modulates prostaglandin secretion in the bovine uterus. Reproduction (cambridge, England) 137:95–105
Yamakoshi S, Bai R, Chaen T, Ideta A, Aoyagi Y, Sakurai T, Konno T, Imakawa K (2011) Expression of mesenchymal-related genes by the bovine trophectoderm following conceptus attachment to the endometrial epithelium. Reproduction (cambridge, England) 143:377–387
Yamauchi N, Yamada O, Takahashi T, Imai K, Sato T, Ito A, Hashizume K (2003) A three-dimensional cell culture model for bovine endometrium: regeneration of a multicellular spheroid using ascorbate. Placenta 24:258–269
Yang C, Lim W, Bae H, Bazer FW, Song G (2018) CC motif chemokine ligand 2 induces proliferation and prevents lipopolysaccharide-induced inflammatory responses in bovine mammary epithelial cells. J Dairy Sci 101:4527–4541
Yun CS, Masaka H, Nishino D, Horaku S, Rahman A-NMI, Khandoker MY, Yamauchi N (2021) Analysis of novel embryonic factors of cattle and effects on endometrial cells in vitro. Anim Reprod Sci 226:106696
Funding
The present study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan (grant no. 19K22311).
Author information
Authors and Affiliations
Contributions
N.Y. conceived the study. C.S.Y., T.S., and H.T. designed the experiments. Y.S., A.I.R., and K.K. performed experiments and analyzed the data. M.A.M.Y.K. wrote the manuscript. All authors read and edited the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The animals used in this study were treated according to the guidelines for Animal Experiments in the Faculty of Agriculture of Kyushu University (no. A19-297-0) and the laws of the Japanese Government (Law no. 105 with notification no. 6).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Summary sentence
Endometrial CCL2 is regulatory factors of implantation process of bovine
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yun, C.S., Saito, Y., Rahman, AN.M.I. et al. C-C motif chemokine ligand 2 regulates prostaglandin synthesis and embryo attachment of the bovine endometrium during implantation. Cell Tissue Res 396, 231–243 (2024). https://doi.org/10.1007/s00441-024-03869-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-024-03869-8