Abstract
Potentially toxic elements (PTEs), including heavy metal exposures, have been associated with numerous negative pregnancy and birth outcomes. However, the association between PTE exposure and gestational diabetes mellitus (GDM) has not yet undergone a comprehensive systematic review. Consequently, this study undertook the first-ever systematic review and meta-analysis of observational studies concerning this association. All relevant articles published in English were searched in Scopus, PubMed, and Web of Science until November 6, 2023, adhering to the MOOSE guidelines. The quality of retrieved studies was evaluated based on the Gascon et al. method. The meta-analysis of association estimates was performed using random effects meta-analysis. Egger’s regression was employed to evaluate publication bias. In total, 16 articles (n = 116,728 participants) were included in our review, with 11 eligible for meta-analysis. Quality assessment categorized five studies (31%) as excellent, nine studies (56%) as good, and two studies (13%) as fair. Maternal high levels of Hg during pregnancy were associated with an increased risk of GDM (for each one-quartile increase in Hg: 1.20, 95% CI 1.08, 1.31), while serum Cd levels during the second trimester were associated with a lower risk of GDM (for each one-quartile increase in Cd: 0.76, 95% CI 0.65, 0.87). Furthermore, exposure to Pb was not associated with higher risk of GDM. In summary, our comprehensive review and meta-analysis underscore the possible negative influence of Hg exposure on GDM.
Similar content being viewed by others
Introduction
Gestational diabetes mellitus (GDM) is a common pregnancy complication characterized by varying degrees of glucose intolerance that emerge or are identified for the first time during pregnancy [1, 2]. Over the past two decades, there has been a global increase in the prevalence of GDM, reaching 16.7% in 2021 [17], 2020). GDM has been linked to immediate maternal complications (such as preeclampsia and primary cesarean section) [16], as well as enduring effects (such as cardiovascular disease, chronic kidney disease, and cancer susceptibility) [60]. Furthermore, an emerging body of evidence suggests that GDM is associated with heightened risks of obesity and overweight [46], insulin resistance [33], and neurocognitive development [76] in the offspring's later life. Noteworthy risk factors for GDM include obesity, hypothyroidism, advanced maternal age, and family history of diabetes [21, 74]. In addition, a growing body of research has indicated a potential link between exposure to potentially toxic elements (PTEs) and elevated GDM risk [12, 66, 69, 70]. These elements, including cadmium (Cd), arsenic (As), mercury (Hg), antimony (Sb), and lead (Pb), can be ingested through contaminated food and water, absorbed through the skin, or inhaled from polluted air [52]. It is hypothesized that PTE exposure may disrupt insulin secretion and increase insulin resistance by damaging pancreatic β cells, resulting in heightened oxidative stress [8, 73]. Nevertheless, the existing body of literature presents contradictory results, as some studies indicate a notable positive association between urinary and serum levels of As [42], Hg [27, 42], and Cd [31, 32] and GDM, while others report inconclusive associations [39, 41, 59]. Moreover, recent research suggests that certain metals such as selenium (Se), zinc (Zn), and copper (Cu) may mitigate oxidative stress and regulate insulin secretion [29, 40] potentially reducing the risk of type 2 diabetes [14, 63] and GDM [79]. However, dissenting findings have been reported, especially regarding Zn [62, 75, 77]. Hence, to arrive at a comprehensive conclusion based on the available data, it is imperative to perform a systematic review and meta-analysis of the existing evidence. This research endeavors to undertake such an analysis of observational studies investigating the link between maternal PTEs exposure during pregnancy and gestational diabetes mellitus (GDM). In addition, this study incorporates an appraisal of the quality of the screened studies and an evaluation of publication bias.
Methods
Strategy for searching and procedures for selection
To conduct this comprehensive review, a systematic exploration was undertaken utilizing Web of Science, PubMed, and Scopus, following the guidelines outlined in the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) framework [64]. The search aimed to identify pertinent articles investigating the correlation between potential toxic elements (PTEs) exposure during pregnancy and GDM. The search spanned until November 6, 2023. Our search strategy, elaborated in Table S1 of the Supplementary Materials, employed key terms related to PTEs and GDM, without temporal limitations. Utilizing the "OR" operator, we combined various PTE-related keywords and GDM terms, while the "AND" operator was used to merge exposure and outcome concepts within our search. To ensure comprehensiveness, the reference lists of retrieved papers were manually checked. The search process was carried out independently by two review authors (MLN and RTH) and any discrepancies were resolved through discussion and consensus between them.
Criteria for inclusion and exclusion and process of data retrieval
This review considered various types of observational study designs, including cohort, cross-sectional, and case–control studies, to explore the relationship between prenatal exposure to PTEs and the development of GDM. Our analysis encompassed a wide array of PTE types, including AS, Pb, Hg, Ni, Cr, and others, without imposing any restrictions or filters. Maternal exposure routes to PTEs, such as occupational, dietary, and ambient exposure, were all considered, as were diverse methods of assessing PTE exposure in pregnant women. The primary study outcome focused on diagnosing GDM during pregnancy, irrespective of factors such as parity, gestational age, maternal age, and the method used to ascertain GDM. To ensure homogeneity, only English language papers were incorporated, while animal studies, reviews, and clinical trials were deliberately excluded from the review.
To extract vital general and methodological details from each study, a data extraction sheet (refer to Table 1) was employed. This sheet encompassed crucial information such as the author's name and publication year, study location, participant demographics, sample size, timing of GDM assessment during gestation, PTE exposure and outcome definitions, PTE concentration, study design, and the measure of association. This measure of association encompassed various forms including correlation coefficient (r), beta-coefficient (β), relative risk (RR), and odds ratio (OR), among others.
Quality assessment
We evaluated the quality of each article included in the review through an extensive 8-criterion checklist (Additional file 1: Table S2) developed by [20] that has been developed for and implemented in the previous systematic reviews of the available evidence on the human health effects of the environmental exposures [19, 37, 38]. After adapting the checklist to align with the present study's objectives, each criterion was assigned a point score ranging from 0 to 2, contributing to a cumulative score of 0 to 11. Subsequently, the total score for each study was converted into a percentage relative to the maximum potential score, enabling the categorization of study quality. Specifically, the percentage ranges were as follows: ≤ 20% denoted very poor quality, 21–40% represented poor quality, 41–60% indicated fair quality, 61–80% signified good quality, and ≥ 81% defined excellent quality [27, 29]. The assessment of article quality was conducted independently by two authors, with any disparities resolved through consensus between them.
Statistical analysis
The associations reported across various studies were harmonized into standardized units [43, 45, 48, 50, 68]. We only combined the results of studies that were reported based on the same measure of association and exposure assessment (method of measurement, media of measurement and exposure metrics). We systematically gathered eligible studies that met our inclusion criteria, extracting the relevant effect estimates along with their standard errors or confidence intervals and sample sizes. We thoroughly assessed heterogeneity among the selected studies using established statistical tests such as Cochran's Q and the I2 statistic. We considered heterogeneity both in the choice of the statistical model and in the interpretation of the results. Based on the degree of heterogeneity, we employed appropriate statistical models. Effect sizes were estimated by the restricted maximum-likelihood approach with weighted random effects and are expressed as standardized odds ratio (ORs) with 95% CIs. We also performed the sub-group analysis to determine the potential impacts of exposure timing during pregnancy and sample type. Because of the limited number of studies included in each meta-analysis and the resulting limited statistical power of Cochran's Q test, a cautious approach was taken, we utilized a random-effect model, allowing for variation both within and between studies [4, 25]. To evaluate potential publication bias, Egger's regression and Funnel plots were utilized [13]. In this study, statistical significance was defined as p < 0.05. The statistical analysis was carried out using Stata software version 16 (Stata Corp LP, 100 College Station, Texas). The leave-one-out sensitivity analyses was conducted for each meta-analysis result to illustrate the robustness of the results.
Results
Characteristics of selected studies
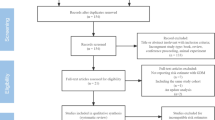
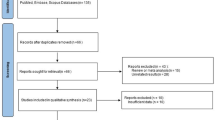
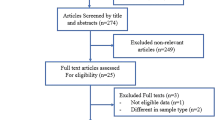
We retrieved a total of 1579 articles in our initial search after omitting 374 duplicate ones. The screening title resulted in extracting 23 articles and among them, 18 remained after completing screening abstracts (Fig. 1). During the full-text screening step, two articles were excluded after careful review due to lack of exposure and outcome of interest, leaving a total of 16 articles (n = 116,728 participants) included in our review. Among these, 11 articles were utilized for the meta-analyses [27, 31, 32, 41, 54, 62, 66, 69, 70, 78].
In the final review and meta-analysis, the selected 16 studies exhibited diverse characteristics. Among these, six studies (37.5%) employed a cross-sectional design [41, 53, 54, 62, 66, 70], five studies (31.3%) had a cohort design [31, 32, 69, 77, 78], and the remaining five studies (31.2%) utilized a case–control design [27, 42, 71, 72, 79]. Geographically, the majority of the studies (n = 13) were conducted in Asia [27, 31, 32, 41, 42, 53, 66, 69,70,71,72, 78, 79], with two studies taking place in the USA [54, 77], and just one study conducted in Europe [62].
The PTEs exposure was assessed through the sample of maternal blood (n = 10) [41, 42, 53, 62, 66, 70, 71, 77,78,79], maternal urine (n = 4) [31, 32, 54, 69], and maternal hair (n = 2) [27, 72].
In terms of GDM diagnosis, 10 studies (62.5%) utilized 75-g OGTT based on the International Association of Diabetes and Pregnancy Study Group criteria (IADPSG) [27, 31, 32, 53, 69, 71, 72, 77,78,79], two studies (12.5%) used 75-g OGTT based on the American College of Obstetricians and Gynecologists criteria (ACOG) [54, 70], two studies (12.5%) used 75-g OGTT based on the Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians (JAOG) criteria [41, 66], and two studies (14%), diagnosed using 100-g OGTT based on Carpenter and Coustan criteria [42, 62]. In all studies, GDM was assessed in 24–28 weeks of pregnancy. IADPSG diagnostic criteria for GDM include fasting glucose ≥ 5.1 mmol/L (92 mg/dl), a 1-h result of ≥ 10.0 mmol/L (180 mg/dl), or a 2-h result of ≥ 8.5 mmol/L (153 mg/dl) [34]. Based on ACOG, GDM is considered when one fasting blood glucose is more than 5.1 mmol/L, or 1-h blood glucose > 10.0 mmol/L, or 2-h blood glucose > 8.5 mmol/L [70]. According to the JSOG and JAOG criteria, GDM is diagnosed when: fasting ≥ 92 mg/ dL (5.1 mmol/L); 1 h ≥ 180 mg/dL (10.0 mmol/L); and 2 h ≥ 153 mg/dL (8.5 mmol/L) [35].
Assessment of the Studies' Quality
Evaluating the quality of individual studies resulted in total scores ranging from 6 (54.5%) to 9 (81.8%) (see Additional file 1: Table S3). Five studies (31%) placed in the excellent grope [32, 42, 71, 77, 78], nine studies (56%) ranked in the good grope [31, 41, 53, 54, 62, 66, 69, 72, 79], and two ones (13%) ranked in the fair group [27, 70]. All studies received the maximum score in items “Statistics”, “Multiplicity”, “Effect size’, and “Confounding factors” (apart from one study). Nevertheless, since no study performed repeated measurements of exposure, they did not gain any score in this item. Concerning item “Study design”, the minimum score of zero and the maximum score of two were obtained by six studies, because of having cross-sectional design [41, 53, 54, 62, 66, 70] and five studies due to having cohort design [31, 32, 69, 77, 78], respectively.
Association with cadmium
The relationship between Cd exposure during pregnancy and the risk of GDM was examined in 13 studies, with four of them involving the collection of maternal urinary samples for Cd analysis [31, 32, 54, 69], seven articles used maternal serum samples [41, 42, 53, 62, 66, 70, 77], and two studies collected maternal hair samples [27, 72]. The maternal levels of Cd were measured in the second trimester of pregnancy by eight studies [27, 41, 42, 53, 54, 62, 66, 70] and at the first trimester by the rest five ones [31, 32, 69, 72, 77]. Five studies documented a statistically significant association between Cd exposure and an elevated risk of GDM [31, 42, 53, 66, 77], while the rest did not discover any notable correlation [27, 32, 41, 54, 62, 69, 70, 72].
Of the 12 reviewed studies that investigated the relationship between the maternal levels of Cd and GDM risk, 10 studies entered into the meta-analysis [27, 31, 32, 41, 54, 62, 66, 69, 70, 72]. The results did not present a statistically significant association between maternal exposure to Cd and increased risk of GDM (for each one-quartile increase in Cd: 1.00, 95% CI 0.83, 1.17). Overall, high heterogeneity was observed between these studies (Cochran's Q test p value = 0.00 and I2 of 69.90%) (Fig. 2).
Moreover, we performed two sensitive meta-analyses first based on the type of collected biological sample (serum vs. urine) and second based on the trimester of pregnancy (first vs. the second trimester) when the Cd was measured. As such, a meta-analysis was performed on three studies that assessed urinary Cd levels during the first trimester [31, 32, 69] and revealed no significant association between elevated Cd levels and an increased risk of GDM (for each one-quartile increase in Cd: 1.68, 95% CI 0.65, 2.70). There was high indication of heterogeneity between these studies (Cochran's Q test p value = 0.00 and I2 of 91.55%) (Additional file 1: Fig. S1). When we combined those studies that assessed the urinary levels of Cd in the first trimester with those in the second trimester [54], similarly, we did not find a significant relationship (for each one-quartile increase: 1.44, 95% CI 0.67, 2.22) with a high heterogeneity between studies (Cochran's Q test p value = 0.00 and I2 of 90.09%) (Fig. 2, part 1).
The meta-analysis of four studies that measured serum levels of Cd in the second trimester [41, 62, 66, 70] showed a significant association between elevated Cd and a lower risk of GDM (for each one-quartile increase in Cd: 0.76, 95% CI 0.65, 0.87) with no indication of heterogeneity between the studies (Cochran's Q test p value = 0.34 and I2 of 0.00%) (Fig. 2, part 2).
Moreover, we performed a subgroup analysis based on study design (i.e., cohort, cross-sectional and case–control). The results were the same as the main analysis in terms of direction and significance (Additional file 1: Fig. S2). The combined results of cross-sectional studies showed that exposure to Cd was associated with decreased risk of GDM. However, the combined results of studies with cohort and case–control designs did not show a significant association.
Association with lead
Among 16 reviewed studies, nine papers assessed the relationship of maternal levels of Pb with the risk of GDM except for two studies (maternal hair) [27, 72], the others measured serum levels of Pb [41, 42, 62, 66, 70, 77, 78]. Furthermore, only three articles assessed the levels of maternal exposure to Pb in the first trimester of pregnancy [72, 77, 78] and the rest of the studies evaluated that in the second trimester [27, 41, 42, 62, 66, 70]. One case–control study showed a significant difference between the mean maternal levels of Pb between GDM and healthy pregnant mothers [42] In contrast, some studies did not observe a statistically significant relationship between Pb exposure and the risk of GDM [27, 41, 62, 66, 70, 72, 77, 78].
We included seven studies [27, 41, 62, 66, 70, 72, 78] in the meta-analysis of the association between maternal levels of Pb and the risk of GDM indicated that the association between exposure to Pb and risk of GDM was not statistically significant (for each one-quartile increase in Pb: 0.95, 95% CI 0.88, 1.03) with no indication of heterogeneity between the studies (Cochran's Q test p value of 0.49 and I2 of 0.00%) (Fig. 3).
The sensitivity analysis was performed on five articles that measured the levels of Pb only using serum samples [41, 62, 66, 70, 78] and four studies that only assessed the serum levels of Pb in the second trimester [41, 62, 66, 70]. The results of the first (for each one-quartile increase in pb: 0.93, 95% CI 0.84, 1.03) (Fig. 3, part 1) and second sensitivity analysis (for each one-quartile increase in Pb: 0.98, 95% CI 0.84, 1.12) (Fig. S3 of Supplemental Materials) indicated a nonsignificant association between the serum levels of Pb and increased risk of GDM with no indication of heterogeneity (Cochran's Q test p value of 0.5 and I2 of 0.00%) and (Cochran's Q test p value of 0.47 and I2 of 0.00%), respectively.
Moreover, the subgroup meta-analysis of exposure to Pb and odds ratio of GDM based on study design showed the same results as the main analysis (Additional file 1: Fig. S4). There was no significant relationship between exposure to Pb and risk of GDM in both cross-sectional and case–control studies.
Association with Mercury
Seven studies examined the relationship between maternal Hg levels and GDM risk, five of them analyzing Hg levels in maternal serum samples [42, 53, 66, 70, 72, 77], except two that collected maternal hair samples [27, 72]. Moreover, two studies assessed the levels of Hg in the first trimester [72, 77], while the others measured Hg in the second trimester [27, 42, 53, 66, 70].
Among the seven reviewed papers, the meta-analysis was performed on four studies [27, 66, 70] that evidenced a significant relationship between the maternal levels of Hg and the increased risk of GDM (for each one-quartile increase in Hg: 1.20, 95% CI 1.08, 1.31). No significant heterogeneity was observed among these studies, as indicated by a Cochran's Q test p value of 0.44 and an I2 value of 0.00% (Fig. 4). When we performed the sensitivity analysis on two articles that measured levels of Hg in serum samples [66, 70], the result was not changed in terms of significance (for each one-quartile increase in Hg: 1.25, 95% CI 1.07, 1.43) and there was no heterogeneity observed among the studies, as evidenced by a Cochran's Q test p value of 0.42 and an I2 value of 0.00% (Additional file 1: Fig. S5).
The results of the subgroup analysis based on the study design showed the same results as the main analysis (Additional file 1: Fig. S6). The combined result of exposure to Hg in cross-sectional studies was significantly associated with higher risk of GDM. However, for case–control studies, this relationship was marginally significant.
Publication bias
We independently assessed publication bias for each of the three meta-analyses, which investigated the association between maternal levels of Cd, Pb, and Hg with the risk of GDM. Our analysis did not reveal any publication bias in the studies that explored the association between Hg and Pb exposures and the risk of GDM, with p values of 0.14 and 0.67, respectively. However, the studies on the association of Cd exposure and risk of GDM indicated a publication bias between investigated studies (p value < 0.01). The results of publication bias for Hg, Pb and Cd have been depicted in Additional file 1: Figs. S4, S5, and S6, respectively.
Studies not included in meta-analyses
We excluded five studies from the meta-analysis for specific reasons. Two studies had a different scope of measured metals exposure (e.g., Se, Zn, Cu) than the others [71, 79]. While in other studies HMs were measured in blood or urine samples. In addition, three studies did not present the same measure of association with other studies (e.g., mean difference) [42, 53, 77]. Therefore, it was not possible to combine the results of this study with other studies. In a nested case–control study conducted in Wuhan, China, Zhu et al. [79] examined the association between serum levels of Mg, Zn, Ca, Fe, Cu, Se, and Cr and the risk of GDM [79]. This study involved 305 GDM cases and 305 non-GDM mothers, with blood samples collected in the first trimester. GDM diagnosis was based on a 75-g OGTT between 24 and 28 weeks, using IADPSG criteria. Their analysis revealed a significant positive association between plasma Fe levels (adjusted OR = 2.04; 95% CI 1.62, 2.57) and Cu levels (adjusted OR = 1.52; 95% CI 1.25, 1.82) with elevated GDM risk. Conversely, elevated plasma Zn levels (adjusted OR = 0.55; 95% CI 0.43, 0.71) and Ca levels (adjusted OR = 0.72; 95% CI 0.56, 0.92) were linked to a decreased GDM risk. Onat et al. [42] conducted a case–control study in Yozgat, Turkey, comparing heavy metals (Cd, Pb, antimony, Hg, and As) and trace elements (chromium-III, chromium-VI, Zn, Cu, and Se) levels between 60 GDM and 52 non-GDM pregnant women [42]. GDM was diagnosed using a 100-g OGTT following Carpenter and Coustan criteria [11]. The GDM group exhibited higher levels of Cd, Pb, Sb, and Hg than the non-GDM group, with the differences being statistically significant for Cd, Pb, and Sb (p < 0.05). Concerning trace elements, while Cu levels were higher among the GDM group, they had significantly lower Cr-III, Zn, and Se levels (p < 0.05) compared to the control group. Rezaei et al. [53] conducted a cross-sectional study in Birjand, Iran, investigating the relationship between As, Cd, Cu, Hg, Mn, Ni, V, Zn levels, and GDM risk among 60 GDM and 42 healthy pregnant women [53]. Second-trimester serum levels of trace elements were measured, and GDM was diagnosed using a 75-g OGTT between 24 and 28 weeks based on IADPSG criteria. GDM women had significantly higher mean levels of As, Cd, and Hg than non-GDM women. After adjusting for confounders, the regression model demonstrated a significant positive association between As (adjusted RD = 0.516; 95% CI 0.355, 0.677), Cd (adjusted RD = 0.719; 95% CI 0.534, 0.904), and Hg (adjusted RD = 0.505; 95% CI 0.276, 0.735) with heightened GDM risk, while V (adjusted RD = − 0.139; 95% CI, − 0.237, − 0.042) significantly reduced GDM risk. Zheng et al. [77] performed a cohort study in Boston, USA, examining the relationship between erythrocyte levels of essential (Cu, Mg, Mn, Se, Zn) and non-essential (As, Ba, Cd, Cs, Pb, Hg) metals and GDM among 1311 pregnant women [77]. Erythrocyte metal levels were measured in the first trimester, and GDM diagnosis was based on a 100-g OGTT in the late second trimester (26–28 weeks), according to Carpenter and Coustan criteria. Their findings showed an inverse U-shaped nonlinear association between erythrocyte Ba levels and GDM risk, along with an inverse association for erythrocyte Hg.
Within a nested case–control study conducted by Zhu et al. in 2021, the correlation between serum levels of Mg, Zn, Ca, Fe, Cu, Se, and Cr with GDM risk was investigated. The study involved a cohort of 305 GDM cases and 305 non-GDM mothers. Diagnosis of GDM was made through a 75-g OGTT conducted between the 24th and 28th weeks of pregnancy, using IADPSG criteria as a basis. Zhu and colleagues observed that an interquartile range (IQR) increase in plasma Fe levels was linked with a significantly heightened GDM risk (OR = 2.04; 95% CI 1.62, 2.57), as was an IQR increment in plasma Cu levels (OR = 1.52; 95% CI 1.25, 1.82). Conversely, an IQR increase in plasma Zn levels was associated with a significantly reduced GDM risk (OR = 0.55; 95% CI 0.43, 0.71), as was an IQR increment in plasma Ca levels (OR = 0.72; 95% CI 0.56, 0.92).
Discussion
To our current understanding, this study stands as the initial systematic review and meta-analysis delving into the relationship between potential toxic element (PTE) exposure during pregnancy and susceptibility to GDM. In total, 16 articles encompassing a collective 116,728 participants and published up until November 2023 were ultimately included in the comprehensive review. The meta-analysis specifically involved 11 of the examined articles. Our meta-analytical findings underscore that exposure to mercury (Hg) is linked with an increased GDM risk. Furthermore, elevated blood levels of cadmium (Cd) during the second trimester of pregnancy correlate with a reduced risk of GDM among pregnant women. However, blood levels of lead (Pb) throughout pregnancy did not exhibit a significant association with GDM risk. The evaluation of our meta-analytical associations through funnel plots revealed no evidence of publication bias.
Assessment of exposure
In our review, four studies examined PTEs using urine samples [31, 32, 54, 69], ten studies based on blood concentration of PTEs [41, 42, 53, 62, 66, 70, 77,78,79] and two studies used maternal hair samples [27] to assess the association of exposure to PTEs and GMD. Exposure assessment based on urine samples has some advantages compared to blood samples. Urine sampling is a noninvasive method and therefore it is mostly welcomed by the participants compared blood sampling method. Moreover, extraction and analysis of PTEs in urine samples are easier compared to blood samples. However, it should be noted that urine levels of PTEs indicated a last 24 h exposure to these pollutants, while blood samples show long-term exposure to PTEs [9, 36, 58]. The advantages of exposure assessment based on hair samples include easier sampling even compared to urine samples, its non-invasiveness and indicating long-term exposure [15, 23]. However, detecting the PTEs needs higher amounts of the hair sample and previous evidence showed that there is a weak association between external exposure to PTEs and hair levels of PTEs compared to urine and blood PTEs levels [44, 47].
Outcome assessment
Regarding GDM diagnosis, near to 60% of studies implemented 75-g OGTT based on the IADPSG. Consensus on using the IADPSG criteria for diagnosing GDM was made in 2010 [34] and it is well known to be associated with more diagnosed GDM cases. The most recent systematic review and meta-analysis study (2021) on 136 705 women (31 studies) evidenced that implementing IADPSG criteria linked to 0.75% increase in the number of cases with GDM [55].
Potential mechanisms
The underlying mechanisms that link potential toxic element (PTE) exposure to gestational diabetes mellitus (GDM) remain unclarified. However, emerging evidence suggests that GDM could potentially share pathways with type 2 diabetes mellitus [5, 49]. GDM is recognized as a multifaceted metabolic disorder influenced by a combination of environmental, lifestyle, and behavioural factors. Among the plausible hypotheses, one pertains to endocrine hormone disruption [65]. PTE exposure could potentially contribute to the dysfunction of beta cells by diminishing the viability of pancreatic islet beta cells, ultimately leading to impaired insulin secretion and insulin resistance, which are crucial components in the development of GDM [7, 56]. This theory is supported by research findings that suggest PTEs could impact the survival and function of islet beta cells. This impact may occur through various mechanisms, including elevated levels of inflammatory markers, such as tumour necrosis factor-alpha and interleukin 6, oxidative stress, and the inhibition of peroxisome proliferator-activated receptor gamma (PPAR-γ). These mechanisms have been demonstrated in both laboratory experiments (in vitro and in vivo) and human studies [10, 22, 67]. Insights from animal model studies reveal that even low doses of mercury (Hg) could activate phosphatidylinositol 3-kinase (PI3K), thereby impacting Akt signalling and triggering dysfunction in pancreatic beta cells. This could potentially lead to impaired maternal beta cells, ultimately resulting in diminished insulin levels and insulin resistance during early pregnancy, contributing to the onset of GDM [18, 28, 57]. Concerning the effects of Cd on GDM, Barregard, et al. [3] did not find a significant relation between levels of blood and urinary Cd concentrations and impaired glucose tolerance, type 2 diabetes, and impaired pancreatic beta-cell function in women [3]. In line with our results, research conducted on cell cultures has revealed that exposure to moderate levels of cadmium can imitate the effects of insulin. This is evidenced by an increase in glucose uptake in adipocytes and fibroblasts, as well as an acceleration in the release of insulin from pancreatic tissue when stimulated by glucose [24].
The potential synergistic effects of exposure to various potentially toxic elements (PTEs), such as cadmium (Cd), mercury (Hg), and lead (Pb), on gestational diabetes during pregnancy could be attributed to their collective impact on endocrine and metabolic pathways. Studies suggest that simultaneous exposure to multiple PTEs may disrupt insulin signalling, induce oxidative stress, and trigger inflammatory responses, collectively contributing to the development of gestational diabetes mellitus (GDM) [26, 61]. The intricate interplay between these elements might amplify their individual adverse effects, leading to a heightened risk of GDM. Furthermore, PTEs may act synergistically to impair pancreatic beta-cell function and insulin sensitivity, crucial factors in glucose homeostasis during pregnancy. Understanding the potential mechanisms of synergistic action among different PTEs is pivotal for unravelling the complex aetiology of gestational diabetes and devising effective preventive strategies [6, 51, 61].
An alternative mechanism potentially linking PTE exposure to GDM lies in the alteration of methylation patterns in genes associated with diabetes [30]. The emergence of GDM during pregnancy as a result of PTE exposure could be attributed to one or a combination of the aforementioned mechanisms.
Limitations
While our evaluation of study quality revealed four studies with an excellent rating and eight with good quality, it's important to acknowledge that certain limitations were acknowledged within these studies, underscoring the need for consideration in future research endeavors. First, the reviewed studies had an observational design therefore a possible causal relationship between PTEs exposures and GDM cannot be established. Second, some studies used urine samples to assess exposure to PTEs during pregnancy which is not a good indicator of long-term exposure to these pollutants. Moreover, the exposure pathways and the sources of PTEs were not assessed in the most of previous studies that are critical for the toxicity of different metals. Furthermore, a limited number of PTEs were assessed in the most of reviewed studies and the synergistic effect of exposure to a mixture of PTEs on GDM has not assessed yet.
Regarding limitations in our review, it should be considered that the distribution of studies was limited to Asia, the USA, and only one study in Europe and we did not observe any study from Africa, South America, and Australia. Moreover, our meta-analysis for some PTEs was only based on a limited number of studies which could lead to higher potential bias in the results. In acknowledging the limitations of our meta-analysis, it is essential to highlight that the majority of studies included were conducted in China, potentially introducing biases and limiting the generalizability of our findings to a more global context. Addressing the observed differences in exposure concentrations among various regions is crucial, as these variations are influenced by environmental, socio-economic, and industrial factors that contribute to disparities in outcomes. This regional heterogeneity should be thoroughly discussed to provide a nuanced understanding of the exposure–outcome relationship. Despite the limited number of studies included, our findings shed light on the need for future research to adopt a more expansive approach, encompassing diverse geographical locations.
Conclusion and future recommendation
We found that exposure to Hg during pregnancy was associated with higher risk of GDM. Moreover, higher serum level of Cd was associated with lower risk of GDM. However, the association of exposure to Pb and GDM was not statistically significant based on overall meta-analysis and sensitivity analysis for studies based on only serum samples and only second-trimester samples. Moreover, we observed a publication bias for studies on Cd exposures, but studies on Hg and Pb had no publication bias. Although the results of this study had some limitations, it could shed light on exposure to PTEs and pregnancy complications and help policy and decision-makers in reducing these adverse health effects, especially during pregnancy. We recommended assessing the exposure to PTEs based on internal and external measurements from different sources (e.g., measuring PTEs in water, foods, air and soil samples), and assessing all exposure pathways including ingestion, dermal and inhalation pathways. Moreover, using advanced PTEs analytical techniques with lower detection limits such as inductively coupled plasma mass spectrometry (ICP-MS) can help to assess a wide range of PTEs in future studies. Assessing based on different biological samples at the same time (i.e., blood, urine, nails, and hair) is recommended for future studies due to providing higher precision and accuracy in internal exposure assessment. Moreover, using advanced statistical analyses on mixture exposure to PTEs and health outcomes can cover the potential synergistic effects of PTEs and get more reliable results.
Data availability
The data of this study will be available on request from corresponding author.
References
2020. IDF Diabetes Atlas.
Association AD (2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37:S81–S90
Barregard L, Bergström G, Fagerberg B (2013) Cadmium exposure in relation to insulin production, insulin sensitivity and type 2 diabetes: a cross-sectional and prospective study in women. Environ Res 121:104–109
Bowden J, Tierney JF, Copas AJ, Burdett S (2011) Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Qstatistics. BMC Med Res Methodol 11:41
Buchanan TA, Xiang AH (2005) Gestational diabetes mellitus. J Clin Invest 115:485–491
Cabrera-Rodríguez R, Luzardo OP, González-Antuña A, Boada LD, Almeida-González M, Camacho M, Zumbado M, Acosta-Dacal AC, Rial-Berriel C, Henríquez-Hernández LA (2018) Occurrence of 44 elements in human cord blood and their association with growth indicators in newborns. Environ Int 116:43–51
Cerf ME (2013) Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne) 4:37
Chang K-C, Hsu C-C, Liu S-H, Su C-C, Yen C-C, Lee M-J, Chen K-L, Ho T-J, Hung D-Z, Wu C-C (2013) Cadmium induces apoptosis in pancreatic β-cells through a mitochondria-dependent pathway: the role of oxidative stress-mediated c-Jun N-terminal kinase activation. PLoS ONE 8:e54374
Choudhury TR, Zaman SZ, Chowdhury TI, Begum BA, Islam MA, Rahman MM (2021) Status of metals in serum and urine samples of chronic kidney disease patients in a rural area of Bangladesh: An observational study. Heliyon 7:e08382
Coelho-Santos V, Gonçalves J, Fontes-Ribeiro C, Silva AP (2012) Prevention of methamphetamine-induced microglial cell death by TNF-α and IL-6 through activation of the JAK-STAT pathway. J Neuroinflammation 9:103
Coustan DR, Nelson C, Carpenter MW, Carr SR, Rotondo L, Widness JA (1989) Maternal age and screening for gestational diabetes: a population-based study. Obstet Gynecol 73:557–561
Didedar R, Rabaninia T, Barmaki B, Dahmardeh S, Nori F, Bagheri S, Malayeri FA (2018) Relation between chromium, iron and copper with gestational diabetes in Zabol Iran. JCDR. https://doi.org/10.7860/JCDR/2018/35574.12268
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
El Dib R, Gameiro OL, Ogata MS, Modolo NS, Braz LG, Jorge EC et al (2015) Zinc supplementation for the prevention of type 2 diabetes mellitus in adults with insulin resistance. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD005525.pub3
Esteban-López M, Arrebola JP, Juliá M, Pärt P, Soto E, Cañas A, Pedraza-Díaz S, González-Rubio J, Castaño A (2022) Selecting the best non-invasive matrix to measure mercury exposure in human biomonitoring surveys. Environ Res 204:112394
Feng H, Zhu W-W, Yang H-X, Wei Y-M, Wang C, Su R-N, Hod M, Hadar E (2017) Relationship between oral glucose tolerance test characteristics and adverse pregnancy outcomes among women with gestational diabetes mellitus. Chin Med J 130:1012–1018
Ferrara A (2007) Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 30:S141–S146
Fujimoto K, Polonsky KS (2009) Pdx1 and other factors that regulate pancreatic beta-cell survival. Diabetes Obes Metab 11(Suppl 4):30–37
Gascon M, Sánchez-Benavides G, Dadvand P, Martínez D, Gramunt N, Gotsens X, Cirach M, Vert C, Molinuevo JL, Crous-Bou M, Nieuwenhuijsen M (2018) Long-term exposure to residential green and blue spaces and anxiety and depression in adults: a cross-sectional study. Environ Res 162:231–239
Gascon M, Triguero-Mas M, Martínez D, Dadvand P, Forns J, Plasència A, Nieuwenhuijsen MJ (2015) Mental health benefits of long-term exposure to residential green and blue spaces: a systematic review. Int J Environ Res Public Health 12:4354–4379
Giannakou K, Evangelou E, Yiallouros P, Christophi CA, Middleton N, Papatheodorou E, Papatheodorou SI (2019) Risk factors for gestational diabetes: an umbrella review of meta-analyses of observational studies. PLoS ONE 14:e0215372
Hagman S, Mäkinen A, Ylä-Outinen L, Huhtala H, Elovaara I, Narkilahti S (2019) Effects of inflammatory cytokines IFN-γ, TNF-α and IL-6 on the viability and functionality of human pluripotent stem cell-derived neural cells. J Neuroimmunol 331:36–45
Hardy EM, Dereumeaux C, Guldner L, Briand O, Vandentorren S, Oleko A, Zaros C, Appenzeller BMR (2021) Hair versus urine for the biomonitoring of pesticide exposure: results from a pilot cohort study on pregnant women. Environ Int 152:106481
Harrison SA, Buxton JM, Clancy BM, Czech MP (1991) Evidence that erythroid-type glucose transporter intrinsic activity is modulated by cadmium treatment of mouse 3T3-L1 cells. J Biol Chem 266:19438–19449
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Ji P, Wei Y, Hua Y, Zhang X, Yao W, Ma Q, Yuan Z, Wen Y, Yang C (2018) A novel approach using metabolomics coupled with hematological and biochemical parameters to explain the enriching-blood effect and mechanism of unprocessed Angelica sinensis and its 4 kinds of processed products. J Ethnopharmacol 211:101–116
Jia X, Zhang L, Zhao J, Ren M, Li Z, Wang J, Wang S, Liu Y, An H, Li Y, Yan L, Li Z, Liu X, Pan B, Ye R (2021) Associations between endocrine-disrupting heavy metals in maternal hair and gestational diabetes mellitus: A nested case-control study in China. Environ Int 157:106770
Kaneko K, Ueki K, Takahashi N, Hashimoto S, Okamoto M, Awazawa M, Okazaki Y, Ohsugi M, Inabe K, Umehara T, Yoshida M, Kakei M, Kitamura T, Luo J, Kulkarni RN, Kahn CR, Kasai H, Cantley LC, Kadowaki T (2010) Class IA phosphatidylinositol 3-kinase in pancreatic β cells controls insulin secretion by multiple mechanisms. Cell Metab 12:619–632
Kong F-J, Ma L-L, Chen S-P, Li G, Zhou J-Q (2016) Serum selenium level and gestational diabetes mellitus: a systematic review and meta-analysis. Nutr J 15:1–10
Kunysz M, Mora-Janiszewska O, Darmochwał-Kolarz D (2021) Epigenetic modifications associated with exposure to endocrine disrupting chemicals in patients with gestational diabetes mellitus. Int J Mol Sci 22:4693
Li X, Huang Y, Xing Y, Hu C, Zhang W, Tang Y, Su W, Huo X, Zhou A, Xia W, Xu S, Chen D, Li Y (2020) Association of urinary cadmium, circulating fatty acids, and risk of gestational diabetes mellitus: a nested case-control study in China. Environ Int. https://doi.org/10.1016/j.envint.2020.105527
Liu W, Zhang B, Huang Z, Pan X, Chen X, Hu C, Liu H, Jiang Y, Sun X, Peng Y, Xia W, Xu S, Li Y (2018) Cadmium body burden and gestational diabetes mellitus: A prospective study. Environ Health Perspect 126:027006
Lowe WL Jr, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, McCance D, Hamilton J, Nodzenski M, Talbot O (2019) Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care 42:372–380
Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI (2010) International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33:676–682
Minakami H, Maeda T, Fujii T, Hamada H, Iitsuka Y, Itakura A, Itoh H, Iwashita M, Kanagawa T, Kanai M, Kasuga Y, Kawabata M, Kobayashi K, Kotani T, Kudo Y, Makino Y, Matsubara S, Matsuda H, Miura K, Murakoshi T, Murotsuki J, Ohkuchi A, Ohno Y, Ohshiba Y, Satoh S, Sekizawa A, Sugiura M, Suzuki S, Takahashi T, Tsukahara Y, Unno N, Yoshikawa H (2014) Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J Obstet Gynaecol Res 40:1469–1499
Miri M, Alahabadi A, Ehrampoush MH, Ghaffari HR, Sakhvidi MJZ, Eskandari M, Rad A, Lotfi MH, Sheikhha MH (2018) Environmental determinants of polycyclic aromatic hydrocarbons exposure at home, at kindergartens and during a commute. Environ Int 118:266–273
Miri M, Nazarzadeh M, Alahabadi A, Ehrampoush MH, Rad A, Lotfi MH, Sheikhha MH, Sakhvidi MJZ, Nawrot TS, Dadvand P (2019) Air pollution and telomere length in adults: a systematic review and meta-analysis of observational studies. Environ Pollut 244:636–647
MoghaddamHosseini V, Ebrahimi Aval H, Lari Najafi M, Lotfi H, Heydari H, Miri M, Dadvand P (2023) The association between exposure to polycyclic aromatic hydrocarbons and birth outcomes: a systematic review and meta-analysis of observational studies. Sci Total Environ 905:166922
Muñoz MP, Valdés M, Muñoz-Quezada MT, Lucero B, Rubilar P, Pino P, Iglesias V (2018) Urinary inorganic arsenic concentration and gestational diabetes mellitus in pregnant women from Arica, Chile. Int J Environ Res Public Health 15:1418
Mwiti Kibiti C, Jide Afolayan A (2015) The biochemical role of macro and micro-minerals in the management of diabetes mellitus and its associated complications: a review. Int J Vitam Nutr Res 85:88–103
Oguri T, Ebara T, Nakayama SF, Sugiura-Ogasawara M, Kamijima M, Kawamoto T, Saito H, Kishi R, Yaegashi N, Hashimoto K, Mori C, Ito S, Yamagata Z, Inadera H, Kamijima M, Nakayama T, Iso H, Shima M, Hirooka Y, Suganuma N, Kusuhara K, Katoh T (2019) Association between maternal blood cadmium and lead concentrations and gestational diabetes mellitus in the Japan Environment and Children’s Study. Int Arch Occup Environ Health 92:209–217
Onat T, Demir Caltekin M, Turksoy VA, Baser E, Aydogan Kirmizi D, Kara M, Yalvac ES (2021) The relationship between heavy metal exposure, trace element level, and monocyte to HDL cholesterol ratio with gestational diabetes mellitus. Biol Trace Elem Res 199:1306–1315
Orsi L, Magnani C, Petridou ET, Dockerty JD, Metayer C, Milne E, Bailey HD, Dessypris N, Kang AY, Wesseling C, Infante-Rivard C, Wünsch-Filho V, Mora AM, Spector LG, Clavel J (2018) Living on a farm, contact with farm animals and pets, and childhood acute lymphoblastic leukemia: pooled and meta-analyses from the Childhood Leukemia International Consortium. Cancer Med 7:2665–2681
Ostrea EM, Villanueva-Uy E, Bielawski DM, Posecion NC, Corrion ML, Jin Y, Janisse JJ, Ager JW (2006) Maternal hair—an appropriate matrix for detecting maternal exposure to pesticides during pregnancy. Environ Res 101:312–322
Passos JDC, Felisbino K, Laureano HA, Guiloski IC (2022) Occupational exposure to pesticides and its association with telomere length—a systematic review and meta-analysis. Sci Total Environ 849:157715
Philipps L, Santhakumaran S, Gale C, Prior E, Logan K, Hyde M, Modi N (2011) The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia 54:1957–1966
Pino A, Chiarotti F, Calamandrei G, Gotti A, Karakitsios S, Handakas E, Bocca B, Sarigiannis D, Alimonti A (2017) Human biomonitoring data analysis for metals in an Italian adolescents cohort: an exposome approach. Environ Res 159:344–354
Pinot de Moira A, Strandberg-Larsen K, Bishop T, Pedersen M, Avraam D, Cadman T, Calas L, Casas M, de Lauzon Guillain B, Elhakeem A, Esplugues A, Estarlich M, Foong RE, Haakma S, Harris JR, Huang R-C, Inskip H, Lertxundi A, Mensink-Bout SM, Nader JLT, Pizzi C, Popovic M, Salika T, Sunyer J, Van Meel ER, Swertz MA, Jaddoe VWV, Burton P, Duijts L, Nybo Andersen A-M (2022) Associations of early-life pet ownership with asthma and allergic sensitization: A meta-analysis of more than 77,000 children from the EU Child Cohort Network. J Allergy Clin Immunol 150:82–92
Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH (2018) The pathophysiology of gestational diabetes mellitus. Int J Mol Sci 19:3342
Polanin JR, Snilstveit B (2016) Converting between effect sizes. Campbell Syst Rev 12:1–13
Pyatha S, Kim H, Lee D, Kim K (2023) Co-exposure to lead, mercury, and cadmium induces neurobehavioral impairments in mice by interfering with dopaminergic and serotonergic neurotransmission in the striatum. Front Public Health 11:1265864
Rehman K, Fatima F, Waheed I, Akash MSH (2018) Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem 119:157–184
Rezaei M, Blaszczyk M, Tinkov AA, Binkowski LJ, Mansouri B, Skalny A, Azadi N, Dosa MD, Bjorklund G (2021) Relationship between gestational diabetes and serum trace element levels in pregnant women from Eastern Iran: a multivariate approach. Environ Sci Pollut Res 28:45230–45239
Romano ME, Gallagher LG, Jackson BP, Baker E, Karagas MR (2019) Maternal urinary cadmium, glucose intolerance and gestational diabetes in the New Hampshire Birth Cohort Study. Environ Res. https://doi.org/10.1016/j.envres.2019.108733
Saeedi M, Cao Y, Fadl H, Gustafson H, Simmons D (2021) Increasing prevalence of gestational diabetes mellitus when implementing the IADPSG criteria: a systematic review and meta-analysis. Diabetes Res Clin Pract 172:108642
Saisho Y (2015) β-cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J Diabetes 6:109–124
Salazar-Petres ER, Sferruzzi-Perri AN (2022) Pregnancy-induced changes in β-cell function: what are the key players? J Physiol 600:1089–1117
Sani A, Abdullahi IL (2017) Evaluation of some heavy metals concentration in body fluids of metal workers in Kano metropolis, Nigeria. Toxicol Rep 4:72–76
Shapiro G, Dodds L, Arbuckle T, Ashley-Martin J, Fraser W, Fisher M, Taback S, Keely E, Bouchard M, Monnier P (2015) Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: the MIREC study. Environ Int 83:63–71
Shou C, Wei Y-M, Wang C, Yang H-X (2019) Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Medicine 1:91–94
Smith D, Gwiazda R, Bowler R, Roels H, Park R, Taicher C, Lucchini R (2007) Biomarkers of Mn exposure in humans. Am J Ind Med 50:801–811
Soomro MH, Baiz N, Huel G, Yazbeck C, Botton J, Heude B, Bornehag CG, Annesi-Maesano I, The, E.m.-c.c.s.g., (2019) Exposure to heavy metals during pregnancy related to gestational diabetes mellitus in diabetes-free mothers. Sci Total Environ 656:870–876
Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, Cappuccio FP, Ceriello A, Reid ME (2007) Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med 147:217–223
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson G (2000) MOOSE guidelines for meta-analyses and systematic reviews of observational studies. JAMA 283:2008–2012
Sun J, Fang R, Wang H, Xu D-X, Yang J, Huang X, Cozzolino D, Fang M, Huang Y (2022) A review of environmental metabolism disrupting chemicals and effect biomarkers associating disease risks: Where exposomics meets metabolomics. Environ Int 158:106941
Tatsuta N, Iwai-Shimada M, Nakayama SF, Iwama N, Metoki H, Arima T, Sakurai K, Anai A, Asato K, Kuriyama S, Sugawara J, Suzuki K, Yaegashi N, Kamijima M, Nakai K, Japan Environment and Children's Study Group. Association between whole blood metallic elements concentrations and gestational diabetes mellitus in Japanese women: The Japan environment and Children's study. Environ Res 2022; 212:113231
Volpe CMO, Abreu LFM, Gomes PS, Gonzaga RM, Veloso CA, Nogueira-Machado JA (2014) The production of nitric oxide, IL-6, and TNF-alpha in palmitate-stimulated PBMNCs is enhanced through hyperglycemia in diabetes. Oxid Med Cell Longev 2014:479587
Wai KM, Swe T, Su Hninn TS, Paing AM, Naing YL, Htay ZW, Ihara K (2024) Prenatal exposure to environmental heavy metals and newborn telomere length: a systematic review and meta-analysis. Environ Pollut 343:123192
Wang X, Gao D, Zhang G, Zhang X, Li Q, Gao Q, Chen R, Xu S, Huang L, Zhang Y, Lin L, Zhong C, Chen X, Sun G, Song Y, Yang X, Hao L, Yang H, Yang L, Yang N (2020) Exposure to multiple metals in early pregnancy and gestational diabetes mellitus: a prospective cohort study. Environ Int. https://doi.org/10.1016/j.envint.2019.105370
Wang Y, Zhang P, Chen X, Wu W, Feng Y, Yang H, Li M, Xie B, Guo P, Warren JL, Shi X, Wang S, Zhang Y (2019) Multiple metal concentrations and gestational diabetes mellitus in Taiyuan, China. Chemosphere 237:124412
Wu T, Li T, Zhang C, Huang H, Wu Y (2023) Association between plasma trace element concentrations in early pregnancy and gestational diabetes mellitus in Shanghai, China. Nutrients 15:115
Xia YY, de Seymour JV, Yang XJ, Zhou LW, Liu Y, Yang Y, Beck KL, Conlon CA, Mansell T, Novakovic B, Saffery R, Han TL, Zhang H, Baker PN (2023) Hair and cord blood element levels and their relationship with air pollution, dietary intake, gestational diabetes mellitus, and infant neurodevelopment. Clin Nutr 42:1875–1888
Yang B, Fu J, Zheng H, Xue P, Yarborough K, Woods CG, Hou Y, Zhang Q, Andersen ME, Pi J (2012) Deficiency in the nuclear factor E2-related factor 2 renders pancreatic β-cells vulnerable to arsenic-induced cell damage. Toxicol Appl Pharmacol 264:315–323
Zhang C, Rawal S, Chong YS (2016) Risk factors for gestational diabetes: is prevention possible? Diabetologia 59:1385–1390
Zhang H, Yan C, Yang Z, Zhang W, Niu Y, Li X, Qin L, Su Q (2017) Alterations of serum trace elements in patients with type 2 diabetes. J Trace Elem Med Biol 40:91–96
Zhao L, Li X, Liu G, Han B, Wang J, Jiang X (2019) The association of maternal diabetes with attention deficit and hyperactivity disorder in offspring: a meta-analysis. Neuropsychiatr Dis Treat 15:675
Zheng Y, Lin PID, Williams PL, Weisskopf MG, Cardenas A, Rifas-Shiman SL, Wright RO, Amarasiriwardena C, Henn BC, Hivert MF, Oken E, James-Todd T (2021) Early pregnancy essential and non-essential metal mixtures and gestational glucose concentrations in the 2nd trimester: Results from project viva. Environ Int 155:106690
Zhou Z, Chen G, Li P, Rao J, Wang L, Yu D, Lin D, Fan D, Ye S, Wu S, Gou X, Wang H, Guo X, Lin L, Suo D, Liu Z (2021) Prospective association of metal levels with gestational diabetes mellitus and glucose: a retrospective cohort study from South China. Ecotoxicol Environ Safety 210:111854
Zhu GJ, Zheng TZ, Xia C, Qi L, Papandonatos GD, Ming Y, Zeng Z, Zhang XC, Zhang HL, Li YY (2021) Plasma levels of trace element status in early pregnancy and the risk of gestational diabetes mellitus: a nested case-control study. J Trace Elements Med Biol. https://doi.org/10.1016/j.jtemb.2021.126829
Acknowledgements
The authors express their gratitude to the Ministry of Education for their support of this study through the Institutional Fund Projects program, with Grant Code: IFP22UQU4331100DSR002.
Funding
None.
Author information
Authors and Affiliations
Contributions
The study's design involved contributions from all authors. RML, AH, AAS, AD, and IBS were responsible for conducting the literature review and drafting the initial manuscript draft. IHK, AHA, and AA conceptualized the project and participated in manuscript editing. RTH, and MLN performed manuscript revisions and conducted a thorough review of the referenced papers. The final version of the paper underwent further revision and received unanimous approval from all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors disclose that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Search keywords and protocols in different databases. Table S2. Criteria for quality assessment of the studies. Table S3. Quality Assessment Results. Fig. S1. Results of meta-analysis for exposure to Cd and risk of GDM based on urine samples in the first trimester. Fig. S2. Results of meta-analysis for exposure to Cd and odds ratio of GDM based on study design (1: cohort, 2: cross-sectional, 3: case–control. Fig. S3. Results of meta-analysis for exposure to Pb and risk of GDM based on blood samples in the second trimester. Fig. S4. Results of meta-analysis for exposure to Pb and odds ratio of GDM based on study design (1: cohort, 2: cross-sectional, 3: case–control). Fig. S5. Results of meta-analysis for exposure to Hg and risk of GDM based on blood samples in the second trimester. Fig. S6. Funnel plot of reviewed studies that investigated risk of GDM-related exposure to Cd.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lefta, R.M., Hjazi, A., Skakodub, A.A. et al. A systematic review and meta-analysis of the association between exposure to potentially toxic elements and gestational diabetes mellitus. Environ Sci Eur 36, 49 (2024). https://doi.org/10.1186/s12302-024-00878-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-024-00878-w