Abstract
The present study aimed to evaluate the effect of vitamin E (VE) supplementation on the reproductive and growth performance, hormonal profile, and biochemical parameters of female hybrid red tilapia before spawning season. Seventy-two female hybrid red tilapia were caught with an average body-weight 272.56 ± 34.84g and an average total length 24.3 ± 2.5 cm. Healthy fish were distributed equally into 4 treatments supplemented with (0, 50, 100, 200 mg/kg) of VE given for 8 weeks. VE significantly improved weight gain, length gain, specific growth rate, average daily gain, feed conversion ratio, and protein efficiency ratio. 50 mg of VE/kg diet could improve the viscerosomatic index, hepatosomatic index, and gonadosomatic index. Also, it could improve the parameters of biochemical (aspartate aminotransferase, alanine transaminase, total protein, albumin, globulin, triglyceride, and alkaline phosphatase) and haematology (total erythrocytic and leucocytic count, hematocrit and hemoglobin) and increase FSH, LH, E2 and, progesterone concentrations significantly. The conclusion revealed that the addition of 50 mg of VE /kg diet has a beneficial impact on reproductive and growth performance, hormonal profile, and biochemical parameters of female hybrid red tilapia. So, it is advisable for adding 50 mg/kg of vitamin E to the fish diet before the spawning season (pre-spawning).
Similar content being viewed by others
Introduction
Vitamins are crucial for fish growth, general health, and successful reproduction like other animals (Watanabe 1985). "Vitamin E" (VE) refers to a class of fat-soluble compounds and the most active homolog of which is -tocopherol (NRC 2011). A vital part of many biological processes, VE is a structural element of cell membranes with a strong antioxidant effect that breaks chains of reactions involved in lipid oxidation. In cultured fish and shrimps, VE stimulates growth and feed utilization; promotes health status and contributes to regulate the immune system and selenium metabolism. It also enhances the reproductive performance and development of larvae and improves fillet quality and shelf life (Hamre 2011).
Animals, including fish, are not known to synthesize tocopherols or to store them in significant quantities in their bodies (El-Sayed and Izquierdo 2022). The maintenance, growth, and physiological processes of animals depend on a constant supply of dietary VE. Several studies concerning the advantages of dietary VE for growth, reproduction, antioxidant activity, feed efficiency, immunological response, and survival of various farmed fish have been reported, including tilapia (Lim et al. 2010a, b).
The dietary VE role in promoting gonadal development, reproductive processes, spawning performance, and larval growth and survival has received considerable attention during the past two decades. These studies showed that high dietary VE supplementation can improve fish fertility, egg development, and larvae quality while reducing egg malformation and abnormality. It can protect eggs against oxidation also (Erdogan and Arslan 2019; El-Sayed and Izquierdo 2022). Additionally, VE can enhance the sperm quality of turbot (S. maximus) and shield sperm cells from oxidation (Xu et al. 2015).
Tilapia is the primary focus of aquaculture in freshwater due to its excellent growth rate, high yield, high disease resistance, tolerance to a variety of environments, and acceptability of both natural and artificial feed (FAO 2016). The hybridization of Mozambique tilapia mutant (O. mossambicus) with other species of tilapia, such as Nile tilapia (O. niloticus) and, blue tilapia (O. aureus), is mainly the cause of genetic variants of red tilapia. Due to its quick growth rate, devoid of a black membrane in the cavity of body, tolerance of salinity, and adaptation to most cultural systems, red tilapia has grown in popularity (Jayaprasad et al. 2011; Pradeep et al. 2014; El-Sayed 2015).
This study was designed to evaluate the effect of vitamin E supplementation on the reproductive and growth performance, hormonal profile and, biochemical parameters of female hybrid red tilapia before spawning season (Pre-spawning).
Material and Methods
The guidelines of the Experimental Animal Care Committee were closely followed during the present study. Moreover, it was also approved by the Scientific Research Ethical Committee of the Faculty of Vet-Medicine, Suez Canal University (Approval No. 2021016).
Female Collection, Maintenance, and Tanks Preparation
Seventy-two female hybrid red tilapia (♂O. niloticus × ♀O. mossambicus) were caught from the sustainable development center and transferred to Aquatic Hatchery Production Department, Fish Farming and Technology Institute, Suez Canal University, Ismailia, Egypt. The fish were transferred in 50 L tanks with portable aerators and at a rate of 1 fish/l. The initial body weight was 272.56 ± 34.84 g and the average total length was 24.3 ± 2.5 cm. Acclimation for fish was done in laboratory conditions for about two weeks before the beginning of the experiment with artificial photoperiod (12 h light/12 h darkness) using (2500 lx) (El-Sayed 2015). The fish were anesthetize using MS-222 (Tricaine methane sulfonate, dose: 100 mg/l, Argent Lab. Inc. Philippines) after feeding stopped (Topic Popovic et al. 2012), and the fish biological measurements were determined.
Fish were kept in indoor fiberglass circular holding tanks of a maximum capacity of 3 m3 (1.7 m diameter and 1.4 m high) filled with 1.5 m3 salt water, under controlled artificial photoperiods and temperature. The used water was filtered with sandy filters and sterilized using ultraviolet units. Aeration was done continuously by an air blower through 3 diffuser stones/tank.
Healthy fish were divided into four equal groups in triplicates which fed four different supplementation levels of VE (0, 50, 100, 200 mg/kg). The selected fish were checked for health which didn’t have tattered fins or missing scales, had bright coloration without abnormally dark spots or light coloration, didn’t seem disinterested, and swam actively with a symmetrical gait. The fish were stocked at a rate of 6 fish/tank (4 fish/m3) (Erdogan and Arslan 2019; Nascimento et al. 2014).
The Experiment Water Physico-Chemical Indicators
The water quality indicators were measured before and during the experiment as showing in Table 1. The water indicators were measured and controlled daily at the indoor holding tanks, using: Thermometer apparatus (Hai million meter, Haiyi instrument Co., LTD, China), DO meter (ExStik II D-0600, FLIR systems, Inc., USA), Digital refractometer (DRBS-300) and pH meter (Milwaukee MW-100).
Feeding Regime
Females were fed manually with a commercial diet (30.2% Crude protein, 6.1% Crude fat, 4.8% Fibre, 6.2% Ash, 0.0 mg VE, pellets 4 mm) 2 times/day for 8 weeks with a 3% feeding rate. The ration was purchased from Skretting Egypt Company. Vitamin E (Karma care co. for veterinary product) was added to the feed with 0 (control), 50 (T1), 100 (T2), and 200 (T3) mg VE/Kg diet. The VE levels were added to experimental diets following the procedure described by Chen et al. (2004), Ibrahim et al. (2020) and Ibrahim et al. (2021).
Biological Measurements
After 8 weeks, all starved fish of each tank were caught, counted and anesthetized in diluted MS-222 at a concentration of 100 mg/l and the final weights and total lengths were recorded. Also, 6 fish from each treatment were dissected and the viscera, liver, and gonad were weighed. Depending on the following formulas the growth parameters and biological measurements were measured:
Average daily gain (g/fish/day):
Specific growth rate (%/day):
Protein efficiency ratio (PER) = \(\frac{Weight\;gain \left(g\right)}{Protein\;intake \left(g\right)}\) (Tekinay and Davies 2001; Asaikkutti et al. 2016; Ibrahim et al. 2022).
The reproductive activities and biological measurements for females were determined from the temporal Fulton’s condition factor (K), Viscerosomatic index % (VSI), Hepatosomatic index % (HSI), and gonadosomatic index (GSI) were measured depending on the following formulas:
Blood Sampling and Analysis
At the experiment end, three females from each replicate were taken and anesthetized in diluted MS-222 at a concentration of (100 mg/l) for collecting two blood samples that were quickly withdrawn from heart puncture into clean and dry screw-copped Eppendorf tubes. One sample was left to clot and centrifuged for 5 min at 5000 rpm for separating serum which was stored at (-25 °C) to determine different hormonal profiles (FSH, LH, Estradiol E2, Progesterone) and, biochemical parameters (aspartate aminotransferase ‘AST’, alanine transaminase ‘ALT’, total protein ‘TP’, albumin ‘ALB’, globulin ‘GLO’, triglyceride ‘TG’, and alkaline phosphatase ‘ALP’). The second sample was taken with an anticoagulant (heparine 20 IU/ ml, Amoun Pharmaceutical Co.) and used directly for the determination of haematological Parameters. It ought to be mentioned that blood sampling of fish was executed in the morning around 8:00 a.m. before providing food (Rinchard et al. 1993).
Haematological Parameters
Under the light microscope, total erythrocytic count (RBCs), total leucocytic count (WBCs) and platelets count were determined using a neubauer hemocytometer after blood dilution with phosphate-buffered saline (pH, 7.2) (Shalaby et al. 2019 and Abdel-Tawwab et al. 2020). Hct (hematocrit) was immediately determined according to (Rehulka 2000) after sampling by placing fresh blood in glass capillary tubes, centrifuged in a micro-hematocrit centrifuge (Centurion Scientific, United Kingdom), and measuring the packed cell volume. Hgb (Hemoglobin concentration) was determined according to (Jain 1993) colorimetrically. According to Haney et al. (1992) MCV, MCH, and MCHC were calculated as follows:
Biochemical Parameters
Serum samples were collected to determine protein profiles, (total protein TP and, albumin ALB) contents (Henry 1964 and Doumas et al. 1997). Globulin (GLO) levels were also determined by subtraction of ALB from TP values. Blood triglycerides (TG) and Alkaline phosphatase (ALP) levels were measured colorimetrically using commercially purchased specific kits (all laboratory procedures were accomplished depending on the manufacturer's protocol) and according to Reitman and Frankel (1957), transaminases (AST & ALT) were determined.
Determination of Hormonal Profile
FSH, LH, E2 (Estradiol), and Progesterone levels were determined using commercial assay ELISA kits as manufactories instructions.
Statistical Analysis
Collected data were presented as mean ± stander error (SE) between all groups. Statistically significant differences between groups were calculated using one-way ANOVA at 5% level of probability followed by post hoc multiple comparison tests (Duncan's 1955). SPSS Program version 20. (SPSS, Richmond, USA) was used in the current study's statistical analysis as described by (Dytham 2011).
Results
The Impact of Different Doses of Vitamin E on the Growth Performance of Female Hybrid Red Tilapia
Female hybrid red tilapia weight gain and length gain were significantly affected (P ≤ 0.05) by dietary vitamin E (VE) (Table 2). The treated groups produced significantly (P ≤ 0.05) better growth performance than the control group. The highest values of weight gain and length gain were detected in T1 (87.09 g) and (3.68 cm) respectively.
SGR, ADG, FCR, and PER values of female hybrid red tilapia supplemented with VE were impacted significantly (P ≤ 0.05) than the control. SGR increased significantly in T1 (50 mg VE) versus the control and other treated groups. Regarding ADG and FCR, all supplemented groups exhibited better significant values than the control. PER in T1 demonstrates a higher significant value than control and other treatments. PER recorded the lower values in control regardless to others (1.27 ± 0.055) (Table 3).
The Impact of Different Doses of Vitamin E on Viscera Somatic Index, Gonadosomatic Index, Hepatosomatic Index and Condition Factors of Female Hybrid Red Tilapia
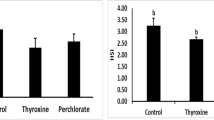
T1 exhibited higher significant values in VSI, GSI and HSI of female hybrid red tilapia when compared to control and other supplemented groups (T2, T3). The highest value of the K factor of female fish specimens was calculated in 50 mg vitamin E treatment (T1) (Table 4).
The Impact of Different Doses of Vitamin E on Haematological Parameters of Female Hybrid Red Tilapia
For Hemoglobin (Hgb) and RBCs, the greatest significant value (P ≤ 0.05) of Hgb and RBCs were recorded in T1. Regarding, Hct and platelets values, no significant variation was observed between the control and treatments. MCV was significantly (P ≤ 0.05) increased in T3 (180.33 fl) than the other treatment. The highest significant values of MCH, and MCHC have been observed in T1 53.67 pg and 33.33% respectively. WBCS were significantly (P ≤ 0.05) increased in T1 and, T2 versus control in (Table 5).
The Impact of Different Doses of Vitamin E on Biochemical Parameters of Female Hybrid Red Tilapia
TP and TG were significantly (P ≤ 0.05) increased in T1 and, T2 than in control and T3. There was no significant variation in ALB and AST values between all treated groups. While GLO showed the greatest significant value in T2 3.55 g/dl. A higher significant level of ALT (12.65 u/l) was recorded in T2 versus control and other treated group (T1 and T3). ALP levels were significantly (P ≤ 0.05) enhanced with increasing VE levels recorded in T2 and, T3 (Table 6).
The Impact of Different Doses of Vitamin E on Hormonal Profile of Female Hybrid Red Tilapia
FSH and LH levels were significantly increased at T1 (50 mg/Kg) and decreased afterward. Regarding the impacts of VE on E2, the highest level was recorded in T1 and, T2 when compared to control and T3. The peak level of progesterone was noticed in T1 (0.12 ng/ml) (Table 7).
Discussion
Finding out the impact of Vitamin E on fish performance, antioxidation, quality, immunity, and, metabolic processes and other biological events has taken up a significant portion of the research in recent years (Hamre 2011; El-Sayed and Izquierdo 2022). In our study, we demonstrate the optimum dose of VE administration and the impacts of this vitamin on reproductive and growth performance, blood analyses, and the hormonal profile of female hybrid red tilapia before the spawning season (pre-spawning).
The current findings showed that the addition of 50, 100, 200 mg/kg of VE significantly improved the growth performance of female red tilapia (weight gain and length gain). Similarly, VE treatments have been demonstrated to considerably modulate the growth performance in female Nile tilapia (O. niloticus). The final weight and length of female Nile tilapia were increased significantly with VE concentrations ranging from 40 to 160 mg/kg feeds (Zhang et al. 2021). Adding 140 mg/kg of VE to the carp feed and 50—100 mg/kg to the catfish (I. punctatus) feed could accelerate weight gain and length gain (Abdel-Hameid et al. 2012; He et al. 2017).
The levels of VE 43.2–45.8 mg/kg (Jiang et al. 2020) and 50—100 mg/kg (Lim et al. 2009) with 6% lipid feeds were required to modulate the performance (final weight gain) and health status of tilapia which consistent with the percentage of lipid in fish feed in current study. The dietary VE supplement could improve WG and WG % significantly of the Nile tilapia (Rohani et al. 2022; Ahmed et al. 2021).
The present results of SGR, ADG, FCR, PER were enhanced significantly by supplementation with 50, 100, 200 mg/kg of VE and, the best values were recorded with the addition of 50 mg/kg of VE treatment. These results agreed with the previous studies of Rohani et al. (2022) who recorded that dietary 50 mg/kg of VE supplement significantly enhance SGR, FCR, and, PER of the Nile tilapia and Saheli et al. (2021) who noted that dietary 35.4 and 78.8 mg/kg of VE supplement significantly improve SGR and PER and decreased FCR of the Caspian trout but fish fed diet unsupplemented with VE had the lower WGR, SGR and PER. The study of Zhang et al. (2021) have shown that 120 and, 160 mg/kg of VE could significantly decrease the FCR in female tilapia, which is constant with previous research.
The current findings showed that supplementing with VE in the right proportions can enhance fish performance, feed assimilation, and absorption with lower the cost and period of hybrid red tilapia pre-spawning preparation which may be due to the VE role as a cofactor of many enzymes that may promote the secretion and activity of digestive enzymes and enhances digestion and nutrient absorption (Swain et al. 2019). Fish species, life stage, feeding regimen, rearing environment, diet composition, and even the sample analysis method may all have an impact on the amount of VE needed which is a vital dietary component for fish (Lee et al. 2015; Lu et al. 2016). Although it had been claimed that vitamin E was necessary for fish to thrive, other research found that excessive amounts of VE in feed which might serve as prooxidants were hazardous to fish and could stunt fish growth (Paul et al. 2004; Li et al. 2013).
The present results regarding biological measurements and reproductive performance including VSI, GSI, and HSI have been improved significantly by supplementation with 50 mg/kg of VE without significant variations in the K factor. similar observations were achieved by Pamungkas et al. (2014) who reported that the peak of female GSI of Nile tilapia was found in broodstock fed with 75 and 375 mg/kg VE. The gonad development index of M. albus was significantly affected by the addition of VE (200 mg/kg) to the feed (Zhang et al. 2007). The best GSI level of female tilapia was achieved at 120 mg/kg of VE (Zhang et al. 2021). While Gammanpila et al. (2010) noticed that the final females’ GSI of Nile tilapia were not influenced by supplementation of the VE. The increase of GSI with 50 mg/kg and, 200 mg /kg of VE may be due to the responsibility of VE in the gonad development process and its impacts on the progress of the vitellogenesis process. Vitamin E with the right amount acts as an antioxidant against fat, preventing the oxidation of fat that happens when vitellogenin is present and will accelerate the vitellogenesis process. As a result, more vitellogenin is present when the oocyte develops, and the gonad weight increases, reflecting an increasing the GSI percentage (Arfah and Setiawat 2013; Tarigan et al. 2021; Ashari et al. 2021).
VSI and, HSI of females in our study were increased with the addition of 50 mg/kg VE. The increase in HSI may be due to fat and VE cumulation in the liver (Amlashi et al. 2011). Hepatosomatic index values are adversely correlated with fecundity and VE content in diet (Nascimento et al. 2014) which explain the decrease in HSI at 200 mg/kg of VE. HSI was increased with dietary VE supplement 35.4 and, 78.8 mg/kg in the study of (Saheli et al. 2021) from 8.9 to 156.9 mg/kg in the study of (Lu et al. 2016). In studies conducted on Nile tilapia by Satoh et al. (1987), HSI dramatically decreased most likely resulting from tissue degradation with an absence of VE treatment and by Lim et al. (2009), supplementation of 50 mg/ kg of VE to diets (6 to 14% lipid) was sufficient to prevent degeneration and/or fat infiltration of liver tissue.
The current results declared that the VE-treated fish cause an increase in their Hgb and RBCs, especially by 50 and 100 mg/kg but without changes in Hct % compared with other treatments. At the same time, 200 mg/kg VE declines Hgb, and RBCs. These results are in agreement with Ispir et al. (2011) who reported that RBCs and Hgb of Nile tilapia were increased by 80 and, 160 mg/kg of VE and decreased by a higher dose (240 mg/kg) of VE. Adding 50 mg/kg of VE improves Hgb and RBCs of Nile tilapia and there are no changes in Hct % when compared to 0 mg/kg of VE and other treatments (Ibrahim 2014). Hct, Hgb and RBCs can be an indicator of oxidative status because erythrocytes are the main sources of free radicals and some of them can cause sutured fatty acids in their membrane phospholipids to peroxide, changing the quality (integrity, size), quantity, and composition of the erythrocytes (Kiron et al. 2004). The obtained value of MCV in the current study represented a significant raising with the VE level of 200 mg/kg followed by 100 mg/kg and decreased at the VE level of 50 mg/kg. However, the peak calculated values of MCH and, MCHC were obtained at the VE level of 50 mg/kg followed by 100 mg/kg. These results are in agreement with Ispir et al. (2011) who reported that 240 mg/kg of VE raised MCV while 80 and, 160 mg/kg of VE raised MCH and, MCHC. The main impact of VE was highly significant at the 28days period for MCV, MCH, and MCHC (Ibrahim 2014).
The total counts of WBC showed that 50 and, 100 mg/kg of VE increased the WBCs significantly, without significant impacts of VE on platelets. Similar results were obtained in a study conducted on Nile tilapia by Ispir et al. (2011) when using 80 and, 160 mg/kg of VE could enhance the WBCs. VE’s main effect was significant for WBC and 50 mg/kg of VE could increase WBCs compared to 0 mg/kg of VE and other treatments (Ibrahim 2014). VE act as a potential antioxidant that protects the leucocyte function, it was discovered that WBC is a vitamin efficiency indicator and a defense mechanism indicator in fish (Sahoo and Mukherjee 2002). VE contributed to the hypothalamic-sympathetic- chromaffin cell axis and interferes in stress responses, where they protect the WBC functions (Ortuño et al. 2003). Supplementation of dietary VE improved haematological parameters (Ibrahim 2014).
In our study, enhanced total protein and globulin values were recorded in fish fed with 50 and 100 mg/kg of VE while they were decreased with supplementation of 200 mg/kg of VE without change in albumin levels. Our results agree with the findings of Saber et al. (2019) who found a significant rise in total protein was recorded in VE-supplemented fish when compared with other groups. Adding 50 mg/kg of VE could enhance the TP, ALB, and, GLO of Nile tilapia compared to 0 mg/kg of VE and other treatments (Ibrahim 2014). Contrary to our results, Lim et al. (2009) and Lim et al. (2010a) noticed that Rising levels of VE from 50 to 100, 200 or 500 mg/kg feed had no impact on serum total protein Nile tilapia. Vitamin E may activate protein phosphatase 2A, diacylglycerol phosphatase, and protein tyrosine phosphatase and inhibit protein kinase A2, phospholipase A2, cyclooxygenase, and lipoxygenases, according to later studies of (Hamre 2011; Zingg 2007, 2019).
In the current study, no significant impacts of different doses of VE supplement were found on AST but 100 mg/kg of VE could increase the ALT value. ALP levels were increased significantly by the increase of VE levels. The blood concentration of hepatic enzymes including ALP, AST, and, ALT is known to be correlated with the functional role of VE (Abdulazeez et al. 2019). The Liver is the primary organ participatory in xenobiotics metabolism (Ibrahim 2014). Activities levels of AST and, ALT could also give evidence about the liver condition (Ghodrati et al. 2021), while ALP is the most important hepatobiliary damage biomarker that catalyze the hydrolysis of organic phosphate esters (Aulbach and Amuzie 2017). The 50 mg/kg addition of VE increases the AST level and decreases the ALP level of Nile tilapia non-significantly after 28 days of treatment but the same dose of VE could decrease the ALP level significantly (Ibrahim 2014). Our findings may be confirmed that VE did not have any adverse impacts on the liver function when used within the suggested dose and these findings could be referred to the following issues, a) VE encourages the synthesis of cytokine, which consider a crucial component of the inflammatory response that protected liver cells., b) the activity of VE as an antioxidant and, anti-inflammatory vitamins agents that keep the hepatic fibrosis and dysfunction that aid making liver act within the normal function (Eddowes et al. 2019).
Supplementation with 50 and 100 mg/kg of VE could increase the TG levels, but 200 mg/kg of VE decreased TG significantly. Similarly, in red seabream raising dietary VE level correlated with reduced TG concentration. The reduction of TG value by VE supplement (200 mg/kg) could be related to TG decrease in chylomicrons (Gao et al. 2012). TG is measured to track metabolism of lipids. High levels of TG can cause nephritic syndrome, glycogen storage disease, and liver failure (Osman et al. 2010). In the study of Taalab et al. (2022), the total lipid profile, was shown to be lower in Nile tilapia-fed diets containing high-level VC, VE, or β-carotene. These conclusions may be due to the role of VE in improving the profiles of lipids in the blood, HDL-C (high-density lipoprotein cholesterol), inhibiting the oxidation of lipids and lowering the risk of atherosclerosis of LDL-C (low-density lipoprotein cholesterol) (Ashor et al. 2016).
In our study, 50 mg/kg of VE could improve the reproductive hormonal levels including (FSH, LH, E2, and Progesterone) significantly. Our findings may be due to the fact of the provision of VE through pituitary cells in vitro triggers the FSH and, LH expression in the fish pituitary (Huang et al. 2019). The increase of E2 and, progesterone in our study may be due to Low-level of VE may stimulate female tilapia to secrete large quantities of steroid hormones (Zhang et al. 2021). Both pituitary FSH LH, and, E2 have major responsibilities in controlling all aspects of the gonadal development and, function across vertebrates (including fish) ( Swanson et al. 2003; Xu et al. 2008; Levavi-Sivan et al. 2010). Progesterone can adjust gonadotropin production and play a role in reproduction (Sun et al. 2020). Similar outcomes were noted in the study of (Zhang et al. 2021) who reported that the FSH of female tilapia was the largest in the group supplemented with 40 mg/kg of VE followed by 80 mg/kg of VE, but the various levels of VE had no impact on E2 and the peak level was recorded in the group supplemented with 120 mg/kg of VE. A level of 120 mg/kg followed by 40 mg/kg of VE could enhance the level of progesterone and demonstrate that these vitamin levels can promote the reproductive performance of female tilapia. In the study of turbot, the mRNA expressions of FSH and, LH in the cells were both increased in vitamin E treated groups when compared to the control group which confirmed that VE promoted the GTHs expression at a molecular level (Huang et al. 2019). Quite a little information has been previously reported about the act of VE in regulating fish FSH, LH, E2, and progesterone. So, the impacts of VE on the hormonal profile of female hybrid red tilapia need additional follow-up investigation.
Conclusion
It was concluded that, the addition of vitamin E in a dose of 50 mg/kg diet has a beneficial impact on the growth and reproductive performing, hormonal profile, and biochemical parameters of female hybrid red tilapia. So, it is advisable that adding 50 mg/kg to the fish diet support the benefits of VE and are recommended for enhancing the health, growth and, reproductive performance of fish before spawning season (pre-spawning).
Data Availability
Data are available on request due to privacy.
References
Abdel-Hameid NAH, Abidi SF, Khan MA (2012) Dietary vitamin E requirement for maximizing the growth, conversion efficiency, biochemical composition and haematological status of fingerling Channa punctatus. Aquac Res 43:226–238
Abdel-Tawwab M, Khalifa E, Diab AM, Khallaf MA, Abdel-Razek N, Khalil RH (2020) Dietary garlic and chitosan alleviated zearalenone toxic effects on performance, immunity, and challenge of European sea bass, Dicentrarchus labrax, to Vibrio alginolyticus infection. Aquac Int 28:493–510
Abdulazeez J, Olusegun AJ, Tanko Y, Abubakar A, Bello UM, Ahmed MK, Aliyu M (2019) Serum liver enzymes and oxidative stress biomarkers in resveratrol treated cholesterol diet induced type 2 diabetes mellitus in rabbits. FASEB J 33. https://doi.org/10.1096/fasebj.2019.33.1_supplement.694.1
Ahmed SAA, Ibrahim RE, Farroh KY, Moustafa AA, Al-Gabri NA, Alkafafy M, Amer SA (2021) Chitosan vitamin E nanocomposite ameliorates the growth, redox, and immune status of Nile tilapia (Oreochromis niloticus) reared under different stocking densities. Aquaculture 541:736804. https://doi.org/10.1016/j.aquaculture.2021.736804
Amlashi AS, Falahatkar B, Sattari M, Gilani MHT (2011) Effect of dietary vitamin E on growth, muscle composition, hematological and immunological parameters of sub-yearling beluga Huso huso L. Fish Shellfish Immunol 30:807–814
Arfah HM, Setiawat I (2013) Vitamin E supplementation with different doses at feed on the reproductive performance of the female brood fish comet (Carassius auratus auratus). J Aquac Indones 12:14–18
Asaikkutti A, Bhavan PS, Vimala K (2016) Effects of different levels of dietary folic acid on the growth performance, muscle composition, immune response and antioxidant capacity of freshwater prawn, Macrobrachium rosenbergii. Aquaculture 464:136–144
Ashari A, Sinaga M, Gde P, Julyantotro S, Ayu D, Pebriani A, Kampus Unud J, Jimbaran B, Selatan K (2021) Effect of giving different doses of vitamin e in feed to the level of gonad maturity of tilapia (Oreochromis niloticus). Adv Trop Biodivers Environ Sci 5:64–68
Ashor AW, Siervo M, van der Velde F, Willis ND, Mathers JC (2016) Systematic review and meta-analysis of randomised controlled trials testing the effects of vitamin C supplementation on blood lipids. Clin Nutr 35:626–637
Aulbach A, Amuzie CJ (2017) Biomarkers in nonclinical drug development. In: a comprehensive guide to toxicology in nonclinical drug development 447–471. https://doi.org/10.1016/B978-0-12-803620-4.00017-7
Chen R, Lochmann R, Goodwin A, Praveen K, Dabrowski K, Lee KJ (2004) Effects of dietary vitamins C and E on alternative complement activity, hematology, tissue composition, vitamin concentrations and response to heat stress in juvenile golden shiner (Notemigonus crysoleucas). Aquaculture 242:553–569
Doumas BT, Watson WA, Biggs HG (1997) Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 258:21–30
Duncan DB (1955) Multiple range and multiple F-Test. Biometrics Sci Res Publ 11:1–5
Dytham C (2011) Statistics, variables and distribution. Choosing and using statistics: A biologist's guide. Wiley-Blackwell, p 320
Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V et al (2019) Accuracy of fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 156:1717–1730
El-Sayed AFM, Izquierdo M (2022) The importance of vitamin E for farmed fish—A review. Rev Aquac 14:688–703
El-Sayed AFM (2015) Tilapia culture, p 1–348. https://doi.org/10.1016/C2017-0-04085-5
Erdogan M, Arslan T (2019) Effects of vitamin E on growth and reproductive performance of pindani (Pseudotropheus socolofi Johnson, 1974). Aquaculture 509:59–66
FAO (2016) The State of World Fisheries and Aquaculture 2016. Contributing to food security and nutrition for all. Rome. 200 pp. CUPUM 2015 - 14th International Conference on Computers in Urban Planning and Urban Management
Gammanpila M, Yakupitiyage A, Bart A (2010) Evaluation of the effects of dietary vitamin C, E and Zinc supplementation on reproductive performance of Nile tilapia (Oreochromis niloticus). Sri Lanka J Aquat Sci 12:39
Gao J, Koshio S, Ishikawa M, Yokoyama S, Mamauag REP, Han Y (2012) Effects of dietary oxidized fish oil with vitamin E supplementation on growth performance and reduction of lipid peroxidation in tissues and blood of red sea bream Pagrus major. Aquaculture 356–357:73–79
Ghodrati M, Rajabi Islami H, Hosseini Shekarabi SP, Shenavar Masouleh A, Shamsaie Mehrgan M (2021) Combined effects of enzymes and probiotics on hemato-biochemical parameters and immunological responses of juvenile Siberian sturgeon (Acipenser baerii). Fish Shellfish Immunol 112:116–124
Hamre K (2011) Metabolism, interactions, requirements and functions of vitamin E in fish. Aquac Nutr 17:98–115
Haney DC, Hursh DA, Mix MC, Winton JR (1992) Physiological and hematological changes in chum salmon artificially infected with erythrocytic necrosis virus. J Aquat Anim Health 4:48–57
He M, Wang K, Liang X, Fang J, Geng Y, Chen Z, Pu H, Hu Y, Li X, Liu L (2017) Effects of dietary vitamin E on growth performance as well as intestinal structure and function of channel catfish (Ictalurus punctatus, Rafinesque 1818). Exp Ther Med 14:5703–5710
Henry RJ (1964) Colorimetric determination of total protein. Clin Chem J
Huang B, Wang N, Wang L, Jia Y, Liu B, Gao X, Liu B, Wang W (2019) Vitamin E stimulates the expression of gonadotropin hormones in primary pituitary cells of turbot (Scophthalmus maximus). Aquaculture 509:47–51
Ibrahim AT (2014) Ameliorative effect of lycopene and vitamin E on some haematological and biochemical parameters of Oreochromis niloticus against diazinon toxicity. Adv Plants Agric Res J 1. https://doi.org/10.15406/apar.2014.01.00014
Ibrahim RE, Amer SA, Shahin SA, Darwish MIM, Albogami S, Abdelwarith AA, Younis EM, Abduljabbar MH, Davies SJ, Attia GA (2022) Effect of fish meal substitution with dried bovine hemoglobin on the growth, blood hematology, antioxidant activity and related genes expression, and tissue histoarchitecture of Nile tilapia (Oreochromis niloticus). Aquac Rep 26:101276. https://doi.org/10.1016/j.aqrep.2022.101276
Ibrahim RE, Ahmed SAA, Amer SA, Al-Gabri NA, Ahmed AI, Abdel-Warith AWA, Younis ESMI, Metwally AE (2020) Influence of vitamin C feed supplementation on the growth, antioxidant activity, immune status, tissue histomorphology, and disease resistance in Nile tilapia, Oreochromis niloticus. Aquac Reports 18
Ibrahim RE, Amer SA, Farroh KY, Al-Gabri NA, Ahmed AI, El-Araby DA, Ahmed SAA (2021) The effects of chitosan-vitamin C nanocomposite supplementation on the growth performance, antioxidant status, immune response, and disease resistance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 534:736269. https://doi.org/10.1016/j.aquaculture.2020.736269
Ispir U, Yonar ME, Oz OB (2011) Effect of di̇etary vitamin e supplementation on the blood parameters of Nile Tilapia (Oreochromis Niloticus). J Anim Plant Sci 21:566–569
Jain NC (1993) Essentials of Veterinary Hematology. Lea and Febiger, Philadelphia, pp 76–250. - References - Scientific Research Publishing
Jayaprasad PP, Srijaya TC, Jose D, Papini A, Hassan A, Chatterji AK (2011) Identification of diploid and triploid red tilapia by using erythrocyte indices. Caryologia 64:485–492
Jiang M, Ma L, Shao H, Wu F, Liu W, Tian J, Yu L, Lu X, Wen H (2020) Dietary vitamin E requirement of sub-adult genetically improved farmed tilapia strain of Nile tilapia (Oreochromis niloticus) reared in freshwater. Aquac Nutr 26:233–241
Kiron V, Puangkaew J, Ishizaka K, Satoh S, Watanabe T (2004) Antioxidant status and nonspecific immune responses in rainbow trout (Oncorhynchus mykiss) fed two levels of vitamin E along with three lipid sources. Aquaculture 234:361–379
Lee CS, Lim C, Gatlin DM, Webster CD (2015) Dietary nutrients, additives, and fish health. Wiley, p 1–355. https://doi.org/10.1002/9781119005568
Levavi-Sivan B, Bogerd J, Mañanós EL, Gómez A, Lareyre JJ (2010) Perspectives on fish gonadotropins and their receptors. Gen Comp Endocrinol 165:412–437
Li M, Chen L, Qin JG, Li E, Yu N, Du Z (2013) Growth performance, antioxidant status and immune response in darkbarbel catfish Pelteobagrus vachelli fed different PUFA/vitamin E dietary levels and exposed to high or low ammonia. Aquaculture 406–407:18–27
Lim C, Yildirim-Aksoy M, Li MH, Welker TL, Klesius PH (2009) Influence of dietary levels of lipid and vitamin E on growth and resistance of Nile tilapia to Streptococcus iniae challenge. Aquaculture 298:76–82
Lim C, Yildirim-Aksoy M, Welker T, Klesius PH, Li MH (2010a) Growth performance, immune response, and resistance to Streptococcus iniae of Nile Tilapia, Oreochromis niloticus, fed diets containing various levels of vitamins C and E. J World Aquac Soc 41:35–48
Lim C, Yildirim-Aksoy M, Shelby R, Li MH, Klesius PH (2010b) Growth performance, vitamin E status, and proximate and fatty acid composition of channel catfish, Ictalurus punctatus, fed diets containing various levels of fish oil and vitamin E. Fish Physiol Biochem 36:855–866
Lu Y, Liang XP, Jin M, Sun P, Ma HN, Yuan Y, Zhou QC (2016) Effects of dietary vitamin E on the growth performance, antioxidant status and innate immune response in juvenile yellow catfish (Pelteobagrus fulvidraco). Aquaculture 464:609–617
Nascimento TSR, de Stéfani MV, Malheiros EB, Koberstein TCD (2014) High levels of dietary vitamin E improve the reproductive performance of female Oreochromis niloticus. Acta Sci - Biol Sci 36:19–26
NRC (2011) Nutrient requirements of fish and shrimp. https://doi.org/10.17226/13039
Ortuño J, Esteban MA, Meseguer J (2003) The effect of dietary intake of vitamins C and E on the stress response of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 14:145–156
Osman AGM, Koutb M, Sayed AEDH (2010) Use of hematological parameters to assess the efficiency of quince (Cydonia oblonga Miller) leaf extract in alleviation of the effect of ultraviolet - A radiation on African catfish Clarias gariepinus (Burchell, 1822). J Photochem Photobiol B Biol 99:1–8
Pamungkas W, Tahapari E, Darmawan J (2014) Gonadal development and spawning frequency of tilapia (Oreochromis niloticus) that feeded by vitamin E supplementation [Perkembangan gonad dan performa pemijahan induk ikan nila (Oreochromis Niloticus) yang diberi pakan dengan penambahan vitamin E supleme. Ber. Biol. 13:64122
Paul BN, Sarkar S, Mohanty SN (2004) Dietary vitamin E requirement of mrigal, Cirrhinus mrigala fry. Aquaculture 242:529–536
Pradeep PJ, Srijaya TC, Hassan A, Chatterji AK, Withyachumnarnkul B, Jeffs A (2014) Optimal conditions for cold-shock induction of triploidy in red tilapia. Aquac Int 22:1163–1174
Rehulka J (2000) Influence of astaxanthin on growth rate, condition, and some blood indices of rainbow trout, Oncorhynchus mykiss. Aquaculture 190:27–47
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Rinchard J, Kestemont P, Kuhn ER, Fostier A (1993) Seasonal changes in plasma levels of steroid hormones in an asynchronous fish the gudgeon Gobio gobio L. (Teleostei, Cyprinidae). Gen Comp Endocrinol 92:168–178
Rohani MF, Bristy AA, Hasan J, Hossain MK, Shahjahan M (2022) Dietary zinc in association with vitamin e promotes growth performance of Nile tilapia. Biol Trace Elem Res 200:4150–4159
Saber TM, ElHady M, Ali HA (2019) Effect of dietary vitamin E on biochemical, oxidative stress and immunological parameters in Nile tilapia exposed to penoxsulam. African J Aquat Sci 44:237–245
Saheli M, Rajabi Islami H, Mohseni M, Soltani M (2021) Effects of dietary vitamin E on growth performance, body composition, antioxidant capacity, and some immune responses in Caspian trout (Salmo caspius). Aquac Reports 21:100857
Sahoo PK, Mukherjee SC (2002) Influence of high dietary α-tocopherol intakes on specific immune response, nonspecific resistance factors and disease resistance of healthy and aflatoxin B1-induced immunocompromised Indian major carp, Labeo rohita (Hamilton). Aquac Nutr 8:159–167
Satoh S, Takeuchi T, Watanabe T (1987) Requirement of Tilapia for α-tocopherol. Pascal-francis inist fr
Shalaby A, Ghareeb A, Abd El-Rahman M, Abd El-Hamid E (2019) Effect of different levels of folic acid on the growth and some physiological aspects of Nile tilapia “Oreochromis niloticus.” Egypt J Aquac 9:33–45
Sun N, Zhang Y, Hou Y, Yi Y, Guo J, Zheng X, Sun P, Sun Y, Khan A, Li H (2020) Effects of osthole on progesterone secretion in chicken preovulatory follicles granulosa cells. Animals 10:1–11
Swain P, Das R, Das A, Padhi SK, Das KC, Mishra SS (2019) Effects of dietary zinc oxide and selenium nanoparticles on growth performance, immune responses and enzyme activity in rohu, Labeo rohita (Hamilton). Aquac Nutr 25:486–494
Swanson P, Dickey JT, Campbell B (2003) Biochemistry and physiology of fish gonadotropins. Fish Physiol Biochem 28:53–59
Taalab HA, Mohammady EY, Hassan TMM, Abdella MM, Hassaan MS (2022) β-Carotene of Arthrospira platensis versus vitamin C and vitamin E as a feed supplement: Effects on growth, haemato-biochemical, immune-oxidative stress and related gene expression of Nile tilapia fingerlings. Aquac Res 53:4832–4846
Tarigan G, Arthana IW, Pebriani DAA (2021) Effect of giving different doses of vitamin E in feed to the level of gonad maturity of tilapia (Oreochromis niloticus). Adv Trop Biodivers Environ Sci 5:64
Tekinay AA, Davies SJ (2001) Dietary carbohydrate level influencing feed intake, nutrient utilisation and plasma glucose concentration in the rainbow trout, oncorhynchus mykiss. Turkish J Vet Anim Sci 25:657–666
Topic Popovic N, Strunjak-Perovic I, Coz-Rakovac R, Barisic J, Jadan M, Persin Berakovic A, Sauerborn Klobucar R (2012) Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J Appl Ichthyol 28:553–564
Watanabe T (1985) Importance of the study of broodstock nutrition for further development of aquaculture. Book: Nutrition and feeding in fish, pp 395–414
Xu H, Huang L, Liang M, Zheng K, Wang X (2015) Effect of dietary vitamin E on the sperm quality of turbot (Scophthalmus maximus). J Ocean Univ China 14:695–702
Xu S, Cheng Y, Keast JR, Osborne PB (2008) 17β-estradiol activates estrogen receptor β-signalling and inhibits transient receptor potential vanilloid receptor 1 activation by capsaicin in adult rat nociceptor neurons. Endocrinology 149:5540–5548
Zhang G, He R, Zhang S, Cao K, Gao H (2007) Effect of vitamin E in broodstock diet on reproductive performance of Monopterus albus. Acta Hydrobiol Sin 02:196–200
Zhang X, Ma Y, Xiao J, Zhong H, Guo Z, Zhou C, Li M, Tang Z, Huang K, Liu T (2021) Effects of vitamin E on the reproductive performance of female and male Nile tilapia (Oreochromis niloticus) at the physiological and molecular levels. Aquac Res 52:3518–3531
Zingg JM (2007) Modulation of signal transduction by vitamin E. Mol Aspects Med 28:481–506
Zingg JM (2019) Vitamin E: Regulatory role on signal transduction. IUBMB Life 71:456–478
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Alaa Sh. Griesh: Study conception and design, material preparation, data collection and analysis and writing. Amal M. El-Nahla: Supervision, Reviewing and Editing. Salah M. Aly: Supervision, Reviewing and Editing. Mohamed F. Badran: Study conception and design, material preparation, data collection and analysis and writing.
Corresponding authors
Ethics declarations
Ethical Approval
Scientific research committee, faculty of veterinary medicine, SCU declares that the research protocol of the present study was approved by the committee with the number (2023045).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Griesh, A.S., El-Nahla, A.M., Aly, S.M. et al. Role of Vitamin E Supplementation on the Reproductive and Growth Performance, Hormonal Profile and Biochemical Parameters of Female Hybrid Red Tilapia. Thalassas (2024). https://doi.org/10.1007/s41208-024-00683-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41208-024-00683-5