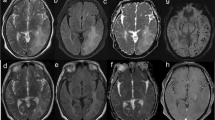
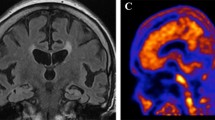
Abstract
Background
Cerebral amyloid angiopathy (CAA) pathology is becoming increasingly important in Alzheimer’s disease (AD) because of its potential link to amyloid-related imaging abnormalities, a critical side effect observed during AD immunotherapy. Identification of CAA without typical magnetic resonance imaging (MRI) markers (MRI-negative CAA) is challenging, and novel detection biomarkers are needed.
Methods
We included 69 participants with high neuritic plaques (NP) burden, with and without CAA pathology (NP with CAA vs. NP without CAA) based on autopsy data from the Alzheimer’s Disease Neuroimaging Initiative. Two participants with hemorrhagic CAA markers based on MRI were excluded and the final analysis involved 36 NP without CAA and 31 NP with CAA. A logistic regression model was used to compare the cerebrospinal fluid (CSF) amyloid-β42 (Aβ42), phosphorylated tau181, and total tau levels, the amyloid positron emission tomography (PET) standardized uptake ratio (SUVR), and cognitive profiles between NP with and without CAA. Regression models for CSF and PET were adjusted for age at death, sex, and the last assessed clinical dementia rating sum of boxes score. Models for cognitive performances was adjusted for age at death, sex, and education level.
Results
NP with CAA had significantly lower CSF Aβ42 levels when compared with those without CAA (110.5 pg/mL vs. 134.5 pg/mL, p-value = 0.002). Logistic regression analysis revealed that low CSF Aβ42 levels were significantly associated with NP with CAA (odds ratio [OR]: 0.957, 95% confidence interval [CI]: 0.928, 0.987, p-value = 0.005). However, amyloid PET SUVR did not differ between NP with CAA and those without CAA (1.39 vs. 1.48, p-value = 0.666). Logistic regression model analysis did not reveal an association between amyloid PET SUVR and NP with CAA (OR: 0.360, 95% CI: 0.007, 1.741, p-value = 0.606).
Conclusions
CSF Aβ42 is more sensitive to predict MRI-negative CAA in high NP burden than amyloid PET.


Similar content being viewed by others
References
Boyle PA, Yu L, Nag S, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015;85(22):1930–1936. DOI: https://doi.org/10.1212/WNL.0000000000002175
Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat Rev Neurol. 2020;16(1):30–42. DOI: https://doi.org/10.1038/s41582-019-0281-2
Boche D, Denham N, Holmes C, Nicoll JA. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer’s disease pathogenesis. Acta Neuropathol. 2010;120(3):369–384. DOI: https://doi.org/10.1007/s00401-010-0719-5
Charidimou A, Boulouis G, Frosch MP, et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022;21(8):714–725. DOI: https://doi.org/10.1016/S1474-4422(22)00208-3
Jäkel L, De Kort AM, Klijn CJM, Schreuder FHBM, Verbeek MM. Prevalence of cerebral amyloid angiopathy: A systematic review and meta-analysis. Alzheimers Dement. 2022;18(1):10–28. DOI: https://doi.org/10.1002/alz.12366
Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. DOI: https://doi.org/10.1007/s00401-011-0910-3
Cairns NJ, Taylor-Reinwald L, Morris JC; Alzheimer’s Disease Neuroimaging Initiative. Autopsy consent, brain collection, and standardized neuropathologic assessment of ADNI participants: the essential role of the neuropathology core. Alzheimers Dement. 2010;6(3):274–279. DOI: https://doi.org/10.1016/j.jalz.2010.03.012
Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. DOI: https://doi.org/10.1002/ana.21610
Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol. 2011;121(5):597–609. DOI: https://doi.org/10.1007/s00401-011-0808-0
Landau SM, Lu M, Joshi AD, et al. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann Neurol. 2013;74(6):826–836. DOI: https://doi.org/10.1002/ana.23908
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. DOI: https://doi.org/10.1016/0022-3956(75)90026-6
Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. DOI: https://doi.org/10.1212/wnl.43.11.2412-a
Crane PK, Carle A, Gibbons LE, et al. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav. 2012;6(4):502–516. DOI: https://doi.org/10.1007/s11682-012-9186-z
Gibbons LE, Carle AC, Mackin RS, et al. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6(4):517–527. DOI: https://doi.org/10.1007/s11682-012-9176-1
Margraf NG, Jensen-Kondering U, Weiler C, et al. Cerebrospinal Fluid Biomarkers in Cerebral Amyloid Angiopathy: New Data and Quantitative Meta-Analysis. Front Aging Neurosci. 2022;14:783996. Published 2022 Feb 14. DOI: https://doi.org/10.3389/fnagi.2022.783996
Sembill JA, Lusse C, Linnerbauer M, et al. Cerebrospinal fluid biomarkers for cerebral amyloid angiopathy. Brain Commun. 2023;5(3):fcad159. Published 2023 May 19. DOI: https://doi.org/10.1093/braincomms/fcad159
Charidimou A, Friedrich JO, Greenberg SM, Viswanathan A. Core cerebrospinal fluid biomarker profile in cerebral amyloid angiopathy: A meta- analysis. Neurology. 2018;90(9):e754–e762. DOI: https://doi.org/10.1212/WNL.0000000000005030
De Kort AM, Kuiperij HB, Marques TM, et al. Decreased Cerebrospinal Fluid Amyloid β 38, 40, 42, and 43 Levels in Sporadic and Hereditary Cerebral Amyloid Angiopathy [published correction appears in Ann Neurol. 2023 Jun 9;:]. Ann Neurol. 2023;93(6):1173–1186. DOI: https://doi.org/10.1002/ana.26675. Epub 2023 Jun 9.
Banerjee G, Ambler G, Keshavan A, et al. Cerebrospinal Fluid Biomarkers in Cerebral Amyloid Angiopathy. J Alzheimers Dis. 2020;74(4):1189–1201. DOI:https://doi.org/10.3233/JAD-191254
Charidimou A, Farid K, Tsai HH, Tsai LK, Yen RF, Baron JC. Amyloid-PET burden and regional distribution in cerebral amyloid angiopathy: a systematic review and meta-analysis of biomarker performance. J Neurol Neurosurg Psychiatry. 2018;89(4):410–417. DOI: https://doi.org/10.1136/jnnp-2017-316851
Jang H, Jang YK, Kim HJ, et al. Clinical significance of amyloid β positivity in patients with probable cerebral amyloid angiopathy markers. Eur J Nucl Med Mol Imaging. 2019;46(6):1287–1298. DOI: https://doi.org/10.1007/s00259-019-04314-7
Banerjee G, Carare R, Cordonnier C, et al. The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice. J Neurol Neurosurg Psychiatry. 2017;88(11):982–994. DOI: https://doi.org/10.1136/jnnp-2016-314697
Case NF, Charlton A, Zwiers A, et al. Cerebral Amyloid Angiopathy Is Associated With Executive Dysfunction and Mild Cognitive Impairment. Stroke. 2016;47(8):2010–2016. DOI: https://doi.org/10.1161/STROKEAHA.116.012999
Acknowledgements
Data collection and sharing for this project were funded by the Alzheimer’s Disease Neuroimaging Initiative (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. This work was supported by the Soonchunhyang University Research Fund.
Funding
Funding: There is no funding.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethical standards: Ethical approval was given by the local ethical committees of all involved sites of ADNI, and the research was conducted in accordance with the Helsinki Declaration.
Conflict of interest: There is nothing to declare.
Additional information
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pyun, JM., Kang, M.J., Baek, S.J. et al. Magnetic Resonance Imaging-Negative Cerebral Amyloid Angiopathy: Cerebrospinal Fluid Amyloid-β42 over Amyloid Positron Emission Tomography. J Prev Alzheimers Dis (2024). https://doi.org/10.14283/jpad.2024.49
Received:
Accepted:
Published:
DOI: https://doi.org/10.14283/jpad.2024.49