Abstract
The sea squirt Ciona robusta (formerly Ciona intestinalis type A) has been the subject of many interdisciplinary studies. Known as a vanadium-rich ascidian, C. robusta is an ideal model for exploring microbes associated with the ascidian and the roles of these microbes in vanadium accumulation and reduction. In this study, we discovered two bacterial strains that accumulate large amounts of vanadium, CD2-88 and CD2-102, which belong to the genera Pseudoalteromonas and Vibrio, respectively. The growth medium composition impacted vanadium uptake. Furthermore, pH was also an important factor in the accumulation and localization of vanadium. Most of the vanadium(V) accumulated by these bacteria was converted to less toxic vanadium(IV). Our results provide insights into vanadium accumulation and reduction by bacteria isolated from the ascidian C. robusta to further study the relations between ascidians and microbes and their possible applications for bioremediation or biomineralization.
Similar content being viewed by others
Introduction
Ascidians, commonly called sea squirts, are sessile marine invertebrates that inhabit shallow ocean water and have a wide geographical distribution. Henze (1911) reported that the blood cells (vanadocytes) of the ascidian Phallusia mammillata contain unexpectedly high levels of vanadium. This unusual phenomenon prompted studies of how ascidians accumulate vanadium (reviewed in Michibata et al. 2003; Michibata and Ueki 2012; Ueki et al. 2015). Our group has extensively investigated vanadium levels and chemical states in a wide range of ascidian species, and has found that ascidians belonging to the Phlebobranchia can generally accumulate high levels of vanadium. The highest vanadium concentration has been found in Ascidia gemmata (350 mM in blood cells) (Michibata et al. 1991), which corresponds to 107 times that in seawater (35 nM) (Collier 1984), and the accumulated vanadium is mostly reduced to the + 3 oxidation state (Hirata and Michibata 1991). While vanadium occurs in + 3 (V(III)), + 4 (V(IV)), and + 5 (V(V)) oxidation states in aqueous solution, V(V) is the most common and stable form (Imtiaz et al. 2015; Wnuk 2023). V(V) is an oxyanion and is usually taken up by cells via anion transporters or the transferrin pathway (Dingley et al. 1982) and reduced to V(IV) in the cytoplasm. This reduction process has been proposed to be mediated by reducing agents, such as glutathione or NADPH, in the cytoplasm (Kanamori et al. 1999). In the vanadocytes of ascidians, V(IV) has been shown to be transported to the acidic organelle, the vacuole, via the Nramp family divalent cation transporter (Ueki et al. 2011), and reduced again to V(III) and stored (Hirata and Michibata 1991; Nette et al. 1999, 2004; Ueki et al. 2002). We identified vanadium-binding proteins, vanabins, from vanadocytes and coelomic fluid of ascidians (Michibata et al. 2003; Trivedi et al. 2003; Yamaguchi et al. 2004; Yoshihara et al. 2005), which can act as V(V) reductase in the cytoplasm of vanadocytes (Kawakami et al. 2006, 2009; Ueki et al. 2014). However, vanadium is also found in other ascidian tissues. In particular, high concentrations have been reported in the intestine, 1.80 mM in Ascidia sydneiensis samea (Romaidi and Ueki 2016) and 0.6 mM in Ciona intestinalis (Hirata and Michibata 1991; Trivedi et al. 2003).
The bacteria in the ascidian intestine are closely related to physiological processes, nutrition, and metabolism of the host (Sommer and Bäckhed 2013; Yang et al. 2022). Several studies have also shown that intestinal bacteria from ascidians are able to accumulate and reduce vanadium. Antipov et al. (2000) discovered Pseudomonas isachenkovii from the intestinal contents of an ascidian worm, and found that it could resist vanadium toxicity after exposure to 6 g/L vanadate and used vanadium as an electron acceptor for anaerobic respiration. Two previous studies found that Shewanella oneidensis was able to grow in the presence of vanadate, using it as the sole electron acceptor, and could reduce vanadate V(V) to vanadyl V(IV) ions (Carpentier et al. 2003, 2005).
Numerous studies have reported that bacteria can accumulate metals as adaptive responses, as bacteria are constantly exposed to unfavorable environmental conditions, such as heavy metal exposure, but they are able to adapt through various protective mechanisms (Mathivanan et al. 2021). For example, Aeromonas sobria accumulates copper and nickel (Qurbani et al. 2022) while Lactobacillus plantarum accumulates cadmium and lead metals (Ameen et al. 2020). Pseudomonas idrijaensis, Marinomonas communis, and Bacillus subtilis can take up mercury, arsenic, and iron, respectively (Takeuchi et al. 2007; dos Reis et al. 2014; Bourdineaud et al. 2020). Micrococcus luteus and Bacillus cohnii have the potential to accumulate zinc, chromium, and cobalt (Benmalek and Fardeau 2016; Marzuki et al. 2021). Three marine bacteria, i.e., Pseudomonas putida, Bacillus cereus, and Pseudomonas pseudoalcaligenes (Shirdam et al. 2006), and Halomonas sp. (Ghazvini and Mashkani 2009) have been reported to accumulate vanadium.
Exposure to high levels of vanadium in the ascidian body triggers its accumulation by some resistant bacteria. Vanadium is present at different levels in the tissues of ascidians, e.g., 1.4 mM in the branchial sac and 1.8 mM in the intestine (Ueki et al. 2015). The ascidian intestine and intestinal contents are exposed to the external environment and harbor highly diverse bacterial communities that play a number of roles in maintenance and regulation of the physiology and environmental adaptation (Dishaw et al. 2012; Sommer and Bäckhed 2013). To determine the contributions of bacteria to accumulation of vanadium in ascidians, our group previously studied vanadium-accumulating bacteria in the intestinal contents of A. sydneiensis samea. Two bacteria, Shewanella strain S-RA-6 and Vibrio strain S-RA-4, were shown to take up large amounts of vanadium (Romaidi and Ueki 2016).
In the present study, we investigated the contributions of bacteria in another vanadium-accumulating ascidian, Ciona robusta (previously known as Ciona intestinalis type A). C. robusta accumulates a moderate level of vanadium (0.6 mM) (Michibata et al. 1991; Trivedi et al. 2003), which is 17,000 times higher than that in seawater. Although the gut microbiota of Ciona has been studied by several groups to examine the pivotal roles of the microbes in shaping the host immune system (Dishaw et al. 2012, 2014; Leigh et al. 2016; Utermann et al. 2020), there have been no previous reports on the relations between microbes and ascidian tissues in C. robusta with regard to vanadium accumulation. This prompted us to isolate and study cultivable bacteria associated with the intestinal contents of C. robusta to explore their vanadium accumulation ability.
Materials and Methods
Isolation of Vanadium-Resistant Bacteria
Adult C. robusta were obtained from the National BioResource Project (NBRP) of MEXT, Japan (https://marinebio.nbrp.jp/marinebio/). Bacterial strains were isolated at two different time points: soon after arriving at our laboratory and after 44 days in aquarium culture. C. robusta specimens were dissected aseptically, and the intestinal contents were collected in 1.5-mL microtubes. The contents were diluted from 10−2 to 10−4 with sterile natural seawater and 10-µL aliquots were inoculated onto agar medium. Three different solid media were prepared in artificial seawater (ASW) (Marine Art SF1; Tomita Pharmaceutical, Kumamoto, Japan): standard medium (yeast extract 2.5 g/L, peptone 5.0 g/L, glucose 1.0 g/L, and agar 15.0 g/L) (Atlas 2005), 1/2TZ medium (yeast extract 0.50 g/L, peptone 2.5 g/L, HEPES 4.8 g/L, MnCl2·4H2O 0.20 g/L, and agar 15.0 g/L) (Maruyama et al. 1993), and Postgate’s B (PB) medium (yeast extract 0.20 g/L, sodium lactate 2.5 g/L, KH2PO4 0.25 g/L, NH4Cl 0.5 g/L, and agar 15.0 g/L) (Antipov et al. 1998). Each medium was supplemented with 10 mM sodium orthovanadate (Sigma-Aldrich, St. Louis. MO, USA) and incubated at 20 °C for 2–4 days. Growing bacterial colonies were randomly selected for further identification.
Identification of Bacterial Strains
Molecular identification of the selected bacterial strains was performed using the 16S ribosomal RNA (rRNA) gene sequencing method with the primers 306F 5′-CCAGACTCCTACGGGAGGCAGC-3′ and 935R 5′-CGAATTAAACCACATGCTCAAC-3′. For polymerase chain reaction (PCR), an appropriate number of bacterial cells picked up from each colony and 1 µM of each primer were mixed with PCR Master Mix (KAPA2G Robust HS ReadyMix; Kapa Biosystems, Wilmington, MA, USA) in a 10 µL reaction volume. The amount of DNA template corresponded to about 5–30 ng. PCR was performed for 30 cycles of 96 °C for 30 s, 50 °C for 40 s, and 72 °C for 40 s. The PCR product (~ 650 bp) was purified using a FastGene™ Gel/PCR Extraction Kit (Nippon Genetics, Tokyo, Japan). DNAs were sent to Fasmac Co. Ltd. (Kanagawa, Japan) to determine the DNA sequence by the Sanger sequencing method. The sequence data were compared to the nucleotide sequences in the NCBI database using BLASTn (https://blast.ncbi.nlm.nih.gov/). The sequences were aligned, and phylogenetic analysis was conducted using ClustalW (https://www.genome.jp/tools-bin/clustalw). The phylogenetic tree was visualized using Interactive Tree of Life (https://itol.embl.de/).
Bioaccumulation Assay of the Vanadium-Resistant Bacteria
Bacterial cells in the log phase were used as an inoculum and cultured in standard medium with shaking at 180 rpm, for 24 h, at 25 °C. The medium used was supplemented with 0.5 mM V(V) or V(IV). The cells were harvested by centrifugation at 3500 rpm and 4 °C for 15 min and washed three times with vanadium-free medium. The pellets were dried overnight at 65 °C, mixed with 500 µL of 1 N nitric acid, and then heated overnight at 65 °C. Each sample was centrifuged at 10,000 rpm for 10 min and the vanadium concentration in the supernatant was measured by atomic absorption spectrometry (AAS) (Shirdam et al. 2006; Romaidi and Ueki 2016). 1/2TZ medium was also used as a comparative medium. Vanadium was expressed as nanograms per milligram dry weight (ng/mg dw) of cells.
Accumulation of Vanadium at Different pH Values
Vanadium accumulation was determined according to variation in pH following the procedure described previously (Romaidi and Ueki 2016). Bacterial cells were prepared by culturing an inoculum in standard medium with rotation at 180 rpm and 25 °C for 24 h. The culture was centrifuged at 3500 rpm and 4 °C for 15 min for harvesting. Harvested cells were suspended in assay buffer (0.5 M NaCl, 0.05 mM sodium phosphate at pH 3, 5, 7, or 9) supplemented with 0.5 mM vanadium. The bacterial cell suspensions were rotated at 180 rpm and 25 °C for 24 h. The cells were collected by centrifugation and washed three times with assay buffer at the appropriate pH. After drying overnight at 65 °C, 0.5 mL 1 N nitric acid was added to the cells and incubated overnight at 65 °C. The mixture was centrifuged at 10,000 rpm for 10 min to obtain the supernatant. Vanadium accumulation was assessed by AAS.
Subcellular Distribution of Vanadium in the Bacterial Cells
The distributions of vanadium in the intracellular and extracellular compartments of bacterial cells were determined according to the previously published methods with some modifications (Desaunay and Martins 2014; Romaidi and Ueki 2016). Bacterial cells were cultured in vanadium-free standard medium for 24 h at 25 °C. Then, cell pellets were incubated in fresh medium containing 50 mM phosphate buffer at the appropriate pH, 0.5 M NaCl, and 0.5 mM vanadium at 25 °C with agitation at 180 rpm. After 24 h of incubation, the bacterial pellets were resuspended with 20 mM EDTA and shaken gently for 5 min to remove vanadium bound to the outer membrane and associated with the periplasmic space. These suspensions were centrifuged at 3500 rpm for 10 min. The supernatant was used directly to assay vanadium in the extracellular compartment. The pellet was treated with 0.5 mM HNO3 and incubated at 90 °C overnight before AAS to assess vanadium in the intracellular compartment.
Vanadium Speciation Assay Using Ion Exchange Chromatography
The ratio of V(V) to V(IV) in bacteria was examined by ion exchange chromatography, as described previously (Ueki et al. 2014). Overnight bacterial cultures were grown in standard medium supplemented with 0.5 mM V(V) at 25 °C and 180 rpm overnight. Harvested bacterial cells were washed with vanadium-free standard medium in triplicate. The bacterial cell pellet was resuspended with IC buffer (12 mM sodium hydrogen carbonate, 4 mM sodium carbonate, and 20 mM EDTA), and the mixture was sonicated to disrupt the cells. After centrifugation (10,000 rpm, 30 min), the pellet was discarded and the supernatant was analyzed for vanadium. Samples were injected into an ion exchange column (SI-50G; Shodex, Munich, Germany), equilibrated with IC buffer at a flow rate of 0.7 mL/min. Vanadium ions were detected by ultraviolet (UV) absorption at 282 nm and recorded with a D-7500 integrator (Hitachi, Ibaraki, Japan). The relative amounts of V(V) and V(IV) were calculated as the relative area ratio calculated from each peak profile.
Results
Isolation and Identification of Vanadium-Resistant Bacteria
We used culture medium containing 10 mM sodium orthovanadate (Na3VO4), which restricted the growth of intestinal bacteria in a previous study (Romaidi and Ueki 2016). We isolated bacteria from the intestinal contents of C. robusta at two different time points, i.e., immediately after arriving at the laboratory from NBRP or after 44 days in culture in the aquarium. We collected 40 bacterial colonies at random from the three different cultivation media; 9 isolates were obtained from the first isolation, and 31 isolates were obtained from the second isolation. All isolates were categorized as vanadium-resistant bacteria because of their ability to grow on medium supplemented with 10 mM sodium orthovanadate.
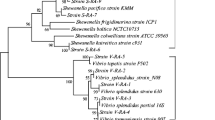
The 40 bacterial isolates were identified by partial 16S rRNA gene sequencing and could be classified into 2 phyla, 8 genera, and 12 strains (Supplementary Table S1). The 40 bacterial isolates formed two main clusters in phylogenetic tree analysis, which suggested that the types of culturable bacteria were different between the first and second isolation methods (Fig. 1). Thirty isolates belonged to the Proteobacteria and were composed of 72% Gammaproteobacteria and 8% Alphaproteobacteria, and eight isolates belonged to Firmicutes. At the genus level, the isolated bacteria were assigned to eight genera: Vibrio (58%), Staphylococcus (18%), Shewanella (8%), Ensifer (8%), Photobacterium (2%), Pseudoalteromonas (2%), Ferrimonas (2%), and Halobacillus (2%). Twelve isolates representing the 12 strains were selected (Table 1) and used to measure their ability to accumulate vanadium.
Phylogenetic tree of vanadium-resistant bacteria from the intestinal contents of C. robusta based on 16S rRNA sequences. After arriving at the laboratory (purple) or after treatment for 44 days (blue), we selected strains (green arrowheads) and identified hyperaccumulator vanadium strains (red arrowheads)
The most culturable strain obtained (23 isolates) belonged to the genus Vibrio, corresponding closely to Vibrio alginolyticus (98.73% ± 1.31%), Vibrio mediterranei (99.32% ± 0.16%), Vibrio diabolicus (99.16%), Vibrio neptunius (99.49%), and Vibrio aerogenes (90.00%). The second most dominant strains (7 isolates) belonged to the genus Staphylococcus and were assigned to Staphylococcus ureilyticus with identity of 98.71% ± 1.08%. These were followed by the genera Shewanella and Ensifer. Three isolates (CD2-95, CD2-101, and CD2-106) showed 99.05% ± 0.34% similarity in 16S rRNA gene sequence to that of Shewanella loihica; three other isolates (CD2-127, CD2-128, and CD2-137) showed 94.01% ± 0.04% similarity to Ensifer alkalisoli. The remaining isolates, CR1-43 and CD2-97, showed 98.83% and 99.33% similarity to Photobacterium damselae and Ferrimonas kyonanensis, respectively, while CD2-130 showed 98.83% similarity to Halobacillus marinus.
Accumulation Assay by the Vanadium-Resistant Bacteria
The abilities of the 12 strains to accumulate vanadium were evaluated by determining the V content per dw of bacterial cells in media containing 0.5 mM V(V) and V(IV). All strains accumulated different amounts of one or both vanadium species. However, only strains CD2-88 and CD2-102 showed high vanadium accumulation ability (Fig. 2). Two strains were potential vanadium hyperaccumulators and were used for further analyses. The vanadium accumulation abilities of these two strains, which were close to the genera Pseudoalteromonas and Vibrio, were examined in standard medium and 1/2TZ medium.
Accumulation of vanadium by selected vanadium-resistant bacterial strains isolated from the intestinal contents of C. robusta. Bacterial cells were cultured in standard medium supplemented with 0.5 mM V(V) or V(IV) for 24 h at 25 °C. The cells were harvested by centrifugation, and the amount of vanadium per dry weight of cells was measured. Error bars correspond to the standard deviation for 3–9 replicates
In the standard medium, strain CD2-102 accumulated 266 ± 77 ng/mg dw of V(V) and 261 ± 99 ng/mg dw of V(IV). No significant differences were observed between the accumulation of V(V) or V(IV) (Student’s t test, p > 0.05). The accumulation pattern of strain CD2-102 differed from that of strain CD2-88, which accumulated only a small amount of V(V) (6 ± 1 ng/mg dw) but a large amount of V(IV) (169 ± 31 ng/mg dw). The remaining strains accumulated various amounts of vanadium, ranging from 0.3 to 62 ng/mg dw for V(V) and from 11 to 32 ng/mg dw for V(IV).
We also investigated vanadium uptake in 1/2TZ medium, which affected the ability of the strains to accumulate vanadium. For strain CD2-88, 1/2TZ medium enhanced the vanadium uptake to 245 ± 67 ng/mg dw for V(V) and 211 ± 45 ng/mg dw for V(IV) (Fig. 3). This difference in V(V) accumulation was significant (p < 0.05), while that of V(IV) accumulation was not (p > 0.05). Interestingly, CD2-102 showed the opposite result, with vanadium accumulation capability decreasing by 98% to 8 ± 5 ng/mg dw for V(IV) and 5 ± 3 ng/mg dw for V(V). Next, we investigated the effects of altering the 1/2TZ medium composition on strain CD2-102. Removing the HEPES and/or manganese(II) chloride did not alter the accumulation ability. The amount of accumulated vanadium was still low at 6–9 ng/mg dw for V(IV) and 3–10 ng/mg dw for V(V) (Fig. 3). After adding glucose, vanadium accumulation increased significantly to 117 ± 10 ng/mg dw for V(V) and 66 ± 15 ng/mg dw for V(IV) (Student’s two-tailed t test).
Accumulation of vanadium in the standard and 1/2TZ medium by A CD2-88 and B CD2-102. Bacterial cells were cultured in each medium supplemented with 0.5 mM V(V) or V(IV) for 24 h at 25 °C. The cells were harvested by centrifugation and the amount of vanadium per dry weight of cells was measured. The symbols − and + indicate removal and addition of substances to the medium, respectively. The error bars correspond to the standard deviation calculated from duplicate data for 3–7 replicates
Vanadium Accumulation at Different pH Levels
To study the influence of pH on vanadium accumulation, we cultured strains CD2-88 and CD2-102 in standard medium and harvested cells were incubated in an assay buffer at pH ranging from 3.0 to 9.0. Under these conditions, bacteria did not grow but could accumulate vanadium. The accumulation of vanadium was greatly affected by the pH (Student’s two-tailed t test; Fig. 4). The results showed that vanadium accumulation increased with decreasing pH. The greatest levels of V(V) accumulation by strains CD2-88 and CD2-102 were observed at pH 3 and were 4342 ± 1900 ng/mg dw and 4905 ± 1767 ng/mg dw, respectively. At pH 5, the V(V) concentration declined to 1132 ± 106 ng/mg dw for CD2-88 and 2150 ± 157 ng/mg dw for CD2-102. Under neutral conditions (pH 7), CD2-88 and CD2-102 took up 25 ± 4 ng/mg dw V(V) and 9 ± 2 ng/mg dw V(V), respectively. A negligible amount of V(V) was accumulated at pH 9. In strains CD2-88 and CD2-102, the V(IV) accumulation differed slightly from the V(V) accumulation pattern. The V(IV) uptake tended to increase with decreasing pH from pH 9.0 to 5.0, and then decreased at pH 3.0. The maximum capacities to accumulate V(IV) for CD2-88 and CD2-102 were 276 ± 110 ng/mg dw and 817 ± 44 ng/mg dw, respectively, which were observed at pH 5.0. At pH 3, the V(IV) accumulation decreased to 188 ± 26 ng/mg dw and 142 ± 49 ng/mg dw for CD2-88 and CD2-102, respectively. The V(IV) accumulation was low at pH 7 and 9. In general, the V(V) and V(IV) uptake were highest at acidic pH.
Effects of pH on vanadium accumulation in sodium chloride-sodium phosphate buffer (A CD2-88 and B CD2-102). Bacterial cells were cultured in standard medium, harvested by centrifugation, and resuspended in assay buffer (0.5 M NaCl, 0.05 mM sodium phosphate at pH 3, 5, 7, or 9) supplemented with 0.5 mM vanadium. The bacterial cell suspensions were incubated for 24 h at 25 °C. The cells were harvested by centrifugation and the amount of vanadium per dry weight of cells was measured. Vertical bars indicate the standard error (n = 3–6)
Subcellular Localization of Vanadium in Bacterial Cells
Experiments were performed to determine in which bacterial cell compartment the vanadium was localized after 24 h in culture. The amount of accumulated vanadium was measured at pH 3, 5, and 7. Bacteria can store metals on the cell surface (extracellular compartment) or inside the cell (intracellular compartment). The relative amount of vanadium was calculated by dividing that in the cytoplasm by the total amount in the cell. The pH and vanadium species greatly affected the localization of the metal in the cells (Fig. 5). At pH 7, the extracellular compartment contained as much as 87–99% of the vanadium, which decreased to 44–49% at pH 5 and 1–9% at pH 3. In contrast, at pH 7, the intracellular compartment contained 76–100% of the vanadium, which decreased to 46–76% and 24–53% at pH 5 and 3, respectively. Strains CD2-88 and CD2-102 showed similar patterns of subcellular localization of vanadium.
Relative amounts of vanadium in the intracellular fraction of A CD2-88 and B CD2-102. Bacterial cells were cultured in standard medium, harvested by centrifugation, and resuspended in assay buffer (0.5 M NaCl, 0.05 mM sodium phosphate at pH 3, 5, or 7) supplemented with 0.5 mM vanadium. The bacterial cell suspensions were incubated for 24 h at 25 °C. The cells were harvested by centrifugation, and the amount of vanadium per dry weight of cells was measured. Error bars correspond to the standard deviation (n = 3)
Vanadium Speciation in Bacterial Cells
Ion chromatography was used to examine vanadium speciation in the cell. V(V) and V(IV) could be differentiated based on peak formation and retention time. The peak area was used to calculate the vanadium concentration. In this analysis, strains CD2-88 and CD2-102 were cultured on standard medium containing 0.5 mM V(V) as the sole vanadium species. A peak of V(IV) would appear if the V(V) in the medium was converted. We detected a large peak of V(IV) compared to V(V) in the bacterial cells. The percentage of V(IV) was 99.4% ± 0.007% for strain CD2-88 and 99.7% ± 0.003% for strain CD2-102, confirming that the bacteria reduced V(V) to V(IV).
Discussion
We screened bacteria from the intestine of C. robusta for vanadium resistance by inoculating three culture media containing 10 mM sodium orthovanadate with aliquots of intestinal contents. Forty strains were obtained and were found to be resistant to vanadium.
Vibrio was the most culturable genus, accounting for 58% of all cultivated strains, and was considered one of the most abundant culturable bacteria present in the aquatic environment (Vezzulli et al. 2015) and marine animals (Sampaio et al. 2022). Vibrio has been reported to be a highly abundant bacterial genus in the gut of Ciona in general (Dishaw et al. 2012) and Ciona intestinalis in particular (Liberti et al. 2019). In addition to resistance to vanadium, some Vibrio species have also been shown to be resistant to other heavy metals. For example, Vibrio parahaemolyticus isolated from oysters and shellfish is resistant to barium, cobalt, cadmium, and copper (Kang et al. 2018; Jo et al. 2020) while V. alginolyticus has been reported to be resistant to arsenic (Takeuchi et al. 2007). Su et al. (2022) reported that Vibrio species from the clam Meretrix meretrix are tolerant to cadmium, zinc, and copper.
The second most dominant genus was Staphylococcus, which accounted for 18% of all cultivated strains, followed by other genera, such as Shewanella, Ensifer, Photobacterium, Pseudoalteromonas, Ferrimonas, and Halobacillus. These genera are commonly found in marine animals, such as ascidians (Dishaw et al. 2014), sponges (Fan et al. 2013; Paul et al. 2021), fish (Campbell et al. 2007), mussels (Beleneva and Maslennikova 2002; Collado et al. 2009), and sea urchins (Yao et al. 2019). Shewanella and Pseudoalteromonas have been reported to be prevalent components of the ascidian gut microbiome (Schreiber et al. 2016; Ueki et al. 2019). Shewanella putrefaciens and Shewanella oneidensis show significant resistance to arsenic, lead, and cadmium (Huang et al. 2011; Jaafar et al. 2016), whereas Pseudoalteromonas citrea and Pseudoalteromonas nigrifaciens show tolerance to copper, cadmium, mercury, and nickel (Ivanova et al. 2001). Staphylococcus shows resistance to arsenic, mercury, silver, and cadmium (Lauková 1994; Lawal et al. 2021) while Ferrimonas kyonanensis, Photobacterium damselae, and Halobacillus sp. have high tolerance to selenium, cadmium, and nickel, respectively (Nakagawa et al. 2006; Yakoubi et al. 2018; Kardel and Torabi 2019). We also obtained isolates belonging to Ensifer, a genus that was originally isolated from plant roots (Li et al. 2016) and shown to have arsenic resistance (Mesa et al. 2017; DiCenzo et al. 2018). No strains belonging to this genus have previously been isolated from marine animals, and this represents the first report of Ensifer isolated from an ascidian.
Two vanadium-resistant bacteria were identified as vanadium accumulators, i.e., CD2-88 (Pseudoalteromonas sp.) and CD2-102 (Vibrio sp.) (Fig. 2). Pseudoalteromonas shows good accumulation of cadmium (Zhou et al. 2013), and this species plays a predominant role in arsenic and lead remediation (Dell’Anno et al. 2020). To the best of our knowledge, however, there have been no previous reports of vanadium accumulation by this genus. In contrast, Vibrio sp. has been reported to be a bioremediation agent for heavy metals, including vanadium. For example, Vibrio fluvialis has the ability to take up mercury (Saranya et al. 2017) while V. alginolyticus and Vibrio rotiferianus have been shown to take up lead and strontium (Parmar et al. 2020). Our research group has also reported that Vibrio strain V-RA-4 can accumulate various metals, especially vanadium (Romaidi and Ueki 2016). Hernández et al. (1998) reported accumulation of high levels of vanadium, 35,004 ng/mg dw and 34,154 ng/mg dw, by Escherichia hermannii CNB50 and Enterobacter cloacae CNB60, respectively. The level of vanadium accumulation in this study was similar to that reported by Romaidi and Ueki (2016) but 100 times lower than reported by Hernández et al. (1998). The present study is the first report that Pseudoalteromonas species can accumulate vanadium, and we found more Vibrio sp. capable of accumulating vanadium.
In addition to accumulating vanadium, the strains CD2-88 and CD2-102 also reduced V(V) to V(IV). More than 99% of the V(V) taken up by both strains was converted into V(IV) in these bacterial cells. Various microorganisms have been reported to possess the ability to reduce V(V) to V(IV), such as Pseudomonas (Lyalkova and Yurkova 1992), Shewanella (Carpentier et al. 2003, 2005; Wang et al. 2017), Geobacter (Ortiz-Bernad et al. 2004; Liu et al. 2017; Yan et al. 2022), Bacillus (Zhang et al. 2019; Zhou et al. 2022), and Polaromonas (Sun et al. 2020). The metal-reducing bacterium Shewanella oneidensis can fully reduce 5 mM V(V) to support its growth (Carpentier et al 2003) while E. cloacae can reduce 55% of the V(V) at a vanadium concentration of 4 mM (van Marwijk et al. 2009). Here, we also found that our vanadium-resistant and vanadium-accumulating bacteria, Pseudoalteromonas sp. strain CD2-88 and Vibrio sp. strain CD2-102, could reduce vanadate V(V) to the lower oxidation state species V(IV). The capability of V(V) bioreduction by bacteria could differ between strains.
The type of growth medium may influence the metal accumulation capability of bacteria. The two strains were examined to determine optimal growth medium conditions, because such information will be necessary for their application to bioremediation. The standard medium and 1/2TZ medium are rich media and have been used to culture bacteria from marine sources (Yamazaki et al. 1993). Strain CD2-88 showed a low level of V(V) accumulation in standard medium, which surprisingly increased by 98% in 1/2TZ medium. In contrast, strain CD2-102 showed the opposite response upon changing to 1/2TZ medium, and addition of glucose to 1/2TZ medium led to 50% recovery of vanadium accumulation. The composition of the medium may have been one factor responsible for the decline in vanadium accumulation by strain CD2-102. This is supported by previous studies by Rathnayake et al. (2013) and El Baz et al. (2015) that medium composition impacts the level of metal tolerance. In this case, the addition of glucose partially restored the vanadium accumulation ability of CD2-102, which uses glucose as its primary source of energy. Bioaccumulation is a cellular energy–dependent process in active metabolic microorganisms (Wróbel et al. 2023). Our results parallel those of Stoll and Duncan (1996) and Bode et al. (1990) who demonstrated that pretreatment of Saccharomyces cerevisiae with glucose enhances heavy metal uptake from wastewater. Glucose is the most appropriate electron donor in the reduction of certain heavy metals (Zeng et al. 2019; Tan et al. 2020). Our results suggest that glucose could play an important role in vanadium accumulation by CD2-88 and CD2-102.
Accumulation is a complex process that depends on various factors, with pH being the most critical parameter in the accumulation of metal ions (Khan et al. 2016). Our results clearly show increasing bioaccumulation of vanadium by CD2-88 and CD2-102 with decreasing pH, with acidic pH 3 being the most favorable condition for vanadium accumulation. Similarly, Romaidi and Ueki (2016) reported optimal vanadium accumulation by Vibrio strain V-RA-4 and Shewanella strain S-RA-6 under acidic conditions. Bode et al. (1990) examined vanadium uptake by S. cerevisiae at low pH. In another study, both cadmium and copper biosorption by Bacillus thuringiensis OSM29 were optimized at low pH (Oves et al. 2013). In contrast, in another study, uptake of other metals was high at neutral and basic pH; e.g., accumulation of lead was maximized under neutral conditions, while chromium and nickel accumulation were maximized under basic conditions (López et al. 2000; Aslam et al. 2020). Taken together, these observations indicate that the influence of pH on metal accumulation varies among bacterial strains and metals.
We found changes in the distribution of V(V) and V(IV) in CD2-88 and CD2-102 strains according to pH. These two strains predominantly accumulated V(V) extracellularly at pH 7, shifting to intracellular storage at pH 3 (Fig. 5). Microorganisms can accumulate metals by either sorption on the cell surface or by intracellular uptake (Ledin 2000; Hansda et al. 2016). In a previous study, our group documented intracellular accumulation of vanadium by Vibrio strain V-RA-4 and Shewanella S-RA-6 (Romaidi and Ueki 2016). Almeida et al. (2020) support intracellular accumulation of vanadate by Ochrobactrum tritici. In contrast to previous studies, we documented both intracellular and extracellular vanadium uptake by bacterial strains isolated from the sea squirt C. robusta. Both accumulation processes could occur in the same organisms. For example, Lactococcus raffinolactis, the Beijerinckiaceae bacterium RH AL1, and Cyclotella sp. accumulate vanadium, lanthanide, and chromium, respectively, in both the extracellular and intracellular compartments (Zhang et al. 2021; Wegner et al. 2021; Li et al. 2021). A few studies have reported that external factors induce a shift in the heavy metal distribution from one compartment to another. Al-Aoukaty et al. (1991) reported that lead accumulated intracellularly in phosphate-deficient medium and extracellularly in phosphate-rich medium. Cadmium was shown to accumulate mainly intracellularly in culture medium with lower metal concentrations and extracellularly in medium containing a higher cadmium concentration (Huang et al. 2014). Consistent with other studies, strains CD2-88 and CD2-102 showed a defense response to external factors by switching of the vanadium accumulation mechanism.
We hypothesized that vanadium-accumulating bacteria could help ascidians absorb vanadium in the intestine. The intestinal contents of C. robusta have pH 6–7 under physiological conditions, and in our study, CD2-88 and CD2-102 accumulated vanadium on the cell surface at pH 7 (Fig. 5). Under these conditions, most of the vanadium was reduced to V(IV) (see the subsection “Vanadium Speciation in Bacterial Cells” of the “Results” section). These results suggest that the bacteria took up V(V) from the intestinal contents and stored it in the extracellular compartment as V(IV), and could help ascidians absorb V(IV) via intestinal epithelial cells. These observations along with determination of the localization of metals provide insights into the interaction between ascidians and bacteria with regard to vanadium accumulation. A previous study on Ascidia sydneiensis samea reported that V(V) could be absorbed directly by strains V-RA-4 (Vibrio tasmaniensis) and S-RA-6 (Shewanella kaireitica) at the same pH (Romaidi and Ueki 2016). There may be different modes of interaction between microorganisms and ascidians in vanadium accumulation.
In summary, 12 bacterial strains were isolated from the intestinal contents of C. robusta and were classified into 8 genera. We identified two prospective bacterial strains with higher levels of vanadium accumulation, designated as CD2-88 and CD2-102. The accumulation and distribution of vanadium was pH-dependent, and nearly all of the accumulated V(V) was converted into V(IV). This research provided new insights into the symbiotic interactions between bacteria and C. robusta. The vanadium-accumulating and vanadium-reducing characteristics of both strains could be useful for application to bioremediation or biomineralization.
Availability of Data and Material
Not applicable.
References
Al-Aoukaty A, Appanna VD, Huang J (1991) Exocellular and intracellular accumulation of lead in Pseudomonas fluorescens ATCC 13525 is mediated by the phosphate content of the growth medium. FEMS Microbiol Lett 83:283–290
Almeida MC, Branco R, Morais PV (2020) Response to vanadate exposure in Ochrobactrum tritici strains. PLoS One 15
Ameen FA, Hamdan AM, El-Naggar MY (2020) Assessment of the heavy metal bioremediation efficiency of the novel marine lactic acid bacterium, Lactobacillus plantarum MF042018. Sci Rep 10:314. https://doi.org/10.1038/s41598-019-57210-3
Antipov A, Lyalikova N, L’vov N, (2000) Vanadium-binding protein excreted by vanadate-reducing bacteria. IUBMB Life Int Union Biochem Mol Biol Life 49:137–141
Antipov AN, Lyalikova NN, Khijniak TV, L’vov NP, (1998) Molybdenum-free nitrate reductases from vanadate-reducing bacteria. FEBS Lett 441:257–260
Aslam F, Yasmin A, Sohail S (2020) Bioaccumulation of lead, chromium, and nickel by bacteria from three different genera isolated from industrial effluent. Int Microbiol 23:253–261
Atlas RM (2005) Handbook of media for environmental microbiology, 2nd edn. CRC Press
Beleneva IA, Maslennikova EF (2002) Opportunistic bacteria detected in cultivated mussels. Zh Mikrobiol Epidemiol Immunobiol 2:81–83 (PMID: 12043163)
Benmalek Y, Fardeau M-L (2016) Isolation and characterization of metal-resistant bacterial strain from wastewater and evaluation of its capacity in metal-ions removal using living and dry bacterial cells. Int J Environ Sci Technol 13:2153–2162
Bode H-P, Friebel C, Fuhrmann GF (1990) Vanadium uptake by yeast cells. Biochim Biophys Acta BBA - Biomembr 1022:163–170
Bourdineaud J-P, Durn G, Režun B et al (2020) The chemical species of mercury accumulated by Pseudomonas idrijaensis, a bacterium from a rock of the Idrija mercury mine. Slovenia Chemosphere 248:126002. https://doi.org/10.1016/j.chemosphere.2020.126002
Campbell S, Harada RM, Li QX (2007) Ferrimonas senticii sp. nov., a novel gammaproteobacterium isolated from the mucus of a puffer fish caught in Kaneohe Bay. Hawai’i Int J Syst Evol Microbiol 57:2670–2673
Carpentier W, De Smet L, Van Beeumen J, Brigé A (2005) Respiration and growth of Shewanella oneidensis MR-1 using vanadate as the sole electron acceptor. J Bacteriol 187:3293–3301
Carpentier W, Sandra K, De Smet I et al (2003) Microbial reduction and precipitation of vanadium by Shewanella oneidensis. Appl Environ Microbiol 69:3636–3639
Collado L, Cleenwerck I, Van Trappen S et al (2009) Arcobacter mytili sp. nov., an indoxyl acetate-hydrolysis-negative bacterium isolated from mussels. Int J Syst Evol Microbiol 59:1391–1396
Collier RW (1984) Particulate and dissolved vanadium in the North Pacific Ocean. Nature 309:441–444
Dell’Anno F, Brunet C, Van Zyl LJ et al (2020) Degradation of hydrocarbons and heavy metal reduction by marine bacteria in highly contaminated sediments. Microorganisms 8:1402. https://doi.org/10.3390/microorganisms8091402
Desaunay A, Martins JMF (2014) Comparison of chemical washing and physical cell-disruption approaches to assess the surface adsorption and internalization of cadmium by Cupriavidus metallidurans CH34. J Hazard Mater 273:231–238
DiCenzo GC, Debiec K, Krzysztoforski J et al (2018) Genomic and biotechnological characterization of the heavy-metal resistant, arsenic-oxidizing bacterium Ensifer sp. M14. Genes 9:379. https://doi.org/10.3390/genes9080379
Dingley AL, Kustin K, Macara IG et al (1982) Vanadium-containing tunicate blood cells are not highly acidic. Biochim Biophys Acta BBA - Mol Cell Res 720:384–389
Dishaw LJ, Flores-Torres J, Lax S et al (2014) The gut of geographically disparate Ciona intestinalis harbors a core microbiota. PLoS One 9
Dishaw LJ, Flores-Torres JA, Mueller MG et al (2012) A basal chordate model for studies of gut microbial immune interactions. Front Immunol 3. https://doi.org/10.3389/fimmu.2012.00096
dos Reis KC, Silva CF, Duarte WF, Schwan RF (2014) Bioaccumulation of Fe3+ by bacteria isolated from soil and fermented foods for use in bioremediation processes. Afr J Microbiol Res 8:2513–2521
El Baz S, Baz M, Barakate M et al (2015) Resistance to and accumulation of heavy metals by Actinobacteria isolated from abandoned mining areas. Sci World J 2015:1–14
Fan L, Liu M, Simister R et al (2013) Marine microbial symbiosis heats up: the phylogenetic and functional response of a sponge holobiont to thermal stress. ISME J 7:991–1002
Ghazvini PTM, Mashkani SG (2009) Effect of salinity on vanadate biosorption by Halomonas sp. GT-83: preliminary investigation on biosorption by micro-PIXE technique. Bioresour Technol 100:2361–2368
Hansda A, Kumar V, Anshumali, (2016) A comparative review towards potential of microbial cells for heavy metal removal with emphasis on biosorption and bioaccumulation. World J Microbiol Biotechnol 32:170. https://doi.org/10.1007/s11274-016-2117-1
Henze M (1911) Untersuchungen über das Blut der Ascidien. I. Mitteilung. Die Vanadiumverbindung der Blutkörperchen. Hoppe-Seyler´s Z Für Physiol Chem 72:494–501. https://doi.org/10.1515/bchm2.1911.72.5-6.494
Hernández A, Mellado RP, Martínez JL (1998) Metal accumulation and vanadium-induced multidrug resistance by environmental isolates of Escherichia hermannii and Enterobacter cloacae. Appl Environ Microbiol 64:4317–4320
Hirata J, Michibata H (1991) Valency of vanadium in the vanadocytes of Ascidia gemmata separated by density-gradient centrifugation. J Exp Zool 257:160–165
Huang F, Guo C-L, Lu G-N et al (2014) Bioaccumulation characterization of cadmium by growing Bacillus cereus RC-1 and its mechanism. Chemosphere 109:134–142
Huang J-H, Voegelin A, Pombo SA et al (2011) Influence of arsenate adsorption to ferrihydrite, goethite, and boehmite on the kinetics of arsenate reduction by Shewanella putrefaciens strain CN-32. Environ Sci Technol 45:7701–7709
Imtiaz M, Rizwan MS, Xiong S et al (2015) Vanadium, recent advancements and research prospects: a review. Environ Int 80:79–88
Ivanova EP, Gorshkova NM, Kurilenko VV (2001) Tolerance of marine Proteobacteria of the genera Pseudoalteromonas and Alteromonas to heavy metals. Microbiology 70:239–241
Jaafar R, Al-Sulami A, Al-Taee A (2016) Bioaccumulation of cadmium and lead by Shewanella oneidensis isolated from soil in Basra governorate, Iraq. Afr J Microbiol Res 10:370–375
Jo S, Shin C, Shin Y et al (2020) Heavy metal and antibiotic co-resistance in Vibrio parahaemolyticus isolated from shellfish. Mar Pollut Bull 156:111246. https://doi.org/10.1016/j.marpolbul.2020.111246
Kanamori K, Sakurai M, Kinoshita T et al (1999) Direct reduction from vanadium(V) to vanadium(IV) by NADPH in the presence of EDTA. A consideration of the reduction and accumulation of vanadium in the ascidian blood cells. J Inorg Biochem 77:157–161
Kang C-H, Shin Y, Yu H et al (2018) Antibiotic and heavy-metal resistance of Vibrio parahaemolyticus isolated from oysters in Korea. Mar Pollut Bull 135:69–74
Kawakami N, Ueki T, Amata Y et al (2009) A novel vanadium reductase, Vanabin2, forms a possible cascade involved in electron transfer. Biochim Biophys Acta BBA - Proteins Proteomics 1794:674–679
Kawakami N, Ueki T, Matsuo K et al (2006) Selective metal binding by Vanabin2 from the vanadium-rich ascidian, Ascidia sydneiensis samea. Biochim Biophys Acta BBA - Gen Subj 1760:1096–1101
Khan Z, Rehman A, Hussain SZ et al (2016) Cadmium resistance and uptake by bacterium, Salmonella enterica 43C, isolated from industrial effluent. AMB Express 6:54. https://doi.org/10.1186/s13568-016-0225-9
Lauková A (1994) Resistance to heavy metals in ruminal staphylococci. Vet Med (praha) 39:389–395. (PMID: 8073587)
Lawal OU, Fraqueza MJ, Worning P et al (2021) Staphylococcus saprophyticus causing infections in humans is associated with high resistance to heavy metals. Antimicrob Agents Chemother 65:e02685-e2720
Ledin M (2000) Accumulation of metals by microorganisms — processes and importance for soil systems. Earth-Sci Rev 51:1–31
Leigh BA, Liberti A, Dishaw LJ (2016) Generation of germ-free Ciona intestinalis for studies of gut-microbe interactions. Front Microbiol 7:2092. https://doi.org/10.3389/fmicb.2016.02092
Li N, Qin L, Jin M et al (2021) Extracellular adsorption, intracellular accumulation and tolerance mechanisms of Cyclotella sp. to Cr(VI) stress. Chemosphere 270:128662. https://doi.org/10.1016/j.chemosphere.2020.128662
Li Y, Yan J, Yu B et al (2016) Ensifer alkalisoli sp. nov. isolated from root nodules of Sesbania cannabina grown in saline–alkaline soils. Int J Syst Evol Microbiol 66:5294–5300
Liberti A, Cannon JP, Litman GW, Dishaw LJ (2019) A soluble immune effector binds both fungi and bacteria via separate functional domains. Front Immunol 10:369. https://doi.org/10.3389/fimmu.2019.00369
Liu H, Zhang B, Yuan H et al (2017) Microbial reduction of vanadium (V) in groundwater: interactions with coexisting common electron acceptors and analysis of microbial community. Environ Pollut 231:1362–1369
López A, Lázaro N, Priego JM, Marqués AM (2000) Effect of pH on the biosorption of nickel and other heavy metals by Pseudomonas fluorescens 4F39. J Ind Microbiol Biotechnol 24:146–151
Lyalkova NN, Yurkova NA (1992) Role of microorganisms in vanadium concentration and dispersion. Geomicrobiol J 10:15–26
Maruyama A, Mita N, Higashihara T (1993) Particulate materials and microbial assemblages around the Izena black smoking vent in the Okinawa trough. J Oceanogr 49:353–367
Marzuki I, Kamaruddin M, Ahmad R et al (2021) Performance of cultured marine sponges-symbiotic bacteria as a heavy metal bio-adsorption. Biodiversitas J Biol Divers 22:5536–5543. https://doi.org/10.13057/biodiv/d221237
Mathivanan K, Chandirika JU, Vinothkanna A et al (2021) Bacterial adaptive strategies to cope with metal toxicity in the contaminated environment – a review. Ecotoxicol Environ Saf 226:112863. https://doi.org/10.1016/j.ecoenv.2021.112863
Mesa V, Navazas A, González-Gil R et al (2017) Use of endophytic and rhizosphere bacteria to improve phytoremediation of arsenic-contaminated industrial soils by autochthonous Betula celtiberica. Appl Environ Microbiol 83:e03411-e3416
Michibata H, Iwata Y, Hirata J (1991) Isolation of highly acidic and vanadium-containing blood cells from among several types of blood cell from Ascidiidae species by density-gradient centrifugation. J Exp Zool 257:306–313
Michibata H, Ueki T (2012) High levels of vanadium in ascidians. In: Michibata H (ed) Vanadium. Springer, Netherlands, Dordrecht, pp 51–71
Michibata H, Yamaguchi N, Uyama T, Ueki T (2003) Molecular biological approaches to the accumulation and reduction of vanadium by ascidians. Coord Chem Rev 237:41–51
Nakagawa T, Lino T, Suzuki K, Harayama S (2006) Ferrimonas futtsuensis sp. nov. and Ferrimonas kyonanensis sp. nov., selenate-reducing bacteria belonging to the Gammaproteobacteria isolated from Tokyo Bay. Int J Syst Evol Microbiol 56:2639–2645
Nette G, Scippa S, De Candia A, De Vincentiis M (2004) Cytochemical localisation of vanadium (III) in the blood cells of the ascidian Phallusia fumigata. Comp Biochem Physiol Part C Toxicol Pharmacol 137:271–279
Nette G, Scippa S, Genovese M, De Vincentiis M (1999) Cytochemical localization of vanadium(III) in blood cells of ascidian Phallusia mammillata Cuvier, and its relevance to hematic cell lineage determination. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 122:231–237
Ortiz-Bernad I, Anderson RT, Vrionis HA, Lovley DR (2004) Vanadium respiration by Geobacter metallireducens: novel strategy for in situ removal of vanadium from groundwater. Appl Environ Microbiol 70:3091–3095
Oves M, Khan MS, Zaidi A (2013) Biosorption of heavy metals by Bacillus thuringiensis strain OSM29 originating from industrial effluent contaminated north Indian soil. Saudi J Biol Sci 20:121–129
Parmar P, Shukla A, Goswami D et al (2020) Comprehensive depiction of novel heavy metal tolerant and EPS producing bioluminescent Vibrio alginolyticus PBR1 and V. rotiferianus PBL1 confined from marine organisms. Microbiol Res 238:126526. https://doi.org/10.1016/j.micres.2020.126526
Paul SI, Rahman MdM, Salam MA et al (2021) Identification of marine sponge-associated bacteria of the Saint Martin’s island of the Bay of Bengal emphasizing on the prevention of motile Aeromonas septicemia in Labeo rohita. Aquaculture 545:737156. https://doi.org/10.1016/j.aquaculture.2021.737156
Qurbani K, Khdir K, Sidiq A et al (2022) Aeromonas sobria as a potential candidate for bioremediation of heavy metal from contaminated environments. Sci Rep 12:21235. https://doi.org/10.1038/s41598-022-25781-3
Rathnayake IVN, Megharaj M, Krishnamurti GSR et al (2013) Heavy metal toxicity to bacteria – are the existing growth media accurate enough to determine heavy metal toxicity? Chemosphere 90:1195–1200
Romaidi Ueki T (2016) Bioaccumulation of vanadium by vanadium-resistant bacteria isolated from the intestine of Ascidia sydneiensis samea. Mar Biotechnol 18:359–371
Sampaio A, Silva V, Poeta P, Aonofriesei F (2022) Vibrio spp.: life strategies, ecology, and risks in a changing environment. Diversity 14:97. https://doi.org/10.3390/d14020097
Saranya K, Sundaramanickam A, Shekhar S et al (2017) Bioremediation of mercury by vibrio fluvialis screened from industrial effluents. BioMed Res Int 2017:1–6
Schreiber L, Kjeldsen KU, Funch P et al (2016) Endozoicomonas are specific, facultative symbionts of sea squirts. Front Microbiol 7:1042. https://doi.org/10.3389/fmicb.2016.01042
Shirdam R, Khanafari A, Tabatabaee A (2006) Cadmium, nickel and vanadium accumulation by three strains of marine bacteria. Iran J Biotechnol 4:180–187
Sommer F, Bäckhed F (2013) The gut microbiota — masters of host development and physiology. Nat Rev Microbiol 11:227–238
Stoll A, Duncan JR (1996) Enhanced heavy metal removal from waste water by viable, glucose pretreated Saccharomyces cerevisiae cells. Biotechnol Lett 18:1209–1212
Su J, Zhang Y, Hu T et al (2022) Prevalence, antibiotic and heavy metal resistance of Vibrio spp. isolated from the clam Meretrix meretrix at different ages in Geligang, Liaohe estuary in China. Front Mar Sci 9:1071371. https://doi.org/10.3389/fmars.2022.1071371
Sun X, Qiu L, Kolton M et al (2020) VV Reduction by Polaromonas spp. in vanadium mine tailings. Environ Sci Technol 54:14442–14454
Takeuchi M, Kawahata H, Gupta LP et al (2007) Arsenic resistance and removal by marine and non-marine bacteria. J Biotechnol 127:434–442
Tan H, Wang C, Zeng G et al (2020) Bioreduction and biosorption of Cr(VI) by a novel Bacillus sp. CRB-B1 strain. J Hazard Mater 386:121628. https://doi.org/10.1016/j.jhazmat.2019.121628
Trivedi S, Ueki T, Yamaguchi N, Michibata H (2003) Novel vanadium-binding proteins (vanabins) identified in cDNA libraries and the genome of the ascidian Ciona intestinalis. Biochim Biophys Acta BBA - Gene Struct Expr 1630:64–70
Ueki T, Fujie M, Romaidi SN (2019) Symbiotic bacteria associated with ascidian vanadium accumulation identified by 16S rRNA amplicon sequencing. Mar Genomics 43:33–42
Ueki T, Furuno N, Michibata H (2011) A novel vanadium transporter of the Nramp family expressed at the vacuole of vanadium-accumulating cells of the ascidian Ascidia sydneiensis samea. Biochim Biophys Acta BBA - Gen Subj 1810:457–464
Ueki T, Takemoto K, Fayard B et al (2002) Scanning X-ray microscopy of living and freeze-dried blood cells in two vanadium-rich ascidian species, Phallusia mammillata and Ascidia sydneiensis samea. Zoolog Sci 19:27–35
Ueki T, Uwagaki M, Yamamoto S, Michibata H (2014) Participation of thioredoxin in the V(V)-reduction reaction by Vanabin2. Biochim Biophys Acta BBA - Gen Subj 1840:3238–3245
Ueki T, Yamaguchi N, Romaidi, et al (2015) Vanadium accumulation in ascidians: a system overview. Coord Chem Rev 301–302:300–308
Utermann C, Blümel M, Busch K et al (2020) Comparative microbiome and metabolome analyses of the marine tunicate Ciona intestinalis from native and invaded habitats. Microorganisms 8:2022. https://doi.org/10.3390/microorganisms8122022
Van Marwijk J, Opperman DJ, Piater LA, Van Heerden E (2009) Reduction of vanadium(V) by Enterobacter cloacae EV-SA01 isolated from a South African deep gold mine. Biotechnol Lett 31:845–849
Vezzulli L, Pezzati E, Brettar I et al (2015) Effects of global warming on Vibrio ecology. Microbiol Spectr 3:1–9
Wang G, Zhang B, Li S et al (2017) Simultaneous microbial reduction of vanadium (V) and chromium (VI) by Shewanella loihica PV-4. Bioresour Technol 227:353–358
Wegner C-E, Westermann M, Steiniger F et al (2021) Extracellular and intracellular lanthanide accumulation in the methylotrophic Beijerinckiaceae bacterium RH AL1. Appl Environ Microbiol 87:e03144-e3220
Wnuk E (2023) Mobility, bioavailability, and toxicity of vanadium regulated by physicochemical and biological properties of the soil. J Soil Sci Plant Nutr 23:1386–1396
Wróbel M, Śliwakowski W, Kowalczyk P et al (2023) Bioremediation of heavy metals by the genus Bacillus. Int J Environ Res Public Health 20:4964. https://doi.org/10.3390/ijerph20064964
Yakoubi L, Benmalek Y, Benayad T, Marie-Laure F (2018) Characterization of cadmium-resistant bacteria isolated from polluted soils in Algeria, and evaluation of cadmium removal, using living free and immobilized cells. Rev Ecol Terre Vie 73:255–268
Yamaguchi N, Kamino K, Ueki T, Michibata H (2004) Expressed sequence tag analysis of vanadocytes in a vanadium-rich ascidian, Ascidia sydneiensis samea. Mar Biotechnol 6:165–174
Yamazaki Y, Maruyama A, Hosono K et al (1993) Asymmetrie reduction of synthetic ketones by marine microorganisms. Z Für Naturforschung C 48:451–456
Yan G, Sun X, Dong Y et al (2022) Vanadate reducing bacteria and archaea may use different mechanisms to reduce vanadate in vanadium contaminated riverine ecosystems as revealed by the combination of DNA-SIP and metagenomic-binning. Water Res 226:119247. https://doi.org/10.1016/j.watres.2022.119247
Yang Y, Zhu Y, Liu H et al (2022) Cultivation of gut microorganisms of the marine ascidian Halocynthia roretzi reveals their potential roles in the environmental adaptation of their host. Mar Life Sci Technol 4:201–207
Yao Q, Yu K, Liang J et al (2019) The composition, diversity and predictive metabolic profiles of bacteria associated with the gut digesta of five sea urchins in Luhuitou fringing reef (northern South China Sea). Front Microbiol 10:1168. https://doi.org/10.3389/fmicb.2019.01168
Yoshihara M, Ueki T, Watanabe T et al (2005) VanabinP, a novel vanadium-binding protein in the blood plasma of an ascidian, Ascidia sydneiensis samea. Biochim Biophys Acta BBA - Gene Struct Expr 1730:206–214
Zeng Q, Hu Y, Yang Y et al (2019) Cell envelop is the key site for Cr(VI) reduction by Oceanobacillus oncorhynchi W4, a newly isolated Cr(VI) reducing bacterium. J Hazard Mater 368:149–155
Zhang B, Li Y, Fei Y, Cheng Y (2021) Novel pathway for vanadium(V) bio-detoxification by gram-positive Lactococcus raffinolactis. Environ Sci Technol 55:2121–2131
Zhang B, Wang S, Diao M et al (2019) Microbial community responses to vanadium distributions in mining geological environments and bioremediation assessment. J Geophys Res Biogeosciences 124:601–615
Zhou D, Liang M, Xia Y et al (2022) Reduction mechanisms of V5+ by vanadium-reducing bacteria in aqueous environments: role of different molecular weight fractionated extracellular polymeric substances. Sci Total Environ 852:158394. https://doi.org/10.1016/j.scitotenv.2022.158394
Zhou W, Zhang H, Ma Y et al (2013) Bio-removal of cadmium by growing deep-sea bacterium Pseudoalteromonas sp. SCSE709-6. Extremophiles 17:723–731
Acknowledgements
Adult ascidian C. intestinalis type A (C. robusta) were obtained from the National BioResource Project (NBRP) of MEXT, Japan (project ID: 2022-413). Some DNA sequence analyses were performed at the Natural Science Center for Basic Research and Development (N-BARD), Hiroshima University.
Funding
Open Access funding provided by Hiroshima University. This work was partly supported by Grants-in-Aid from the Japan Society for Promoting Science (JSPS) (19K06740 and 22K06317 to Tatsuya Ueki). Dewi Yuliani is supported by an Indonesia Bangkit Scholarship (BIB) from the Ministry of Religious Affairs and Indonesia Endowment Fund for Education (LPDP) from the Ministry of Finance, the Republic Indonesia.
Author information
Authors and Affiliations
Contributions
Dewi Yuliani and Tatsuya Ueki contributed all the experiments and wrote the main manuscript. Dewi Yuliani prepared all table and figures. Fumihiro Morishita and Takuya Imamura confirmed some of experimental data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
We used adult specimens of an invertebrate animal, Ciona robusta. Although the research on the invertebrate does not require animal ethics approval by the animal ethics committee of Hiroshima University, ascidians were handled with care to avoid unnecessary stress in accordance with guideline for the animal welfare by the committee.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuliani, D., Morishita, F., Imamura, T. et al. Vanadium Accumulation and Reduction by Vanadium-Accumulating Bacteria Isolated from the Intestinal Contents of Ciona robusta. Mar Biotechnol 26, 338–350 (2024). https://doi.org/10.1007/s10126-024-10300-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-024-10300-4