Abstract
Purpose
Several retrospective studies and meta-analyses of Peptide Radionuclide Radiation Therapy in meningiomas suggest six-month progression-free survival improvement for WHO grade 1 and 2 meningiomas. In the present study, we aimed to evaluate the impact of such treatment on three-dimensional volume growth rate (3DVGR) in nonanaplastic meningiomas.
Methods
The authors performed a retrospective study including eight patients treated with Lutathera®. Millimetric 3D T1-weighted with gadolinium enhancement magnetic resonance imaging sequences were requested for volume measurement. Then, tumor growth rate was classified following a previously described 3DVGR classification (Graillon et al.).
Results
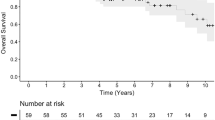
Patients harbored seven WHO grade 2 meningiomas and one aggressive WHO grade 1. All patients, except one, underwent four treatment cycles. 3DVGR significantly decreased at 3, 6, and 12 months after treatment initiation analyzing each lesion separately. Mean and median 3DVGR from all patients were respectively at 29.5% and 44.5%/6 months before treatment initiation, then at 16.5% and 25%/6 months at three months post-treatment initiation, 9.5% and 4.5%/6 months after 6 months, as well as 9.5% and 10.5%/6 months after 12 months. At 3, 6, and 12 months after treatment initiation, 4/8, 6/7, and 5/6 patients were class 2 (stabilization or severe 3DVGR slowdown), respectively. No patient was class 1 at 6 and 12 months, suggesting a lack of drug response.
Conclusion
In nonanaplastic meningiomas, Lutathera®’s antitumoral activity appeared delayed and more likely observed at six months, while no major response was observed under treatment. Moreover, its antitumoral activity persisted for 12–18 months following treatment initiation.






Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Graillon T, Ferrer L, Siffre J, Sanson M, Peyre M, Peyriere H, Mougel G, Autran D, Tabouret E, Figarella-Branger D, Barlier A, Kalamarides M, Dufour H, Colin T, Chinot O (2021) Role of 3-D volume growth rate for drug activity evaluation in meningioma clinical trials: the example of the CEVOREM study. Neuro oncol doi: noab019 [pii]. https://doi.org/10.1093/neuonc/noab019. 6131353 [pii]
Graillon T, Sanson M, Campello C, Idbaih A, Peyre M, Peyriere H, Basset N, Autran D, Roche C, Kalamarides M, Roche PH, Fuentes S, Tabouret E, Barrie M, Cohen A, Honore S, Boucekine M, Baumstarck K, Figarella-Branger D, Barlier A, Dufour H, Chinot OL (2020) Everolimus and Octreotide for patients with recurrent Meningioma: results from the phase II CEVOREM trial. Clin Cancer Res 26:552–557. https://doi.org/10.1158/1078-0432.CCR-19-21091078– 0432.CCR-19-2109 [pii]
Lou E, Sumrall AL, Turner S, Peters KB, Desjardins A, Vredenburgh JJ, McLendon RE, Herndon JE 2nd, McSherry F, Norfleet J, Friedman HS, Reardon DA (2012) Bevacizumab therapy for adults with recurrent/progressive meningioma: a retrospective series. J Neurooncol 109:63–70. https://doi.org/10.1007/s11060-012-0861-0
Nayak L, Iwamoto FM, Rudnick JD, Norden AD, Lee EQ, Drappatz J, Omuro A, Kaley TJ (2012) Atypical and anaplastic meningiomas treated with bevacizumab. J Neurooncol 109:187–193. https://doi.org/10.1007/s11060-012-0886-4
Graillon T, Romano D, Defilles C, Saveanu A, Mohamed A, Figarella-Branger D, Roche PH, Fuentes S, Chinot O, Dufour H, Barlier A (2017) Octreotide therapy in meningiomas: in vitro study, clinical correlation, and literature review. J Neurosurg 127:660–669. https://doi.org/10.3171/2016.8.JNS16995
Barresi V, Alafaci C, Salpietro F, Tuccari G (2008) Sstr2A immunohistochemical expression in human meningiomas: is there a correlation with the histological grade, proliferation or microvessel density? Oncol Rep 20:485–492
Fodi C, Skardelly M, Hempel JM, Hoffmann E, Castaneda S, Tabatabai G, Honegger J, Tatagiba M, Schittenhelm J, Behling F (2022) The immunohistochemical expression of SSTR2A is an independent prognostic factor in meningioma. Neurosurg Rev 45:2671–2679. https://doi.org/10.1007/s10143-021-01651-w [pii]. 1651 [pii]
Horowitz T, Salgues B, Padovani L, Farah K, Dufour H, Chinot O, Guedj E, Graillon T (2023) Optic nerve sheath meningiomas: solving Diagnostic challenges with (68)Ga-DOTATOC PET/CT. Diagnostics (Basel) 13. https://doi.org/10.3390/diagnostics13132307. 2307. diagnostics13132307 [pii]. diagnostics-13-02307 [pii]
Graillon T, Regis J, Barlier A, Brue T, Dufour H, Buchfelder M (2020) Parasellar Meningiomas Neuroendocrinol. https://doi.org/10.1159/000509090. 000509090 [pii]
Chamberlain MC, Glantz MJ, Fadul CE (2007) Recurrent meningioma: salvage therapy with long-acting somatostatin analogue. Neurology 69:969–973. https://doi.org/10.1212/01.wnl.0000271382.62776.b7. doi:69/10/969 [pii]
Norden AD, Ligon KL, Hammond SN, Muzikansky A, Reardon DA, Kaley TJ, Batchelor TT, Plotkin SR, Raizer JJ, Wong ET, Drappatz J, Lesser GJ, Haidar S, Beroukhim R, Lee EQ, Doherty L, Lafrankie D, Gaffey SC, Gerard M, Smith KH, McCluskey C, Phuphanich S, Wen PY (2015) Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma. Neurology 84:280–286. https://doi.org/10.1212/WNL.0000000000001153. WNL.0000000000001153 [pii]
Schulz C, Mathieu R, Kunz U, Mauer UM (2011) Treatment of unresectable skull base meningiomas with somatostatin analogs. Neurosurg Focus 30:E11. https://doi.org/10.3171/2011.1.FOCUS111
Galldiks N, Hattingen E, Langen KJ, Tonn JC (2023) Imaging characteristics of meningiomas. Adv Exp Med Biol 1416:21–33. https://doi.org/10.1007/978-3-031-29750-2_3
Strosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar A, Mittra E, Wolin EM, Yao JC, Pavel ME, Grande E, Van Cutsem E, Seregni E, Duarte H, Gericke G, Bartalotta A, Mariani MF, Demange A, Mutevelic S, Krenning EP (2021) (177)Lu-Dotatate plus long-acting octreotide versus high–dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol 22:1752–1763:S1470-2045(21)00572-6 [pii]. https://doi.org/10.1016/S1470-2045(21)00572-6
Bartolomei M, Bodei L, De Cicco C, Grana CM, Cremonesi M, Botteri E, Baio SM, Arico D, Sansovini M, Paganelli G (2009) Peptide receptor radionuclide therapy with (90)Y-DOTATOC in recurrent meningioma. Eur J Nucl Med Mol Imaging 36:1407–1416. https://doi.org/10.1007/s00259-009-1115-z
Mirian C, Duun-Henriksen AK, Maier A, Pedersen MM, Jensen LR, Bashir A, Graillon T, Hrachova M, Bota D, van Essen M, Spanjol P, Kreis C, Law I, Broholm H, Poulsgaard L, Fugleholm K, Ziebell M, Munch T, Walter MA, Mathiesen T (2021) Somatostatin receptor-targeted Radiopeptide Therapy in Treatment-Refractory Meningioma: individual Patient Data Meta-analysis. J Nucl Med 62:507–513. https://doi.org/10.2967/jnumed.120.249607.jnumed.120.249607. [pii]
Salgues B, Graillon T, Horowitz T, Chinot O, Padovani L, Taieb D, Guedj E (2022) Somatostatin receptor theranostics for refractory meningiomas. Curr Oncol 29:5550–5565. https://doi.org/10.3390/curroncol29080438. curroncol29080438 [pii]. curroncol-29-00438 [pii]
Seystahl K, Stoecklein V, Schuller U, Rushing E, Nicolas G, Schafer N, Ilhan H, Pangalu A, Weller M, Tonn JC, Sommerauer M, Albert NL (2016) Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro Oncol 18:1538–1547:now060 [pii]. https://doi.org/10.1093/neuonc/now060
van Essen M, Krenning EP, Kooij PP, Bakker WH, Feelders RA, de Herder WW, Wolbers JG, Kwekkeboom DJ (2006) Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J Nucl Med 47: 1599–1606 doi:47/10/1599 [pii]
Marincek N, Radojewski P, Dumont RA, Brunner P, Muller-Brand J, Maecke HR, Briel M, Walter MA (2015) Somatostatin receptor-targeted radiopeptide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in progressive meningioma: long-term results of a phase II clinical trial. J Nucl Med 56:171–176. https://doi.org/10.2967/jnumed.114.147256.jnumed.114.147256. [pii]
Gerster-Gillieron K, Forrer F, Maecke H, Mueller-Brand J, Merlo A, Cordier D (2015) 90Y-DOTATOC as a therapeutic option for Complex Recurrent or Progressive meningiomas. J Nucl Med 56:1748–1751. https://doi.org/10.2967/jnumed.115.155853. jnumed.115.155853 [pii]
Parghane RV, Talole S, Basu S (2019) Prevalence of hitherto unknown brain meningioma detected on (68)Ga-DOTATATE positron-emission tomography/computed tomography in patients with metastatic neuroendocrine tumor and exploring potential of (177)Lu-DOTATATE peptide receptor radionuclide therapy as single-shot treatment approach targeting both tumors. World J Nucl Med 18:160–170. https://doi.org/10.4103/wjnm.WJNM_39_18. WJNM-18-160 [pii]
Kreissl MC, Hanscheid H, Lohr M, Verburg FA, Schiller M, Lassmann M, Reiners C, Samnick SS, Buck AK, Flentje M, Sweeney RA (2012) Combination of peptide receptor radionuclide therapy with fractionated external beam radiotherapy for treatment of advanced symptomatic meningioma. Radiat Oncol 7:99. https://doi.org/10.1186/1748-717X-7-99. [pii]
Kaley T, Barani I, Chamberlain M, McDermott M, Panageas K, Raizer J, Rogers L, Schiff D, Vogelbaum M, Weber D, Wen P (2014) Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol 16:829–840. https://doi.org/10.1093/neuonc/not330. not330 [pii]
Nestle U, Kremp S, Schaefer-Schuler A, Sebastian-Welsch C, Hellwig D, Rube C, Kirsch CM (2005) Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-small cell lung cancer. J Nucl Med 46:1342–1348:46/8/1342 [pii]
Nioche C, Orlhac F, Boughdad S, Reuze S, Goya-Outi J, Robert C, Pellot-Barakat C, Soussan M, Frouin F, Buvat I (2018) LIFEx: a freeware for Radiomic feature calculation in Multimodality Imaging to accelerate advances in the characterization of Tumor Heterogeneity. Cancer Res 78:4786–4789. https://doi.org/10.1158/0008-5472.CAN-18-0125. [pii]
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: [TG, OC]; Methodology: [TG, BS, ET, OC]; Formal analysis and investigation: [TG, BS, TH, RA, EG]; Writing - original draft preparation: [TG, ET, OC]; Writing - review and editing: [TG, BS, TH, LP, RA, ET, EG, OC]; Supervision: [OC, EG]
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the French National College of Neurosurgery n° IRB00011687.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Graillon, T., Salgues, B., Horowitz, T. et al. Peptide radionuclide radiation therapy with Lutathera in multirecurrent nonanaplastic meningiomas: antitumoral activity study by growth rate analysis. J Neurooncol (2024). https://doi.org/10.1007/s11060-024-04622-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11060-024-04622-5