Abstract
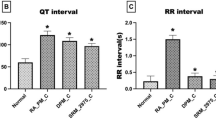
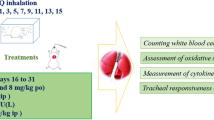
Intravenous injection of capsaicin produces vagal-mediated protective cardio-pulmonary (CP) reflexes manifesting as tachypnea, bradycardia, and triphasic blood pressure (BP) response in anesthetized rats. Particulate matter from diesel engine exhaust has been reported to attenuate these reflexes. However, the effects of gaseous constituents of diesel exhaust are not known. Therefore, the present study was designed to investigate the effects of gaseous pollutants in diesel exhaust, on capsaicin-induced CP reflexes in rat model. Adult male rats were randomly assigned to three groups: Non-exposed (NE) group, filtered diesel exhaust-exposed (FDE) group and N-acetyl cysteine (NAC)-treated FDE group. FDE group of rats (n = 6) were exposed to filtered diesel exhaust for 5 h a day for 5 days (D1–D5), and were taken for dissection on day 6 (D6), while NE group of rats (n = 6) remained unexposed. On D6, rats were anesthetized, following which jugular vein was cannulated for injection of chemicals, and femoral artery was cannulated to record the BP. Lead II electrocardiogram and respiratory movements were also recorded. Results show that intravenous injection of capsaicin (0.1 ml; 10 µg/kg) produced immediate tachypneic, hyperventilatory, hypotensive, and bradycardiac responses in both NE and FDE groups of rats. However, these capsaicin-induced CP responses were significantly attenuated in FDE group as compared to the NE group of rats. Further, FDE-induced attenuation of capsaicin-evoked CP responses were diminished in the N-acetyl cysteine-treated FDE rats. These findings demonstrate that oxidant stress mechanisms could possibly be involved in inhibition of CP reflexes by gaseous pollutants in diesel engine exhaust.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article at relevant places. Data from the study will be made available by the corresponding author upon reasonable request.
References
Kelly, F. J., & Fussell, J. C. (2011). Air pollution and airway disease. Clinical and Experimental Allergy, 41(8), 1059–1071. https://doi.org/10.1111/j.1365-2222.2011.03776.x
Riedl, M., & Diaz-Sanchez, D. (2005). Biology of diesel exhaust effects on respiratory function. Journal of Allergy Clinical Immunology, 115(2), 221–228. https://doi.org/10.1016/j.jaci.2004.11.047
Lewis, A. C., Carslaw, D. C., & Kelly, F. J. (2015). Vehicle emissions: Diesel pollution long underreported. Nature, 526(7572), 195. https://doi.org/10.1038/526195c
Bernstein, D. I. (2012). Diesel exhaust exposure, wheezing and sneezing. Allergy, Asthma and Immunology Research, 4(4), 178–183. https://doi.org/10.4168/aair.2012.4.4.178
Samoli, E., Atkinson, R. W., Analitis, A., Fuller, G. W., Green, D. C., Mudway, I., Anderson, H. R., & Kelly, F. J. (2016). Associations of short-term exposure to traffic-related air pollution with cardiovascular and respiratory hospital admissions in London, UK. Occupational and Environmental Medicine, 73(5), 300–307. https://doi.org/10.1136/oemed-2015-103136
Chen, H., Zhang, Z., van Donkelaar, A., Bai, L., Martin, R. V., Lavigne, E., Kwong, J. C., & Burnett, R. T. (2020). Understanding the joint impacts of fine particulate matter concentration and composition on the incidence and mortality of cardiovascular disease: A component-adjusted approach. Environmental Science and Technology, 54(7), 4388–4399. https://doi.org/10.1021/acs.est.9b06861
Brook, R. D., Franklin, B., Cascio, W., Hong, Y., Howard, G., Lipsett, M., Luepker, R., Mittleman, M., Samet, J., et al. (2004). Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation, 109(21), 2655–2671. https://doi.org/10.1161/01.CIR.0000128587.30041.C8
Bautista, D. M., Jordt, S. E., Nikai, T., Tsuruda, P. R., Read, A. J., Poblete, J., Yamoah, E. N., Basbaum, A. I., & Julius, D. (2006). TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell, 124(6), 1269–1282. https://doi.org/10.1016/j.cell.2006.02.023
Bauer, R. N., Diaz-Sanchez, D., & Jaspers, I. (2012). Effects of air pollutants on innate immunity: The role of toll-like receptors and nucleotide- binding oligomerization domain-like receptors. Journal of Allergy and Clinical Immunology, 129(1), 14–24. https://doi.org/10.1016/j.jaci.2011.11.004
Bass, V., Gordon, C. J., Jarema, K. A., MacPhail, R. C., Cascio, W. E., Phillips, P. M., Ledbetter, A. D., Schladweiler, M. C., Andrews, D., et al. (2013). Ozone induces glucose intolerance and systemic metabolic effects in young and aged Brown Norway rats. Toxicology and Applied Pharmacology, 273(3), 551–560. https://doi.org/10.1016/j.taap.2013.09.029
Berger, A., Zareba, W., Schneider, A., Rückerl, R., Ibald-Mulli, A., Cyrys, J., Wichmann, H. E., & Peters, A. (2006). Runs of ventricular and supraventricular tachycardia triggered by air pollution in patients with coronary heart disease. Journal of Occupational and Environmental Medicine, 48(11), 1149–1158. https://doi.org/10.1097/01.jom.0000245921.15916.03
Bennett, B. A., Spannhake, E. W., Rule, A. M., Breysse, P. N., & Tankersley, C. G. (2018). The acute effects of age and particulate matter exposure on heart rate and heart rate variability in mice. Cardiovascular Toxicology, 18(6), 507–519. https://doi.org/10.1007/s12012-018-9461-3
Lee, L. Y., & Lundberg, J. M. (1994). Capsazepine abolishes pulmonary chemoreflexes induced by capsaicin in anesthetized rats. Journal of Applied Physiology, 76(5), 1848–1855. https://doi.org/10.1152/jappl.1994.76.5.1848
Pingle, S. C., Matta, J. A., & Ahern, G. P. (2007). Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handbook of Experimental Pharmacology, 179, 155–171. https://doi.org/10.1007/978-3-540-34891-7_9
Toh, C. C., Lee, T. S., & Kiang, A. K. (1955). The pharmacological actions of capsaicin and analogues. British Journal of Pharmacology and Chemotherapy, 10(2), 175–182. https://doi.org/10.1111/j.1476-5381.1955.tb00079.x
Coleridge, H. M., Coleridge, J. C. G., & Kidd, C. (1964). Role of the pulmonary arterial baroreceptors in the effects produced by capsaicin in the dog. Journal of Physiology, 170(2), 272–285. https://doi.org/10.1113/jphysiol.1964.sp007330
Ravi, K., & Singh, M. (1996). Role of vagal lung C-fibres in the cardiorespiratory effects of capsaicin in monkeys. Respiratory Physiology, 106(2), 137–151. https://doi.org/10.1016/s0034-5687(96)00070-9
Donnerer, J., & Lembeck, F. (1982). Analysis of the effects of intravenously injected capsaicin in the rat. Naunyn-Schmiedebergs Archives of Pharmacology, 320(1), 54–57. https://doi.org/10.1007/BF00499072
Skofitsch, G., Saria, A., & Lembeck, F. (1983). Phenyldiguanide and capsaicin stimulate functionally different populations of afferent C-fibres. Neuroscience Letters, 42(1), 89–94. https://doi.org/10.1016/0304-3940(83)90427-5
Dutta, A., & Deshpande, S. B. (2010). Mechanisms underlying the hypertensive response induced by capsaicin. International Journal of Cardiology, 145(2), 358–359. https://doi.org/10.1016/j.ijcard.2010.02.034
Widdicombe, J. G. (1998). The J Reflex. Journal of Physiology, 511, 2. https://doi.org/10.1111/j.1469-7793.1998.002bi.x
Deering-Rice, C. E., Romero, E. G., Shapiro, D., Hughen, R. W., Light, A. R., Yost, G. S., Veranth, J. M., & Reilly, C. A. (2011). Electrophilic components of diesel exhaust particles (DEP) activate transient receptor potential ankyrin-1 (TRPA1): A probable mechanism of acute pulmonary toxicity for DEP. Chemical Research in Toxicology, 24(6), 950–959. https://doi.org/10.1021/tx200123z
Li, J., Kanju, P., Patterson, M., Chew, W. L., Cho, S. H., Gilmour, I., Oliver, T., Yasuda, R., Ghio, A., et al. (2011). TRPV4-mediated calcium influx into human bronchial epithelia upon exposure to diesel exhaust particles. Environmental Health Perspectives, 119(6), 784–793. https://doi.org/10.1289/ehp.1002807
Kumar, S., Bhagat, P., Pandey, S., & Pandey, R. (2022). The role of antioxidant agent (N-acetylcysteine) in oleic acid-induced acute lung injury in a rat model. Cureus, 14(9), e29478. https://doi.org/10.7759/cureus.29478
Revand, R., & Singh, S. K. (2020). Retrograde cannulation of femoral artery: A novel experimental design for the precise elicitation of vasosensory reflexes in anesthetized rats. MethodsX, 7, 101017. https://doi.org/10.1016/j.mex.2020.101017
Revand, R., & Singh, S. K. (2021). Ipsilateral somatic nerves mediate histamine-induced vasosensory reflex responses involving perivascular afferents in rat models. Scientific Reports, 11(1), 14648. https://doi.org/10.1038/s41598-021-94110-x
Singh, S. K., Mandal, M. B., & Ravindran, R. (2020). Instillation of bradykinin into femoral artery elicits cardiorespiratory reflexes involving perivascular afferents in anesthetized rats. Physiology International, 107(1), 40–54. https://doi.org/10.1556/2060.2020.00009
Dutta, A., Akella, A., & Deshpande, S. B. (2013). A study to investigate capsaicin-induced pressure response in vagotomized rats. Indian Journal of Pharmacology, 45(4), 365–370. https://doi.org/10.4103/0253-7613.115019
Cavallari, J. M., Fang, S. C., Eisen, E. A., Schwartz, J., Hauser, R., Herrick, R. F., & Christiani, D. C. (2008). Time course of heart rate variability decline following particulate matter exposures in an occupational cohort. Inhalation Toxicology, 20(4), 415–422. https://doi.org/10.1080/08958370801903800
Park, S. K., O’Neill, M. S., Vokonas, P. S., Sparrow, D., & Schwartz, J. (2005). Effects of air pollution on heart rate variability: The VA normative aging study. Environmental Health Perspectives, 113(3), 304–309. https://doi.org/10.1289/ehp.7447
Tsuji, H., Larson, M. G., Venditti, F. J., Manders, E. S., Evans, J. C., Feldman, C. L., & Levy, D. (1996). Impact of reduced heart rate variability on risk for cardiac events. The Framingham heart study. Circulation, 94(11), 2850–2855. https://doi.org/10.1161/01.cir.94.11.2850
Thorén, P. (1979). Role of cardiac vagal C-fibers in cardiovascular control. Reviews of Physiology, Biochemistry and Pharmacology, 86, 1–94. https://doi.org/10.1007/BFb0031531
Coleridge, J. C., & Coleridge, H. M. (1984). Afferent vagal c fibre innervation of the lungs and airways and its functional significance. Reviews of Physiology, Biochemistry and Pharmacology, 99, 1–110. https://doi.org/10.1007/BFb0027715
Zoccal, D. B., Furuya, W. I., Bassi, M., Colombari, D. S., & Colombari, E. (2014). The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Frontiers in Physiology, 5, 238. https://doi.org/10.3389/fphys.2014.00238
Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D., & Julius, D. (1997). The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature, 389(6653), 816–824. https://doi.org/10.1038/39807
Ho, C. Y., Gu, Q., Lin, Y. S., & Lee, L. Y. (2001). Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respiratory Physiology, 127(2–3), 113–124. https://doi.org/10.1016/s0034-5687(01)00241-9
Kollarik, M., & Undem, B. J. (2004). Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild-type and TRPV1−/− mice. Journal of Physiology, 555(1), 115–123. https://doi.org/10.1113/jphysiol.2003.054890
Clifford, P. S., Litzow, J. T., & Coon, R. L. (1987). Pulmonary depressor reflex elicited by capsaicin in conscious intact and lung-denervated dogs. American Journal of Physiology, 252, R394–R397. https://doi.org/10.1152/ajpregu.1987.252.2.R394
Li, N., Wang, M., Oberley, T. D., Sempf, J. M., & Nel, A. E. (2002). Comparison of the pro-oxidative and proinflammatory effects of organic diesel exhaust particle chemicals in bronchial epithelial cells and macrophages. Journal of Immunology, 169(8), 4531–4541. https://doi.org/10.4049/jimmunol.169.8.4531
Hetland, R. B., Cassee, F. R., Refsnes, M., Schwarze, P. E., Låg, M., Boere, A. J., & Dybing, E. (2004). Release of inflammatory cytokines, cell toxicity and apoptosis in epithelial lung cells after exposure to ambient air particles of different size fractions. Toxicology In Vitro, 18(2), 203–212. https://doi.org/10.1016/s0887-2333(03)00142-5
Tao, F., Gonzalez-Flecha, B., & Kobzik, L. (2003). Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radical Biology and Medicine, 35(4), 327–340. https://doi.org/10.1016/s0891-5849(03)00280-6
Dick, C. A., Singh, P., Daniels, M., Evansky, P., Becker, S., & Gilmour, M. I. (2003). Murine pulmonary inflammatory responses following instillation of size-fractionated ambient particulate matter. Journal of Toxicology and Environ Health, Part A, 66(23), 2193–2207. https://doi.org/10.1080/716100636
Suter, P. M., Domenighetti, G., Schaller, M. D., Laverrière, M. C., Ritz, R., & Perret, C. (1994). N-acetylcysteine enhances recovery from acute lung injury in man. A randomized, double-blind, placebo-controlled clinical study. Chest, 105(1), 190–194. https://doi.org/10.1378/chest.105.1.190
Särnstrand, B., Tunek, A., Sjödin, K., & Hallberg, A. (1995). Effects of N-acetylcysteine stereoisomers on oxygen-induced lung injury in rats. Chemico-Biological Interaction, 94(2), 157–164. https://doi.org/10.1016/0009-2797(94)03332-3
Fan, E., Brodie, D., & Slutsky, A. S. (2018). Acute respiratory distress syndrome: Advances in diagnosis and treatment. JAMA, 319(7), 698–710. https://doi.org/10.1001/jama.2017.21907
Raghu, G., Berk, M., Campochiaro, P. A., Jaeschke, H., Marenzi, G., Richeldi, L., Wen, F. Q., Nicoletti, F., & Calverley, P. M. A. (2021). The multifaceted therapeutic role of N-acetylcysteine (NAC) in disorders characterized by oxidative stress. Current Neuropharmacology, 19(8), 1202–1224. https://doi.org/10.2174/1570159x19666201230144109
Mohanty, R. R., Padhy, B. M., Das, S., & Meher, B. R. (2021). Therapeutic potential of N-acetyl cysteine (NAC) in preventing cytokine storm in COVID-19: Review of current evidence. European Review for Medical and Pharmacological Sciences, 25(6), 2802–2807. https://doi.org/10.26355/eurrev20210325442
Acknowledgements
The authors convey their thankfulness to Prof. S. B. Deshpande and Prof. Sanjeev K. Singh from the Department of Physiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India, whose earlier works have inspired the methodology of the present study. Further, the authors also acknowledge the Department of Mechanical Engineering, Indian Institute of Technology, New Delhi, for their inputs regarding the development and fabrication of the animal whole body exposure chamber.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The study was conceptualized by RR and AP. Data collection and data analysis were performed by RR, AD and SS. The first draft of the manuscript was written by RR, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical Approval
The present study was approved by the Institutional Animal Ethics Committee of the All India Institute of Medical Sciences, New Delhi, India (Ref. No. 391/IAEC-1/2022 dated 17.03.2023).
Consent to Participate and Publication
Not applicable.
Additional information
Handling Editor: Matthew Campen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Revand, R., Dontham, A., Sarkar, S. et al. Subacute Exposure to Gaseous Pollutants from Diesel Engine Exhaust Attenuates Capsaicin-Induced Cardio-Pulmonary Reflex Responses Involving Oxidant Stress Mechanisms in Adult Wistar Rats. Cardiovasc Toxicol 24, 396–407 (2024). https://doi.org/10.1007/s12012-024-09842-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-024-09842-9