Abstract
Background
Fecal microbiota transplants can be administered orally in encapsulated form or require invasive procedures to administer liquid formulations. There is a need for an oral liquid formulation of fecal microbiota for patients who are unable to swallow capsules, especially if they require multiple, repeated administrations.
Aims
These studies were conducted to develop a protocol to manufacture an organoleptically acceptable powdered fecal microbiota formulation that can be suspended in a liquid carrier and used for fecal microbiota transplantation.
Methods
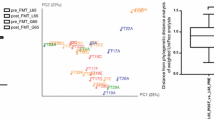
Several processing steps were investigated, including extra washes of microbiota prior to lyophilization and an addition of a flavoring agent. The viability of bacteria in the transplant formulation was tested using live/dead microscopy staining and engraftment into antibiotic-treated mice. After development of a clinical protocol for suspension of the powdered microbiota, the new formulation was tested in three elderly patients with recurrent Clostridioides difficile infections and who have difficulties in swallowing capsules. Changes in the microbial community structure in one of the patients were characterized using 16S rRNA gene profiling and engraftment analysis.
Results
The processing steps used to produce an organoleptically acceptable suspension of powdered fecal microbiota did not result in loss of its viability. The powder could be easily suspended in a liquid carrier. The use of the new formulation was associated with abrogation of the cycle of C. difficile infection recurrences in the three patients.
Conclusion
We developed a novel organoleptically acceptable liquid formulation of fecal microbiota that is suitable for use in clinical trials for patients with difficulties in swallowing capsules.

Similar content being viewed by others
References
Brandt LJ, Aroniadis OC. An overview of fecal microbiota transplantation: techniques, indications, and outcomes. Gastrointest Endosc. 2013;78:240–249.
Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect. 2003;36:580–585.
Hamilton MJ, Weingarden AR, Sadowsky MJ et al. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:761–767.
Staley C, Hamilton MJ, Vaughn BP et al. Successful resolution of recurrent clostridium difficile infection using freeze-dried, encapsulated fecal microbiota; pragmatic cohort study. Am J Gastroenterol. 2017;112:940–947.
Vaughn BP, Fischer M, Kelly CR et al. Effectiveness and safety of colonic and capsule fecal microbiota transplantation for recurrent Clostridioides difficile infection. Clin Gastroenterol Hepatol. 2022;21:1330.
Kang DW, Adams JB, Gregory AC et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:10.
Haifer C, Paramsothy S, Kaakoush NO et al. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2022;7:141–151.
Staley C, Kaiser T, Beura LK et al. Stable engraftment of human microbiota into mice with a single oral gavage following antibiotic conditioning. Microbiome. 2017;5:87.
Caporaso JG, Lauber CL, Walters WA et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624.
Gohl DM, Vangay P, Garbe J et al. Systematic improvement of amplicon marker gene methods for increased accuracy in microbiome studies. Nat Biotechnol. 2016;34:942–949.
Schloss PD, Westcott SL, Ryabin T et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541.
Staley C, Kaiser T, Vaughn BP et al. Predicting recurrence of Clostridium difficile infection following encapsulated fecal microbiota transplantation. Microbiome. 2018;6:166.
Pruesse E, Quast C, Knittel K et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–7196.
Edgar RC, Haas BJ, Clemente JC et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200.
Cole JR, Wang Q, Cardenas E et al. The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141-145.
Knights D, Kuczynski J, Charlson ES et al. Bayesian community-wide culture-independent microbial source tracking. Nat Methods. 2011;8:761–763.
Dettmar PW, Strugala V, Fisher J et al. Antacid efficacy of maalox in comparison to a range of antacids. J Pharm Sci Therap. 2019;5:301–325.
Martinez-Guryn K, Hubert N, Frazier K et al. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe. 2018;23:458-469.e455.
de Vos P, Mujagic Z, de Haan BJ et al. Strains can enhance human mucosal and systemic immunity and prevent non-steroidal anti-inflammatory drug induced reduction in T regulatory. Cells Front Immunol. 2017;8:1000.
Khoruts A, Staley C, Sadowsky MJ. Faecal microbiota transplantation for Clostridioides difficile: mechanisms and pharmacology. Nat Rev Gastroenterol Hepatol. 2021;18:67–80.
Acknowledgments
Research funding for this work was supported by Finch Therapeutics and philanthropic funding from the Frank J. and Eleanor A. Maslowski Trust and non-profit Achieving Cures Together.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sadowsky, M.J., Matson, M., Mathai, P.P. et al. Successful Treatment of Recurrent Clostridioides difficile Infection Using a Novel, Drinkable, Oral Formulation of Fecal Microbiota. Dig Dis Sci (2024). https://doi.org/10.1007/s10620-024-08351-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10620-024-08351-7