Abstract
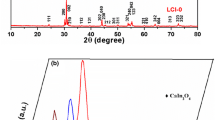
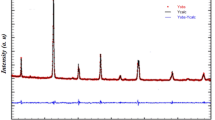
Co-doped LaInO3-based materials have been studied. Strontium-sustituted solid solutions have high conductivity values but exhibit a low level of oxygen deficiency. Mg2+ and Ca2+ ions have been selected as the B-sublattice co-dopant. Both series of the solid solutions—La0.9Sr0.1In1 – xCaxO2.95 – 0.5x and La0.9Sr0.1In1 – yMgyO2.95 – 0.5y—crystallize in orthorhombic symmetry with space group Pnma. Ionic conductivity in a dry atmosphere is determined by the transport of oxygen ions. Oxygen-ion transport in solid solutions is ~30–40% at high temperatures (T > 700°C) and increases to >80% when the temperature decreases to 400–300°C. The substitution of In3+ by Ca2+ makes it possible to increase the oxygen-ion electrical conductivity; the highest values are achieved for the compositions La0.9Sr0.1In0.95Ca0.05O2.925 and La0.9Sr0.1In0.9Ca0.1O2.9. The introduction of the Mg2+ co-dopant into the In3+ sites leads to a decrease in ionic electrical conductivity compared to La0.9Sr0.1InO2.95. The effects of changes in oxygen mobility with changes in geometric factors (cell volume, critical radius) are considered.










Similar content being viewed by others
REFERENCES
A. Buonomano, G. Barone, and C. Forzano, Energy Rep. 8, 4844 (2022). https://doi.org/10.1016/j.egyr.2022.03.171
S. S. Kumar and H. Lim, Energy Rep. 8, 13793 (2022). https://doi.org/10.1016/j.egyr.2022.10.127
M. D. Scovell, Int. J. Hydrogen Energy 47, 10441 (2022). https://doi.org/10.1016/j.ijhydene.2022.01.099
O. Corigliano, L. Pagnotta, and P. Fragiacomo, Sustainability 14, 15276 (2022). https://doi.org/10.3390/su142215276
A. I. Klyndyuk and Ya. Yu. Zhuravleva, Russ. J. Inorg. Chem. 67, 2084 (2022). https://doi.org/10.1134/S0036023622601404
F. Pişkin, Russ. J. Inorg. Chem. 67, 1239 (2022). https://doi.org/10.1134/S0036023622080216
E. Filonova and D. Medvedev, Nanomaterials 12, 1991 (2022). https://doi.org/10.3390/nano12121991
Z. Chen, Q. Jiang, F. Cheng, et al., J. Mater. Chem. A 7, 6099 (2019). https://doi.org/10.1039/C8TA11957K
A. Y. Stroeva, V. P. Gorelov, and V. B. Balakireva, Russ. J. Electrochem. 46, 552 (2010). https://doi.org/10.1134/S1023193510070116
A. V. Kuz’min, A. Yu. Stroeva, and V. P. Gorelov, Russ. J. Electrochem. 54, 43. https://doi.org/10.1134/S1023193518010056
A. V. Egorova, K. G. Belova, and I. E. Animitsa, Int. J. Hydrogen Energy 48, 22685 (2023). https://doi.org/10.1016/j.ijhydene.2023.03.263
M. Gambino, S. D. Tommaso, F. Giannici, et al., J. Chem. Phys. 147, 144702 (2017). https://doi.org/10.1063/1.4993705
H.-L. Kim, S. Kim, K.-H. Lee, et al., J. Power Sources 267, 723 (2014). https://doi.org/10.1016/j.jpowsour.2014.06.006
P. Dhanasekaran and N. M. Gupta, Mater. Res. Bull. 47, 1217 (2012). https://doi.org/10.1016/j.materresbull.2012.01.031
K. Sood, K. Singh, and O. P. Pandey, Physica B 456, 250 (2015). https://doi.org/10.1016/j.physb.2014.08.036
K. Sood, K. Singh, S. Basu, et al., Ionics 21, 2839 (2015). https://doi.org/10.1007/s11581-015-1461-8
H. He, X. Huang, and L. Chen, Solid State Ionics 130, 183 (2000). https://doi.org/10.1016/S0167-2738(00)00666-4
H. He, X. Huang, and L. Chen, Electrochim. Acta 46, 2871 (2001). https://doi.org/10.1016/S0013-4686(01)00508-4
B. Bakiz, F. Guinneton, M. Arab, et al., Adv. Mater. Sci. Eng. 2010, 360597 (2010). https://doi.org/10.1155/2010/360597
R. D. Shannon, Acta Crystallogr., Sect. A: Found. Crystallogr. 32, 751 (1976). https://doi.org/10.1107/S0567739476001551
S. Nishiyama, M. Kimura, and T. Hattori, Key Eng. Mater. 216, 65 (2001). https://doi.org/10.4028/www.scientific.net/KEM.216.65
D. M. Smyth, Solid State Ionics 129, 5 (2000). https://doi.org/10.1016/S0167-2738(99)00312-4
S. Lany and A. Zunger, Phys. Rev. B 80, 085202 (2009). https://doi.org/10.1103/PhysRevB.80.085202
Ya. Dong, Yi. Huang, D. Ding, et al., Acta Mater. 203, 116487 (2021). https://doi.org/10.1016/j.actamat.2020.116487
J. A. Kilner and R. J. Brook, Solid State Ionics 6, 237 (1982). https://doi.org/10.1016/0167-2738(82)90045-5
A. F. Sammells, R. L. Cook, J. H. White, et al., Solid State Ionics 52, 111 (1992).
Chr. Tantardini and A. R. Oganov, Nature Commun. 12, 2087 (2021). https://doi.org/10.1038/s41467-021-22429-0
V. N. Voronov, Ionic Mobility and Properties of Perovskite-Type Compounds ABH (Krasnoyarsk, 2006) [in Russian].
Funding
The study was supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of the Development Program of the Ural Federal University named after the first President of Russia B.N. Yeltsin in accordance with the strategic academic leadership program “Priority 2030.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Additional information
Translated by G. Kirakosyan
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Belova, K.G., Egorova, A.V., Pachina, S.P. et al. Electrical Properties of Co-doped LaInO3 Perovskite. Russ. J. Inorg. Chem. (2024). https://doi.org/10.1134/S0036023623602763
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S0036023623602763