Abstract
Background
Acute pancreatitis (AP) is one of the most common acute abdominal disorders; due to the lack of specific treatment, the treatment of acute pancreatitis, especially serious acute pancreatitis (SAP), is difficult and challenging. We will observe the changes of Interleukin -22 levels in acute pancreatitis animal models, and explore the mechanism of Interleukin -22 in acute pancreatitis.
Objective
This study aims to assess the potential protective effect of Interleukin -22 on caerulein-induced acute pancreatitis and to explore its mechanism.
Methods
Blood levels of amylase and lipase and Interleukin -22 were assessed in mice with acute pancreatitis. In animal model and cell model of caerulein-induced acute pancreatitis, the mRNA levels of P62 and Beclin-1 were determined using PCR, and the protein expression of P62, LC3-II, mTOR, AKT, p-mTOR, and p-AKT were evaluated through Western blot analysis.
Results
Interleukin -22 administration reduced blood amylase and lipase levels and mitigated tissue damage in acute pancreatitis mice model. Interleukin -22 inhibited the relative mRNA levels of P62 and Beclin-1, and the Interleukin -22 group showed a decreased protein expression of LC3-II and P62 and the phosphorylation of the AKT/mTOR pathway. Furthermore, we obtained similar results in the cell model of acute pancreatitis.
Conclusion
This study suggests that Interleukin -22 administration could alleviate pancreatic damage in caerulein-induced acute pancreatitis. This effect may result from the activation of the AKT/mTOR pathway, leading to the inhibition of autophagy. Consequently, Interleukin -22 shows potential as a treatment.
Similar content being viewed by others
Introduction
Mild acute pancreatitis (MAP) is a self-limited condition with a favorable prognosis. However, severe acute pancreatitis (SAP) is associated with transient or persistent organ failure and local or systemic complications. The mortality rate in SAP ranges from 15 to 30%, making it a significant threat to human health [1,2,3,4]. In normal circumstances, the pancreas secretes digestive enzymes as inactive zymogens that become activated upon reaching the duodenum. During acute pancreatitis (AP), premature activation of trypsinogen in the acinar cells leads to pancreatic self-digestion, a crucial pathogenesis that involves the release of various inflammatory mediators, resulting in pancreatic acinar injury, parenchymal necrosis, and systemic inflammation. Acid inhibition, anti-inflammation, and symptomatic support constitute the primary treatments against AP. Given the unclarified pathological mechanism, AP lacks specific treatments despite medical advancements. Therefore, investigating factors that can predict AP severity or prognosis and exploring new targeted therapeutic drugs are imperative.
Autophagy, a lysosomal degradation pathway, is pivotal in cell differentiation and apoptosis, involving two primary steps: autophagosome formation and its subsequent fusion with lysosomes to form autolysosomes. Autophagy is a dynamic process under normal physiological conditions, autophagosome accumulation may result from either an uptick in autophagosome formation or a defect in lysosomal fusion, and autolysosome accumulation suggests lysosomal degradation defects. Dysregulation of autophagy is implicated in the pathogenesis of numerous diseases, including neurodegenerative and inflammatory diseases and cancer [5,6,7]. Numerous studies conducted on animal experimental models have revealed that during the early stage of AP, autophagy activation is excessive due to impaired autophagy flow and reduced degradation of autophagy lysosomes [8,9,10,11]. This aberrant accumulation and impairment of autophagy play a significant role in activating pancreatic enzymes. The imbalance between autophagosome formation and lysosome degradation results in aberrant activation of autophagy flux, subsequently triggering abnormal activation of pancreatic enzymes. Hence, rectifying the anomalously activated autophagic flux is intrinsically linked to mitigating the inflammatory damage of AP, especially SAP. The mTOR pathway, an autophagy regulatory pathway, impedes the activation of autophagy flux through its augmented expression and has been explored as an intervention target in various autophagy-related diseases.
Interleukin-22 (IL-22), a member of the interleukin-10 family, predominantly acts on epithelial cells, and its specificity determined by its unique receptor, IL-22R1. IL-22 binds to IL-22R1, forming a distinct receptor complex (IL-22/IL-22R1 subunit), which then interacts with the common subunit IL-10R2 to initiate downstream signaling. IL-10R2 is widely distributed in various tissues without specificity. IL-22R1 is exclusively expressed in stromal and epithelial cells in specific tissues, and its expression is particularly abundant in pancreatic acinar cells, the intestinal tract and skin [12]. Upon binding to the receptor, IL-22-IL-22R1-IL-10R2 complex activates the JAK1/TYK2 kinase associated with the receptor, resulting in phosphorylation of STAT proteins, primarily STAT3. Furthermore, IL-22 has been shown to activate MAPK and AKT pathways [13,14,15]. Experiments have shown that IL-22 can induce epithelial cell proliferation, inhibit apoptosis, repair tissue damage, regulate intestinal epithelial homeostasis during inflammation, and play a vital role in inducing anti-microbial immunity and maintaining the integrity of intestinal, lung, skin, and other mucosal barriers [16,17,18]. Therefore, IL-22, presumed to act as a protective cytokine, is believed to inhibit inflammation and progression throughout various acute inflammatory diseases. This implication suggests a potential involvement of IL-22 in AP, where it may exert a protective effect.
In this investigation, we observed elevated blood IL-22 levels in mice with AP, which subsequently declined. The elevation of IL-22 in AP prompts inquiry: does it merely signify inflammation, or does it represent a protective bodily response against AP? To explore this question, we examined the impact of IL-22 utilizing in vitro and in vivo models of experimental AP. Our findings indicate that exogenous IL-22 mitigates autophagy in caerulein-induced AP. Further results suggest that the protective role of IL-22 in AP may be achieved by activating the AKT/mTOR pathway.
Materials and Methods
Animals, Cell Line, Reagents, and Antibodies
Male C57BL/6 J mice from Beijing Vital River (China) were used in this study. The AR42J cell line and F-12 K medium were obtained from the BeNa culture collection (China). Recombinant mouse Interleukin -22 (Miltenyi, Germany) was used, along with Caerulein (Sigma, America) and Lipopolysaccharide (Solarbio, China). AKT, mTOR, anti-mTOR (phosphor s2448), and anti-AKT (Ser473) antibodies were purchased from Proteintech(China). LC3 and P62 antibodies were obtained from Abclonal(China), and GAPDH Rabbit Polyclonal antibody and goat anti-rabbit IgG(H + L) HRP conjugate were from Proteintech(China). Trizol reagent, PCR primers, Reverse transcription kit, and SYBR Green qPCR kit were purchased from Agbio(China).
Animal Models
Healthy male mice, weighing 18-22 g and aged 6–8 weeks, were maintained in a controlled environment, with temperatures of 15–25 °C and relative humidity of 45–55%. The mice were exposed to a 12-h light and 12-h dark cycle for one week, following a 12-h fasting period, with unrestricted access to water before experimentation. The animal procedures performed in this study were approved by the Animal Ethics Committee of Shandong University and adhered to the NIH guidelines for the Care and Use of Laboratory Animals. The mice were randomly assigned to one of three groups: (1) NS group (n = 10), which received saline treatment; (2) AP group (n = 30): treated with 50 μg/kg/body weight caerulein intraperitoneally for seven times at hourly intervals, followed by immediate administration of 10 mg/kg/body weight lipopolysaccharide after the final caerulein injection; and (3) IL-22 group (n = 30): where mice were subjected to the same caerulein and lipopolysaccharide treatment as the AP group, but with an additional injection of 200 ng IL-22 given 2 h before and after the initial caerulein injection for each mouse. Additionally, the AP group and IL-22 group were further divided into three subgroups based on the time points after AP induction: 12 h, 24 h, and 48 h subgroup (n = 10/group).
Mice were euthanized through cervical dislocation, and blood samples were subsequently collected from the posterior orbital plexus, followed by centrifugation at 1500×g and 4 °C. The resulting serum was preserved at − 80 °C pending further analysis. The pancreas specimens, obtained 24 h after caerulein induction, were divided into three tissue samples. One sample was fixed in formalin for histological analysis, while the other two samples were frozen in liquid nitrogen and stored at − 80 °C for Western Blot analysis and RT-PCR analysis, respectively.
Cell Culture
The AR42J cell line was cultured in F-12 K medium, supplemented with 20% fetal bovine serum, 50 units/ml penicillin, and 50 µg/ml streptomycin, and maintained at 37 °C in a 5% CO2 atmosphere. Cells in the logarithmic growth phase were seeded into 6-well plates at a density of 0.8 × 106 cells per well, with triplicate wells for each experimental condition. Cells were divided into three groups: the control group (Ctrl group), the caerulein treatment group (Cae group), and the caerulein + IL-22 treatment group (Cae + IL-22 group). 100 nmol/L caerulein was added 48 h after IL-22 treatment (10 ng/ml). LDH activity in the supernatant of the culture medium for each group was assessed using an LDH cytotoxicity assay kit, while the cell growth curve was monitored using a CCK8 kit. Total protein and total RNA were extracted from all groups according to the provided instructions.
Serological Assay
Serum amylase and lipase of samples were detected utilizing an automatic biochemical analyzer.
Enzyme-Linked Immunosorbent Assay (ELISA)
IL-22 levels were detected utilizing the ELISA kit according to the instructions of the kit.
Histopathological Examination
Pancreatic tissue underwent fixation in 4% paraformaldehyde for 24 h, followed by paraffin embedding and sectioning into 3 μm slices. Sections were subjected to dewaxing and hydration before staining with hematoxylin and eosin. The severity of pancreatitis was evaluated by grading inflammation, edema, and necrosis within the pancreatic tissue sections on a scale from 0 to 3. Experienced pathologists, blinded to the experimental groups, conducted assessments of pancreatic tissue damage, randomly selecting various microscopic fields from each group for analysis.
Real-Time Quantitative PCR (RT-PCR)
Total RNA was isolated from pancreatic tissues and AR42J cells utilizing Trizol reagent, adhering to the manufacturer’s protocol. RNA concentration and quality were ascertained by measuring the A260/A280 and A260/A230 OD ratios, respectively. Reverse transcription and RT-PCR were conducted utilizing a Reverse Transcription Kit and a SYBR Green qPCR Kit. Employing GAPDH as the reference gene, the comparative CT (2 − ΔΔCT) method was utilized to calculate the relative expression levels of target genes. Three replicates were conducted for each target gene in each biological sample. Experimental primers for RT-PCR are listed in Table 1.
Western Blot
Pancreatic tissue and AR42J cells were homogenized in RIPA buffer, incubated for 30 min on ice, and centrifuged at 12,000 g, 4 °C for 15 min. After centrifugation, supernatants were harvested, and protein concentrations were determined employing the BCA protein assay according to the manufacturer's instructions. Proteins underwent SDS-PAGE on a 10% gel and were transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was subsequently incubated with primary antibodies at 4 °C overnight, followed by a 1-h room temperature incubation with horseradish peroxidase-conjugated secondary antibodies. Signal detection was achieved via enhanced chemiluminescence (ECL) and analyzed with the ChemiDoc™ Touch Imaging System. Protein expression levels were quantified and normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistical Processing
All data were analyzed utilizing GraphPad Prism 8.0 and presented as mean ± SEM. Statistical analyses were performed using one-way ANOVA or the Kruskal–Wallis H test to compare three groups. Tukey’s multiple comparison tests were then applied to identify significant differences between pairs. The unpaired t test was utilized to compare the two groups. Statistical significance was defined as a p-value below 0.05. The results presented in this study were based on a minimum of three independent experiments.
Results
Serum Lipase, Amylase, and IL-22 Level Are Elevated in AP Mice Induced by Caerulein Plus Lipopolysaccharide (Fig. 1)
The level of amylase, lipase, and IL-22 in the blood were notably elevated in the AP group compared to the NS group at 12 h and 24 h post-induction of AP (p < 0.05).
Additionally, the levels of IL-22 reached their highest point at 24 h after AP induction, and decreased at 48 h (p < 0.05), with a similar trend to amylase and lipase.
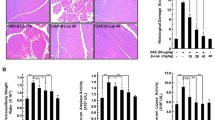
Il-22 Relieves Inflammation of the AP Mice (Fig. 2)
Il-22 relieves inflammation of the AP in mice. A The blood amylase levels in each group at the 12 h after inducing AP. B The blood amylase levels in each group at the 24 h after inducing AP. C The blood lipase levels in each group at the 12 h after inducing AP. D The blood lipase levels in each group at the 24 h after inducing AP. E Pancreatic tissue lesions scores of each group. F Hematoxylin and eosin-stained images of pancreatic for each group (100×)
Blood amylase and lipase levels of the IL-22 group increased at 12 h after inducing AP, but they decreased significantly at 24 h, reaching levels markedly lower than those in the AP group (p < 0.05) (Fig. 2A–D).
Histological examination revealed that the pancreatic structure and morphology of acinar cells were normal in the NS group. In the AP group, there was evidence of pancreatic tissue edema, neutrophil infiltration, unclear acinar cell structure, and visible necrosis. In the IL-22 group, acinar cells were swelling, tissue edema, and a small amount of neutrophil infiltration. The pathological score in the IL-22 group was lower than that in the AP group (p < 0.05) (Fig. 2E, F).
IL-22 Alleviated Caerulein-Induced Cell Damage in AR42J Cell
Caerulein stimulation decreased AR42J cell viability and increased the necrosis rate; concurrently, IL-22 enhanced cell viability and inhibited caerulein-induced necrosis in AR42J cells (p < 0.05). (Fig. 3).
IL-22 Alleviates Autophagy Damage in AP Mice Model
In the AP mouse model, the IL-22 group decreased the relative mRNA levels of P62, Beclin-1, and ATG5 compared to the AP group (Fig. 4A–C). Furthermore, the IL-22 group displayed lower levels of P62 protein expression and a decreased LC3-II compared to the AP group (Fig. 4D).
IL-22 Alleviates Autophagy Damage in AP Cell Model
In AR42J cells, the relative mRNA levels of P62 and Beclin-1 were found to be decreased in the Cae + IL-22 group compared to the Cae group (Fig. 5A, B). Additionally, the protein expression of P62 and the LC3-II were lower in the Cae + IL-22 group when compared to the Cae group (Fig. 5C, D).
IL-22 Activates the AKT/mTOR Signaling Pathway (Fig. 6)
The Western Blot analysis revealed that the addition of IL-22 induced phosphorylation of AKT/mTOR in both the AP animal and cell models (Fig. 6).
Discussion
AP is a common inflammatory disorder of pancreas with considerable morbidity and certain mortality. AP is characterized by pancreatic self-digestion, leading to tissue damage due to the premature activation of digestive enzymes. This can further cause the release of a variety of inflammatory mediators, further induce both local and systemic inflammatory responses [19, 20]. Despite medical advances, AP, especially SAP, remains a therapeutic challenge and life-threatening because no specific and effective therapies are available.
Autophagy is an evolutionarily conserved cellular process that involves the degradation and recycling of dysfunctional organelles or aggregated proteins within cells. Three major autophagy pathways have been identified, including macroautophagy, chaperone-mediated autophagy, and microautophagy. We will focus here on macroautophagy, and basal autophagy plays an essential role in maintaining the physiological function of pancreatic acinar cells [21,22,23]. However, abnormal or defective autophagy can exacerbate pancreatic inflammation [24, 25]. Increasing evidence suggests that impaired autophagy is associated with trypsinogen activation, and regulating autophagy could help reduce AP inflammation [26]. Currently, three primary pathways regulate autophagy, including the inhibitory mTOR pathway, the stimulating pathway via beclin-1 (also known as autophagy-related gene 6/Atg6), and the stimulation of the light chain 3II (LC3-II) / Atg5-Atg12-Atg16 pathway [15, 27, 28].
LC3 is the commonly used name for microtubule-associated protein 1 light chain 3, which is an autophagy-specific marker that exists in two forms:LC3-I (cytoplasmic type) and LC3-II (membrane-bound type). During autophagy, LC3-I is conjugated by Atg7, Atg5, Atg12, and Atg16L to the highly lipophilic phosphatidylethanolamine moiety to generate LC3-II, and LC3-II is involved in the extension and closure of autophagosome membrane [29]. P62 (also known as SQSTM1) is a specific degradation product of the autophagy pathway and is responsible for recognizing and delivering ubiquitinated proteins to the autophagy degradation pathway. The increase of LC3-II and P62 suggested that the autophagy degradation pathway was blocked and autophagy completion was inhibited [10]. A study [30] shown that “LC3 excess” in GFP-LC3 mice perturbed basal pancreatic autophagy and shown the physiological importance of autophagy for acinar cell function. Beclin1, the first mammalian autophagy gene discovered, binds to Class III phosphatidylinositol 3-kinase (PI3K C3) to form a complex that promotes autophagy. Atg5 and Atg12 bind to form the autophagy conjugated ligand Atg5-Atg12, which is necessary for autophagy formation. The AP model induced by Atg5 knockout mice showed decreased autophagy and reduced AP inflammation [26].
IL-22 is a cytokine with anti-inflammatory properties, and it is first identified in IL-9 treated mouse lymphoma cells [31]. IL-22 binds to receptor complexes (composed of IL-10R2 and IL-22R1) and activates various signaling pathways. Pancreatic acinar cells are a target of IL-22. Studies have shown that the serum IL-22 level increases in patients with AP [32], while IL-22 RA1 expression is upregulated during AP but returns to baseline as the pancreas recovers [33]. These findings suggest that IL-22 may be involved in the pathogenesis of AP.
Regulating autophagy can ameliorate systemic organ injury and alter the severity of L-arginine or caerulein-induced AP in mice [34]. A study [35] suggested that taurocholate-induced AP exacerbated by over-activating autophagy via activation of AMPK and subsequently inhibition of mTOR. Another study demonstrated that IL-22 attenuated liver injury in mice treated with ethanol and CCl4 by regulating the expression of autophagy-related genes and inhibiting the PI3K/AKT/mTOR pathway [36]. Feng et al. [37] found that IL-22 inhibited the autophagy pathway, making mice resistant to caerulein-induced AP, and ameliorated AP by combining B-cell lymphoma-2(Bcl-2) and Bcl-XL with Beclin-1.
Our study demonstrated that the serum IL-22 level is increased in AP model mice, peaking 24 h after AP inducting, with an increasing trend similar to that of serum amylase. These results indicate that IL-22 is involved in the inflammatory process of AP, and it may be utilized as a diagnostic indicator for AP. A study [9] suggested that trypsinogen activation and acinar cell vacuole formation were related to impaired autophagic flux in AP models. Thus, it is necessary to explore the effect of IL-22 on autophagy in caerulein-induced AP.
We also observed that exogenous IL-22 can ameliorate the severity of caerulein-induced AP in mice, further research found that the expression of Beclin-1, P62, and LC3-II was upregulated in caerulein-induced AP, and their expression decreased in the IL-22 group. This suggests that autophagy was activated in AP mice, but there was autophagy degradation dysfunction and autophagosome accumulation [38], in line with previous studies [25, 39]. It also indicated that IL-22 restored autophagic flux in caerulein-induced AP, and then alleviated pancreatic inflammation. A study [40] has suggested that mTOR activation plays a potential protective role in the later recovery of AP through the increased S6RP phosphorylation at 36 h and 48 h after AP induction. We observed AKT/mTOR phosphorylation at 24 h after AP induction in IL-22 group, this indicated IL-22 may advance the recovery of AP. And we got the same results in cells test. So, we speculated that IL-22 may regulate autophagy through the AKT/mTOR signal pathway, activation of mTOR can phosphorylates Atg13 and reduces its affinity to Atg1, then inhibits autophagy and attenuates the severity of caerulein-induced AP. These findings suggest that IL-22 may be a potential drug for the treatment of pancreatitis in the future.
Conclusions
In conclusion, our study revealed that autophagy was activated in AP mice induced by caerulein and LPS, while autophagy flux was impaired. Exogenous IL-22 attenuated the impaired autophagic flux, and the AKT/mTOR pathway could play a crucial role in restoring autophagy.
References
Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175–184.
Li L, Li YQ, Sun ZW et al. Qingyi decoction protects against myocardial injuries induced by severe acute pancreatitis. World J Gastroenterol. 2020;26:1317–1328.
Portelli M, Jones CD. Severe acute pancreatitis: pathogenesis, diagnosis and surgical management. Hepatobiliary Pancreat Dis Int. 2017;16:155–159.
Saxena R, Kumar S, Nafe Z et al. Clinical, biochemical, and radiological correlation in the severity of acute pancreatitis: a retrospective study. Cureus. 2023;15:e34996.
Grimm Wesley A, Messer Jeannette S, Murphy Stephen F et al. The Thr300Ala variant in ATG16L1 is associated with improved survival in human colorectal cancer and enhanced production of type I interferon. Gut. 2016;65:456–464.
Eshraghi M, Adlimoghaddam A, Mahmoodzadeh A et al. Alzheimer’s disease pathogenesis: role of autophagy and mitophagy focusing in microglia. Int J Mol Sci. 2021;22:3330.
Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021;54:437–453.
Fortunato F, Kroemer G. Impaired autophagosome-lysosome fusion in the pathogenesis of pancreatitis. Autophagy. 2009;5:850–853.
Mareninova OA, Hermann K, French SW et al. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340–3355.
Gukovskaya Anna S, Ilya G, Hana A et al. Autophagy, Inflammation, and immune dysfunction in the pathogenesis of pancreatitis. Gastroenterology. 2017;153:1212–1226.
Wang S, Ni HM, Chao X et al. Impaired TFEB mediated lysosomal biogenesis promotes the development of pancreatitis in mice and is associated with human pancreatitis. Autophagy. 2019;15:1954–1969.
Feng D, Wang Y, Wang H et al. Acute and chronic effects of IL-22 on acetaminophen-induced liver injury. J Immunol. 2014;:2512–2518.
Pickert G, Neufert C, Leppkes M et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 2009;206:1465–1472.
Lejeune D, Dumoutier L, Constantinescu S et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–33682.
Wang S, Yao Y, Yao M et al. Interleukin-22 promotes triple negative breast cancer cells migration and paclitaxel resistance through JAK-STAT3/MAPKs/AKT signaling pathways. Biochem Biophys Res Commun. 2018;503:1605–1609.
Zhang X, Liu S, Wang Y et al. Interleukin-22 regulates the homeostasis of the intestinal epithelium during inflammation. Int J Mol Med. 2019;43:1657–1668.
Patnaude L, Mayo M, Mario R et al. Mechanisms and regulation of IL-22-mediated intestinal epithelial homeostasis and repair. Life Sci. 2021;271:119195.
Ye J, Liu L, Ji Q et al. Anti-interleukin-22-neutralizing antibody attenuates angiotensin II-induced cardiac hypertrophy in mice. Mediators Inflamm. 2017;2017:5635929.
Saluja A, Dudeja V, Dawra R et al. Early intra-acinar events in pathogenesis of pancreatitis. Gastroenterology. 2019;156:1979–1993.
Habtezion A, Gukovskaya AS, Pandol SJ. Acute pancreatitis: a multifaceted set of organelle and cellular interactions. Gastroenterology. 2019;156:1941–1950.
Antonucci L, Fagman JB, Kim JY et al. Basal autophagy maintains pancreatic acinar cell homeostasis and protein synthesis and prevents ER stress. Proc Natl Acad Sci USA. 2015;112:E6166–E6174.
Feng Y, He D, Yao Z et al. The machinery of macroautophagy. Cell Res. 2014;24:24–41.
Hale AN, Ledbetter DJ, Gawriluk TR et al. Autophagy: regulation and role in development. Autophagy. 2013;9:951–972.
Chinzei R, Masuda A, Nishiumi S et al. Vitamin K3 attenuates cerulein-induced acute pancreatitis through inhibition of the autophagic pathway. Pancreas. 2011;40:84–94.
Xiao J, Feng X, Huang XY et al. Spautin-1 ameliorates acute pancreatitis via inhibiting impaired autophagy and alleviating calcium overload. Mol Med. 2016;22:643–652.
Hashimoto D, Ohmuraya M, Hirota M et al. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol. 2008;181:1065–1072.
Czaja MJ. Functions of autophagy in hepatic and pancreatic physiology and disease. Gastroenterology. 2011;140:1895–1908.
Gukovskaya AS, Gorelick FS, Groblewski GE et al. Recent insights Into the pathogenic mechanism of pancreatitis: role of acinar cell organelle disorders. Pancreas. 2019;48:459–470.
Voronina S, Chvanov M, De Faveri F et al. Autophagy, acute pancreatitis and the metamorphoses of a trypsinogen-activating organelle. Cells. 2022;11:2514.
Mareninova OA, Jia W, Gretler SR et al. Transgenic expression of GFP-LC3 perturbs autophagy in exocrine pancreas and acute pancreatitis responses in mice. Autophagy. 2020;16:2084–2097.
Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–1819.
Vasseur P, Devaure I, Sellier J et al. High plasma levels of the pro-inflammatory cytokine IL-22 and the anti-inflammatory cytokines IL-10 and IL-1ra in acute pancreatitis. Pancreatology. 2014;14:465–469.
Xue J, Nguyen DT, Habtezion A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology. 2012;143:1670–1680.
Wan J, Chen J, Wu D et al. Regulation of Autophagy Affects the Prognosis of Mice with Severe Acute Pancreatitis. Dig Dis Sci. 2018;63:2639–2650.
Ji L, Li L, Qu F et al. Hydrogen sulphide exacerbates acute pancreatitis by over-activating autophagy via AMPK/mTOR pathway. J Cell Mol Med. 2016;20:2349–2361. https://doi.org/10.1111/jcmm.12928.
Meng YX, Zhao R, Huo LJ. Interleukin-22 alleviates alcohol-associated hepatic fibrosis, inhibits autophagy, and suppresses the PI3K/AKT/mTOR pathway in mice. Alcohol Clin Exp Res. 2023;47:448–458.
Feng D, Park O, Radaeva S et al. Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int J Biol Sci. 2012;8:249–257.
Klionsky DJ, Abdel-Aziz AK, Abdelfatah S et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy. 2021;17:1–382.
Cao Y, Yang W, Tyler MA et al. Noggin attenuates cerulein induced acute pancreatitis and the impaired autophagy. Pancreas. 2013;42:301–307.
Hu YY, Zhou CH, Dou WH et al. Improved autophagic flux is correlated with mTOR activation in the later recovery stage of experimental acute pancreatitis. Pancreatology. 2015;15:470–477.
Acknowledgments
This work was supported by the General program of National Natural Science Foundation of China (No. 82170650) and the Natural Science Foundation of Shandong Province (No. ZR2020MH057).
Funding
This work was supported by the General program of National Natural Science Foundation of China (No. 82170650) and the Natural Science Foundation of Shandong Province (No. ZR2020MH057).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Fu Xinjuan, Xiu zhigang, Xu qianqian, and Yuerui. The first draft of the manuscript was written by Fu xinjuan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures involving animals comply with the NIH guidelines for the Care and Use of Laboratory Animals, and ethical approval was granted by Shandong University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fu, X., Xiu, Z., Xu, Q. et al. Interleukin-22 Alleviates Caerulein-Induced Acute Pancreatitis by Activating AKT/mTOR Pathway. Dig Dis Sci (2024). https://doi.org/10.1007/s10620-024-08360-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10620-024-08360-6