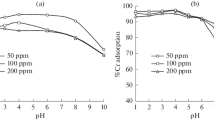
In hydrometallurgical processing of oxidized gold-bearing ores, copper, cobalt, zinc, and other metals, along with gold, dissolve in sodium cyanide alkaline solutions forming metal cyanide complexes, which have a negative impact on the gold leaching process. One method for removing copper from cyanide solutions is sorption using anion exchange resins. This article discusses the choice of a sorbent for sorption of gold, copper and cobalt from production and barren gold-containing solutions. Sorption studies are conducted on production solutions obtained during heap leaching of gold from ore (gold 1.13-1.20 mg/liter; copper 60.8-63.2 mg/liter; cobalt 1.73-1.78 mg/liter; iron 0.1 mg/liter), and on barren solutions after gold extraction from production solutions using activated carbon (gold 0.19-0.26 mg/liter; copper 49.6-59.5 mg/liter; cobalt 1.61-1.83 mg/liter; iron < 0.1 mg/liter). Sorption studies are conducted using resins such as AM-2B, Purolite A-500 and AV-17-8. Research results show that sorption characteristics of the resins tested for sorption of metals (copper, cobalt) from production and barren solutions are similar. For sorption of copper and cobalt strongly basic resins AV-17-8 and Purolite A-500 demonstrate the best sorption characteristics. The mixed-base anion exchange resin AM-2B exhibits enhanced selectivity for gold.





Similar content being viewed by others
References
O. Alonso-González, F. Nava-Alonso, and A. Uribe-Salas, “Copper removal from cyanide solutions by acidification,” Minerals Engineering, 22, No. 4, 324–329 (2009); https://doi.org/10.1016/j.mineng.2008.09.004.
X. Dai, A. Simons, and P. Breuer, “A review of copper cyanide recovery technologies for the cyanidation of copper containing gold ores,” Minerals Engineering, 25, No. 1, 1–13 (2012); https://doi.org/10.1016/j.mineng.2011.10.002.
B. Surimbayev, A. Akcil, L. Bolotova, S. Shalgymbayev, and A. Baikonurova, “Processing of refractory gold-bearing sulfide concentrates: a rev.,” Mineral Processing and Extractive Metallurgy Review, 1–19 (2023); https://doi.org/10.1080/08827508.2023.2230344.
M. A. Meretukov and K. I. Strukov, “Advanced hydrometallurgical techniques applicable to gold-copper and copper-gold ores. Practices adopted abroad,” Tsvetnye Met., No. 1 (2023); https://doi.org/10.17580/tsm.2023.01.03.
S. Shalgymbayev, I. Bolotova, and B. Surimbayev, “Kazmekhanobr’s technologies in the field of processing of low-grade goldbearing ores and technogenic raw materials,” Tsvetnye Met., 38–45 (2021); https://doi.org/10.17580/tsm.2021.09.03.
Breuer P. L., Dai X., and M. I. Jeffrey, “Leaching of gold and copper minerals in cyanide deficient copper solutions,” Hydrometallurgy, 78, No. 3-4, 156–165 (2005); https://doi.org/10.1016/j.hydromet.2005.02.004.
X. Dai and P. L. Breuer, “Cyanide and copper cyanide recovery by activated carbon,” Minerals Engineering, 22, No. 5, 469–476 (2009); https://doi.org/10.1016/j.mineng.2008.12.007.
X. Dai, M. I. Jeffrey, and P. L. Breuer, “A mechanistic model of the equilibrium adsorption of copper cyanide species onto activated carbon,” Hydrometallurgy, 101, No. 3-4, 99–107 (2010); https://doi.org/10.1016/j.hydromet.2009.12.005.
V. Z. Arens, L. V. Shumilova, M. I. Fazlullin, et al., “Promising areas of chemical and microbiological treatment of nonferrous and precious metal mineral resources,” Metallurgist, 61, 800–806 (2018); https://doi.org/10.1007/s11015-018-0567-4.
M. I. Fazullin, Precious Metal Piecewise Leaching [in Russian], Izd. Akadem. Gorn. Nauk, Moscow (2001).
F. Xie, D. Dreisinger, and F. Doyle, “A review on recovery of copper and cyanide from waste cyanide solutions,” Mineral Processing and Extractive Metallurgy Review, 34, No. 6, 387–411 (2013); https://doi.org/10.1080/08827508.2012.695303.
G. Larrabure and J. C. F. Rodríguez-Reyes, “A review on the negative impact of different elements during cyanidation of gold and silver from refractory ores and strategies to optimize the leaching process,” Minerals Engineering, 173, 107194 (2021); https://doi.org/10.1016/j.mineng.2021.107194.
D. Medina and C. G. Anderson, “A review of the cyanidation treatment of copper-gold ores and concentrates,” Metals, 10, No. 7, 897 (2020); https://doi.org/10.3390/met10070897.
V. V. Barchenkov, Hydrometallurgical Treatment Technology for Gold-Containing Flotation Concentrates Using Activated Carbon [in Russian], Poisk, Chita (2004).
M. Mariana et al., “Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption,” J. of Water Process Engineering, 43, 102221 (2021); https://doi.org/10.1016/j.jwpe.2021.102221.
X. Dai, P. L. Breuer, and M. I. Jeffrey, “Comparison of activated carbon and ion-exchange resins in recovering copper from cyanide leach solutions,” Hydrometallurgy, 101, No. 1-2, 48–57 (2010); https://doi.org/10.1016/j.hydromet.2009.11.019.
A. Dabrowski et al., “Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method,” Chemosphere, 56, No. 2, 91–106 (2004); https://doi.org/10.1016/j.chemosphere.2004.03.006.
V. A. Leao et al., “The dependence of sorbed copper and nickel cyanide speciation on ion exchange resin type,” Hydrometallurgy, 61, No. 2, 105–119 (2001); https://doi.org/10.1016/S0304-386X(01)00159-1.
A. B. Botelho Junior, D. B. Dreisinger, and D. C. R. Espinosa, “A review of nickel, copper, and cobalt recovery by chelating ion exchange resins from mining processes and mining tailings,” Mining, Metallurgy & Exploration, 36, 199–213 (2019); https://doi.org/10.1007/s42461-018-0016-8.
J. Van Deventer, “Selected ion exchange applications in the hydrometallurgical industry,” Solvent Extraction and Ion Exchange, 29, No. 56, 695–718 (2011); https://doi.org/10.1080/07366299-2011.595626.
L. S. Bolotova and V. P. Gladyshev, “Features of metal behaviour during ion exchange extraction of gold and silver from cyanide solutions,” Kompleks. Ispol. Mineral. Syr’ya, No. 3, 29–33 (1982).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 67, No. 10, pp. 34–38, October, 2023. Russian DOIhttps://doi.org/10.52351/00260827_2023_10_34.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanaly, E.S., Surimbaev, B.N., Bolotova, L.S. et al. Choice of a Sorbent for Copper and Cobalt Sorption from Gold-Containing Heap Leaching Solutions. Metallurgist 67, 1457–1465 (2024). https://doi.org/10.1007/s11015-024-01638-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-024-01638-0