Abstract
In this paper, dietary patterns are reconstructed across two phases represented at the Copper Age of Scaba ’e Arriu (Sardinia, Italy) via isotopic analysis of human and animal skeletal remains. Collagen carbon, nitrogen, and bioapatite carbon, and oxygen isotopes were used to infer diet, economic practices, and possibly different climatic conditions. Differential management of cattle, sheep/goats, and pigs was detected, with scarce animal products in the human diet in the Early Copper Age, followed by an increase in animal products identifiable in herbivore livestock, coincident with more rainy conditions in the Late Copper Age. Dietary information was then considered in light of the climatic periods already known for the period and compatible with the data presented. The study’s outcome indicates generally sedentary and endogamous groups with only a few outliers in both phases.
Similar content being viewed by others
Introduction
Sardinian prehistory has attracted scholars for its monumental aspects, centered in the rock-carved tombs during the Neolithic and Copper Age (ca. 4200–2500 BC) and in the stone towers known as nuraghi in the Bronze Age (ca. 1700–1100 BC). Such a strong perception has led to the widespread use of the terms Nuragic and Pre-Nuragic to define two periods, based on the inception of the stone towers building tradition (Lilliu 1988; Webster 2016), with Pre-Nuragic encompassing several distinct cultural aspects from the Early Neolithic through the Early-Middle Bronze Age (ca. 5500–1700 BC). Much less is known, and much less has been investigated, of the economic practices and complex interaction between human groups and the natural and cultural landscape constituting the physical and social coordinates of their daily lives. Overall patterns of resource exploitation recorded up to the Bronze Age in Sardinia can be described as fully within the boundaries of general Neolithic tradition as concerns plants (Ucchesu et al. 2014) and animal species (Wilkens 2012) essential for daily subsistence, with some variation in relative proportions but within a remarkably consistent pool of resources that changed substantially only in the Iron Age. Among the notable facts highlighted by stable isotopy in the last two decades, it is essential to underline that the Neolithization process effectively led to the marginalization of marine resources in the Mediterranean (Craig et al. 2006; Richards 2003; Richards et al. 2003), and Sardinia, where this occurred ca. 5500 BC, was no exception (Lai et al. 2014; Lai 2016). Therefore, the relevant questions under the subsistence umbrella regard mainly the proportion of vegetal and animal resources, with all their social, cultural, and health implications, and the occurrence of the so-called secondary products revolution elements (Sherratt 1983). The latter have been shown not to co-occur but to be adopted step-wise and in varying degrees of intensity in the different components (Evershed et al. 2008; Greenfield 2010; Sherratt 1983).
Several other social aspects intersect in complex ways with practices such as plowing, grazing, milking, manuring, transporting, and wool shearing: the making of individual and collective identities and the relationships among them, involving gender, age, ethnicity, mobility, and social standing. The 3rd millennium BC in the Central-Western Mediterranean has been recognized as a period of heightened residential mobility (Furholt 2020; Price et al. 1998, 2004), accompanied by the gradual, patchy rise of generalized sources of authority and prestige through wealth accumulation and metals, although in diverse and experimental ways (Beck 2020; Costa Caramé et al. 2010; Waterman et al. 2014, 2016b). An increasingly distinct and uneven gender definition (Robb 1994) took shape, which fits local evidence (Hayden 1998). A novel emphasis on highlands has been documented (Melis 2000), likely connected with the use of marginal lands for grazing or farming, with continued use of collective burials, which suggests the absence or the disguise of intense social differentiation (Cámara Serrano and Spanedda 2002). The analysis of faunal remains has shown some incipient evidence for older bovines, pointing to varying degrees of utilization of their labor force (Lai et al. 2011; Wilkens 2012).
On the background of these processes are climatic conditions, which BC underwent profound changes in the 3rd millennium, making this fundamental to understanding paleoecology and historical developments in the Mediterranean. During this period, the present-day Mediterranean climatic pattern was established, with drier overall conditions, sharper rainfall seasonality, and summer droughts (Magri 1997, 1999; Magri and Sadori 1995; Ramrath et al. 2000; Tuccimei et al. 2003; Zielhofer et al. 2004), which interact with human practices leading to the establishment of plants that had been confined to refugia during the last glacial period and spread into the Corsica-Sardinia landmass (Lumaret et al. 2002; Reille 1992). Such climatic changes appear independent of human activities and are connected to the intensity of the westerlies that bring rain to the Mediterranean (Damnati 2000; Magny and Haas 2004; Weiss 1997). This long-term drier trend can also be articulated into two peaks separated by milder conditions (Jalut et al. 2000). The first, around 2900–2700 BC, corresponds to the sudden worsening identified in Tunisia (Zielhofer et al. 2004). The second, around 2300–2100 BC, is indicated by multiple proxies in the Western Mediterranean (Giraudi 2004; Magny et al. 2002; Rimbu et al. 2004) and seems correlated to evidence across the Northern hemisphere (Dalfes et al. 1997). These phases in Sardinia seem to coincide with radical shifts in settlement patterns and material culture. Stone architecture, new ceramic styles, and new burial rituals appear after the first event (Moravetti 2009; Webster and Webster 2017: 76–111), and the end of village life follows the second event. At Scaba ’e Arriu, the Early Copper Age phase (ECA hereafter: ca. 3400–2600 BC) overlaps with the first dry peak, the Late Copper Age phase (LCA hereafter: ca. 2700–2350 BC) with milder, wetter conditions.
Questions addressed in this study are as follows: (i) Was there was any dietary variation across the two phases? (ii) How can we describe it? (iii) Is there any evidence for climate change? (iv) How do these factors fit the overall evidence from the site toward a comprehensive understanding of economic dynamics? With these aims, stable isotopic analyses were applied to the bone remains from the burial at Scaba ’e Arriu (Siddi, Sardinia).
Archaeological context
The site of Scaba ’e Arriu lies at 200 m. a.s.l., approximately 60 km from the South coast and 30 from the West coast of Sardinia. It consists of a tomb carved in the local marly sandstone between a hilly landscape to the South and East and a narrow and elongated basaltic plateau to the West, from which small seasonal streams flow. The fertility of the soils in a radius of over 20 km made this area historically dedicated to cereal cultivation.
The tomb is articulated into the actual burial room and a small antechamber, preceded by a corridor/entryway. In the Late Copper Age (LCA hereafter: ca. 2700–2350 BC), the antechamber, whose roof had collapsed, was turned into a passageway flanked by upright slabs, after the clearing of earlier human remains from the chamber (see Fig. 1). Such slabs were laid on top of layers of soil, stones, animal bones, and human bones from earlier burials. The stratigraphy in the corridor and the surrounding area includes two thick layers (US4 and US5) characterized by human and animal bones, and by Early Copper Age (ECA hereafter: ca. 3400–2600 BC) cultural materials, resulting from ritual activity (Ragucci and Usai 1999; Usai 1998) and/or from emptying the earlier remains and grave goods. Thinner lower layers yielded Neolithic pottery dating to the early 4th millennium BC. The two main phases, the subject of this study, were radiocarbon dated (Lai 2009) (Table 1).
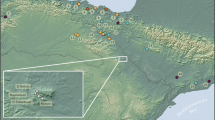
Top left (a): Sardinia in the Mediterranean. Top right (b): Scaba ’e Arriu in Sardinia—both elaborated based on maps from maps-for-free.com. Bottom left (c): section and plan of the rock-carved burial (elaboration L. Lai, permission from MiBAC – Soprintendenza Archeologia, Belle Arti e Paesaggio città metropolitana di Cagliari, provincie di Oristano e Sud Sardegna): left to right, the terminal part of the corridor and the antechamber, which together with the whole surrounding area contained the animal and human remains from the Filigosa phase (early 3rd mill. BC), and further right, the burial chamber with the remains from phase Monte Claro (mid-to-late 3rd mill. BC). Bottom right (d): outline of stratigraphy in the different areas
Preliminary averages of the isotopic values have been presented previously (Lai et al. 2011; Usai et al. 2011). Additional radiocarbon dates are now available (Olivieri et al. 2017), and analyses concerning pathologies and cross-sectional geometry of long bones have also been carried out (Chilleri et al. 2012; Murgia et al. 2021, 2020; Parkinson 2019; Parkinson et al. 2018). All isotopic values are presented here, with new data from FTIR measurements on bioapatite.
The two phases investigated are Filigosa (ECA, US4 and US5), dating to the early 3rd mill. BC, Monte Claro (LCA, inner chamber), mid-to-late 3rd mill. BC. The radiocarbon dates from the University of Arizona, Beta Analytics, and the University of Heidelberg agree on this overall chronology (Table 1, Fig. 2), corroborating the stratigraphic and behavioral break recorded in burial depositions. The clearing of the tomb chamber and the addition of the slabs in the antechamber from previous remains occurred between them.
Oxcal-generated chart of calibrated radiocarbon dates from Scaba ’e Arriu (see Table 1), both unmodelled (black filled) and modeled (grey filled). Raw dates (BP) are from three different laboratories (Lai 2008; Olivieri et al. 2017; Ragucci and Usai 1999), and the two phases were modeled according to evidence of a hiatus between them, with distinct end and start
Materials and methods
A total of 46 bone samples were analyzed, all except one representing different individuals: 15 humans and 18 animals from the outer corridor and surrounding area (ECA phase, except two animal specimens from the Neolithic layers) and 13 humans from the inner chamber (LCA phase). Human sample selection, aiming to optimize data on sex and age, targeted cranial and pelvic bones. No substantial systematic difference is expected between these elements (Fahy et al. 2017). Hydrological regimes must have been different across the site, with the former more exposed to rainwater but perhaps protected by soil and the latter protected from direct rainwater. This suggests different microenvironmental and taphonomic conditions for the two assemblages, which need to be considered in assessing signal preservation in bioapatite (Lee-Thorp and Sponheimer 2003; Wang and Cerling 1994). The ECA and LCA human remains had a similar taphonomic history for the first few hundred years after deposition but a different one for the last four millennia.
The animal remains, including Bos taurus, Ovis aries/Capra hircus, Sus scrofa domesticus/ferus, and Canis familiaris, residues of ritual meals or ritual offerings (Usai et al. 2011), appear to have been found in layers US5 and US6 in the antechamber, and US4 and US5 in the corridor and in the area facing it and surrounding it, parallel to a similar ritual elsewhere (Cocco and Usai 1988; Lai et al. 2017), but mixed with human remains, which may be the residue of special ritual activities or more likely of the clearing carried out later. Therefore, their taphonomic history is expected to be consistent with the contemporary human remains after being cleared out, followed by soil accumulation. Two specimens, Prolagus sardus (ochotonid endemic to Sardinia and Corsica, now extinct) and Vulpes vulpes, are from levels compatible with the Neolithic layer US8. Since they are burrowers, they may be intrusive, but they have been included to strengthen the local faunal baseline.
To extract collagen, ca. 1 g of bone per individual was physically cleaned, ultrasonicated, and dried. Preparation (Tykot 2004) followed an established procedure (Ambrose 1990): humic acid contaminants were removed by soaking in 50 mL of 0.1 mol/L NaOH for ~ 24 h. Then, collagen was extracted by soaking in 50 mL of 2% HCl in two or more ~ 24-h baths, based on need. If necessary, samples were cut into small pieces to help the solution permeate the tissue. A further ~ 24-h bath in NaOH removes any contaminants not exposed to the reaction in the whole bone. Samples were finally soaked for ~ 24 h in 50 mL of a methanol-chloroform-distilled water solution (proportion 2:1:0.8) to remove lipids—a step that has consequences for results comparability (Liden et al. 1995). Samples were rinsed at every change of reagent. The extracted pellets were dried overnight at ~ 65 °C. Two replicates per sample were weighed into tin capsules and analyzed in continuous-flow mode with a Carlo-Erba 2500 Series II CHN analyzer, coupled with a Thermo Finnigan Delta + XL stable isotope ratio mass spectrometer, with precision (2σ) better than ± 0.3‰ for δ15N and ± 0.2‰ for δ13C, located at the Paleolab, St. Petersburg campus, University of South Florida.
To isolate the bioapatite carbonate (phosphate can also be extracted from bioapatite), ~ 10 mg of bone powder was treated with a ~ 72-h bath in 1.5 mL of 2% sodium hypochlorite to dissolve the organic portion. Non-structural carbonate was removed by soaking the sample for ~ 24 h in a 1.0 mol/L buffered acetic acid/sodium acetate solution. While preparation was ongoing, new studies showed that soaking times of ~ 4 h in less concentrated acetic acid (0.1 M) are sufficient and even better to prevent recrystallization, which introduces a possible differential fractionation (Berna et al. 2004; Garvie-Lok et al. 2004: 771–775; see also Nielsen-Marsh and Hedges 2000b; Zazzo and Saliège 2011). However, changing preparation times on route was considered less beneficial vs. losing comparability within the dataset, so the same method was employed for all samples. Later research appears to reach inconclusive results: short acid pretreatments may be unable to remove authigenic carbonate (Zazzo 2014), but long pretreatments in enamel may alter the isotopic signature significantly (Skippington et al. 2019), to the point that no pretreatment has more recently been suggested as potentially preferable (Pellegrini and Snoeck 2016; Snoeck and Pellegrini 2015). In any case, attention was paid to consistency with soaking times to ensure comparability (Garvie-Lok et al. 2004; Grimes and Pellegrini 2013; Koch et al. 1997; Lee-Thorp and Sponheimer 2003). One milligram per sample of the resulting bioapatite powder was analyzed on a Thermo Finnigan Delta + XL mass spectrometer, in dual-inlet configuration, equipped with a Kiel III individual acid bath carbonate system, with precision (2σ) better than ± 0.04‰ for δ13C and ± 0.06‰ for δ18O.
Bioapatite has a specific turnover rate, estimated to be slightly faster than collagen, in the order of several years (Hedges et al. 2007). All macronutrients contribute to its synthesis, which makes it a unique proxy for overall diet, unlike collagen which records mainly the protein portion (Ambrose 2000; Jim et al. 2004; Tieszen and Fagre 1993), and unlike tooth enamel, which derives from all macronutrients but only during the few years of formation for each tooth type (Koch et al. 2009; Reid and Dean 2006). Therefore, considering that in the Western Mediterranean context, between the Mesolithic and the Bronze Age, there were no nutritionally significant C4 plants nor marine foods (Craig et al. 2006; Tafuri et al. 2009), bioapatite is extremely valuable. Unlike collagen, there are no precise indicators of preservation of the isotopic signature, but there is general evidence for better reliability in remains from dry climates compared to temperate areas (Zazzo and Saliège 2011). Concerns regarding the bone bioapatite ability to preserve its biogenic signal have emerged early (reviews in Lee-Thorp 2008; Lee-Thorp and Sponheimer 2003; Schoeninger and DeNiro 1982), and problems were recorded in several contexts (see Trueman et al. 2003), which made highly contentious its use as an archive of isotopic information (Kohn and Cerling 2002). However, confidence in the preservation of the isotopic signal has been supported by recent research using 14C: due to the different dynamics governing early diagenesis, for samples younger than 40,000 years, bone shows isotopic reliability similar to tooth enamel (Zazzo 2014).
Several indirect tools were employed to detect possible diagenetic trends and to estimate the reliability of the isotopic signal. Bioapatite yields were measured at each preparation step (Chesson et al. 2021; Nielsen-Marsh and Hedges 2000b), and parameters measured by FTIR, such as the Crystallinity Index or Splitting Factor (IR-SF) and Carbonate to Phosphate ratio (C/P), were explored, together with possible presence of calcite and fluorapatite peaks (Shemesh 1990; Weiner 2010: 286–292; Weiner and Bar-Yosef 1990; Wright and Schwarcz 1996). Different studies have shown the possibility of a moderate increase in crystallinity coupled with intense isotopic alteration or vice versa (Koch et al. 2000; Lee-Thorp and Sponheimer 2003; Pucéat et al. 2004; Trueman et al. 2008; White et al. 1998). These measurements are consequently to be taken as indirect tools to understand potential processes at play in specific taphonomic environments. Whereas acidity of the soil matrix appears to be the most prominent factor for bone degradation (Berna et al. 2004; Nielsen-Marsh et al. 2007), other elements should be considered in rocks from neutral to alkaline such as Scaba ’e Arriu’s marly sandstone. FTIR-derived values may allow the identification of possible indicators of diagenetic processes.
Results: preservation
Preservation and diagenesis indicators are in Supplementary Materials 1, 2, and 3. Conditions of collagen were quite variable but overall acceptable. Most samples had C:N ratios and C and N concentrations within the range accepted as representative of well-preserved, isotopically reliable collagen (Ambrose 1990; DeNiro 1985). Some samples were considered for paleoecological reconstruction even though a single subsample represented them. Replicates excluded for possible instrumental detection errors were as follows: #8687; #8679, r. 1, which yielded normal C:N ratio, but N% = 36.35 and C% = 82.93; #6989, r. 1 (ECA phase), #7010, r. 2, and #7014, r. 2 (LCA phase). In one case (#8685, replicate 2), the C:N ratio was abnormal because N had no signal, but the value was retained since C% and isotopic values were very close to the replicate with all parameters within the acceptable range. Only sample #7020 had a C:N ratio of 2.4 and was not considered for discussion.
Bioapatite δ18O and δ13C (Fig. 3) ECA phase values show that, despite a central area of minimal overlap and the exception of one human outlier, the different species maintain the signal that is expected according to their physiology and diet (Kohn 1996): obligate drinkers such as humans and pigs have more negative δ18O, and moderately drought-tolerant taxa such as sheep and goats have more enriched values. The two samples of extinct Prolagus show the most distinct values, with very enriched δ18O and depleted δ13C. The preservation of species-specific signals is the strongest argument in favor of the reliability of the isotopic signal (Koch 1998; Kohn 1996; Sponheimer and Lee-Thorp 1999).
For the ECA phase, an ANOVA exploration of possible correlations between parameters that might suggest systematic diagenetic processes at work such as IR-SF and C/P and 1096 cm−1 shoulders, yields, and isotopic values themselves (Wright and Schwarcz 1996) did not lead to identifying any, except the expected one between IR-SF and C/P, which reflects some degree of diagenesis and recrystallization, since bone crystallinity generally co-varies inversely with preservation of organic matter (Hedges et al. 1995; Lee-Thorp and Sponheimer 2003; Person et al. 1995; Weiner and Bar-Yosef 1990; Wright and Schwarcz 1996). Some statistically significant correlations (between: δ13Ccol and IR-SF; δ15N and bioapatite yield; δ18O and both δ13Capa and the deriving Δδ13Capa-col) were weak and likely caused by random effects of small numbers. Removing one observation, the p value is in the non-significant range. No significant correlations were found in IR-SF and δ18O values (ANOVA), nor any significant difference in δ18O associated with minimal presence vs. absence of a shoulder at 1096 cm−1 measured through FTIR (Mann–Whitney test). Furthermore, setting aside the one outlier (identified visually with a boxplot based on the interquartile method) with δ18O = − 1.4‰, the two groups have an identical mean (− 3.4‰). Whether the IR 1096 cm−1 peak is linked to fluorapatite uptake or reflects an overall increase in crystallinity (Weiner 2010: 288–289), such few and minimal shoulders and their lack of correlation with either IR-SF or δ18O do not point to any significant isotopic alteration, so the recrystallization suggested by IR-SF and C/P must have been limited, and/or mainly from the bone matrix itself, with no meaningful contribution from exogenous carbonate.
Similarly, among the samples from the LCA phase, no significant correlation was found between the different measures related to possible diagenetic processes, or the correlation is weak and becomes non-significant by removing one of the extreme values (δ13Ccol and both δ15N and the related Δδ13Capa-col; δ15N and δ18O; δ13Capa and collagen yield), except for the one between δ13Ccol and collagen yield, which is perhaps due to a random effect of small numbers, considering that the standard parameters for the collagen samples are entirely acceptable.
No detectable difference was noticed between the two specimens from individuals found inside ceramic vessels (#7005, 7012) and those from the remaining individuals. This further suggests no significant alteration of isotopic values, and it also probably reflects the sharing of a substantial portion of the taphonomic history before the secondary deposition of the remains inside jars. IR-SF and C/P in both human groups and the faunal samples can be compared with the intact context of Santa Caterina di Pittinuri (Lai et al. 2017), dating to the Filigosa ECA phase, contemporaneous to Scaba ’e Arriu’s, where animal remains were recovered outside, and human remains still in their original burial chamber. Whereas domestic animal species are relatively consistent, FTIR-derived human values from both phases are closer to faunal ones at Scaba ’e Arriu, unlike at Santa Caterina di Pittinuri, where a much higher IR-SF in humans matches the concretions observed on the human remains: both can be read as outcomes of a different hydrological regime inside and outside the burial chamber. Notably, at Santa Caterina di Pittinuri, bioapatite δ18O and δ13C values clustered by species as at Scaba ’e Arriu. This examination points to the absence of overall diagenetic processes that could have substantially altered the isotopic signature of the samples analyzed from both phases.
Collagen yield (“concentration” in Ambrose 1990) of the two sets of human samples is broadly comparable: 4.8 ± 1.6% for ECA phase depositions and 5.7 ± 2.2% for LCA phase depositions. Bioapatite carbonate yields are also similar: 65.8 ± 4.7% for the former and 61.6 ± 6.5% for the latter. Recent assessments of such indicator (Chesson et al. 2021) would place these values across the upper limit of the acceptable range, but without similar significant correlations with other indicators as IR-SF and C/P. Such a slight difference points to similar diagenetic factors for both human collections, processes which must have been concentrated in the first centuries of their taphonomic history and then slowed significantly for the ECA group.
Collagen yields of faunal specimens (average 2.0 ± 1.3‰ for all animal specimens; 2.3% for Ovis/Capra sp.; 2.2% for Bos sp.; 1.5% for Sus sp.) are lower and more variable than humans, which can be connected to the following: (a) different pre-burial treatment, unlike humans (Jans et al. 2004; Nielsen-Marsh et al. 2007; Roberts et al. 2002); (b) different depositional history: Scaba ’e Arriu’s animal remains may have lost their organic matter in the few centuries of exposure in the surrounding area, sharing the ensuing situation with the human remains for the next ~ 4500 years; (c) a combination of the two. Cooking is an analog of diagenesis since no method to differentiate their effect has been devised. If carried out before burial (for ritual consumption), it could help speed up the mechanical break-up of the bone structure, opening the way to microbial or chemical action (Roberts et al. 2002).
Bioapatite yields for animal samples are somewhat higher: 69.1 ± 4.3%, which fits the trend that where taphonomy, and not soil pH, is the primary diagenetic agent, animal bones are generally better preserved (Jans et al. 2004), showing prevalent fungus-related diagenesis, vs. human samples where microbial attack was dominant. This is compatible with animal bones undergoing faster diagenesis before being covered and mixed into compact soil layers when the inner room was cleared for reuse. The latter was protected from water, and such a consistently drier environment must have favored the preservation of collagen and of biogenic apatite along with it (Lee-Thorp and Sponheimer 2003; Zazzo and Saliège 2011). Moreover, the bedrock where the tomb was carved is marly sandstone, a calcium carbonate-rich sedimentary rock. The two combined factors are predictors of good overall preservation (Nielsen-Marsh and Hedges 2000a; Nielsen-Marsh et al. 2007) and bone mineral isotopic preservation (Lee-Thorp and Sponheimer 2003). Bone tissue from one cranium (n.8003) from the LCA phase recovered in the chamber inside a ceramic vessel showed collagen and bioapatite yield and δ13C and δ18O values fully consistent with the rest of the individuals, further supporting the inference that most diagenetic processes had already occurred before the discontinuity in burials. The microclimate inside the room probably caused the combination of slow collagen loss and fast increase in crystallinity suggested as conducive to preservation (Lee-Thorp and Sponheimer 2003). Similar conditions will likely have been in place for the ECA human remains that later continued their depositional history outside the tomb.
A smaller set of tooth enamel isotopic measurements (Tables 2 and 3; Fig. 4) shows that beyond some variability in the relationship between bone and tooth, the average values for δ13C and δ18O are fully comparable with bone (ECA phase: n = 8, δ13C = − 12.5 ± 0.7‰, δ18O = − 3.4 ± 0.2‰; LCA phase: n = 3, δ13C = − 12.0 ± 0.4‰, δ18O = − 4.4 ± 0.2‰). Furthermore, the average difference between bioapatite and enamel values is within instrumental precision range, and δ18O values maintain their significant difference between the two phases (Mann–Whitney test: p = 0.01783), which suggests no significant contamination in bone apatite signature and corroborates the significance of variation between the two phases.
Scaba ’e Arriu (Siddi). Chart showing all values of enamel δ13C and δ18O, and values of bone bioapatite δ13C and δ18O for corresponding individuals. Beyond some degree of variation, especially in δ13C from Monte Claro samples, the overall difference between the two sets is slight, and the difference in δ18O values between the two phases remains significant, which corroborates the reliability of bone apatite values
Results: isotopic values
Collagen isotope values (Tables 2, 3, and 4; Fig. 5) are generally within the expected range of fully terrestrial, C3-based ecosystems. The few wild species specimens from Late Neolithic layers show collagen isotope values similar to those at the contemporaneous site of Santa Caterina di Pittinuri (Lai et al. 2017). Relative to the diagonal line that should represent the vegetal-animal protein continuum within a C3-based domestic ecology, Vulpes sp. shows depleted δ15N and enriched δ13Ccol. The difference between Vulpes sp. and both humans and Canis sp.—the latter also sharing a similar physiology—suggests at both sites some wild resources so far unidentified. Prolagus shows at both sites significantly depleted values, to be associated with forest litter environments (at Santa Caterina, only one individual of Cervus had comparable values), which might indicate which ecosystem change eventually led to its extinction.
Among the herbivores, sheep/goats have the lowest δ13C and δ15N average values (− 20.2 ± 0.3‰; 7.1 ± 0.3‰), reflecting their entirely herbivorous diet, likely a mix of grass and leaves, whereas cattle yielded more diverse and on average more enriched values (− 19.7 ± 0.7‰; 8.4 ± 0.8‰). Plotting δ15N for the two groups (Mann–Whitney test: p = 0.02) with the δ13Capa-col spacing (averages for sheep/goat 9.1 ± 0.6‰; for cattle 9.8 ± 0.5‰, Mann–Whitney test: p = 0.06) shows some overlap but a clear average difference (Fig. 6). The bioapatite values (sheep/goats: δ13C = − 11.1 ± 0.3‰ and δ18O = − 1.7 ± 0.5‰; cattle: δ13C = − 9.9 ± 1.0‰ and δ18O = − 2.6 ± 0.7‰) confirm the expected physiology-dependent variation among species, but also the difference between the two herbivorous groups, which points to the controlled management of cattle in smaller plots closer to the village, more fertile and progressively manured (Bogaard et al. 2007), for use as draft animals and perhaps for dairy products, unlike caprines. This is in line with the advanced age of many cattle specimens, which led to infer their use for secondary products (Usai et al. 2011). Also possible is an effect of the topographic difference in pasture location for sheep/goats and cattle, with sheep/goats feeding on ridges, slopes, or the nearby plateau of Pranu’e Siddi: higher nitrogen concentration as in rich, fertile soils can inhibit atmospheric N-fixing, leading to enriched values, whereas N-poor vegetal communities would uptake isotopically depleted N from the air (Garten 1993). This applies to cultivated fields, especially if manured (Amundson et al. 2003). Fire has also been shown to potentially enrich δ15N values (Cook 2001; Saito et al. 2007). Whereas fires in a high-biomass primary forest would enrich δ15N values of the young new plants, frequent fires in shrubby, dry vegetal communities as the Mediterranean lowlands near Scaba ’e Arriu would increase the need for bacterial N-fixing, leading to lower values. This, in turn, overlaps with the possibility of caprines feeding in distant fields periodically cleared by fire, unlike those closest to the village, plausibly employed for horticulture and pasture of draft animals. Such a dichotomy between caprines and draft oxen is historically and ethnographically documented in Sardinia in Medieval through Modern rural organization (Ferrante and Mattone 2004; Le Lannou 1941: 117): tame cattle, vegetable gardens, and tree crops were near the village. The outer area was divided into sections and alternatively cultivated with cereal grains, legumes, or left fallow. The farthest, marginal land was pasture for sheep and goats.
Concerning pig values, while average collagen δ13C (− 20.0 ± 0.4‰) is similar to the herbivores, average δ15N is about half a trophic level above caprines (8.8 ± 0.7‰), as expected in omnivorous diets, but close to cattle (see above), possibly due to being kept near or in the village on a mainly plant-based diet. Bioapatite values (δ13C = − 10.8 ± 1.3‰; δ18O = − 4.1 ± 0.4‰) align with water-related physiology and are closer to humans while not overlapping with any herbivore. The greater internal variation aligns with the blurred distinction between wild and domesticate (Albarella et al. 2006; Rowley Conwy et al. 2012), with part of the year spent in forested areas. However, no value is recorded at Scaba ’e Arriu, compatible with full forest environments as at Santa Caterina (Lai et al. 2017). This also fits the morphology-based identification of remains from the former as consistent with the domestic pig, unlike those from the latter (Usai et al. 2011).
Collagen values for humans are within overall expected ranges for C3-based ecosystems: ECA phase: δ13C = − 19.2 ± 0.2‰; δ15N = 10.5 ± 0.8‰; LCA phase: δ13C = − 19.1 ± 0.2‰; δ15N = 10.9 ± 0.8‰. Values of the two phases are similar (Mann–Whitney: δ13C: p = 0.5195; δ15N: p = 0.548) and would be attributed to a similar diet, other conditions being equal. However, despite our lack of direct indication from fauna for the LCA phase, we can infer different climatic conditions from δ18O (see below). The baseline can be assumed to be more depleted for δ13C and δ15N, and animal protein consumption can be assessed as higher for this phase. Bioapatite values are also within the expected overall ranges: ECA phase: δ13C = − 11.4 ± 1.3‰; δ18O = − 3.3 ± 0.6‰; LCA phase: δ13C = − 13.0 ± 0.7‰; δ18O = − 3.9 ± 0.3‰. Statistically, they are both significantly different: δ13C: p = 0.003246; δ18O: p = 0.003175. The former can be read in terms of dietary variation, per se and especially through the derived interval δ13Capa-col (ECA phase = 7.7 ± 0.6‰; LCA phase = 6.0 ± 0.3‰; p = 0.00005), whereas δ18O values as reflecting variation in climatic conditions (see below).
At Scaba ’e Arriu, only a few outliers have been recorded, pointing to a low proportion of individuals who perhaps spent part of their lives far from the group: n. 5949 and possibly n. 5950 (adults), and cranium 5 (subadult). Whereas none of the cranial pathological markers in the LCA group, i.e., cribra orbitalia and trephinations (Chilleri et al. 2012) had any association with isotopic values, as concerns sex there is a statistically significant difference between males and females in δ15N in the ECA phase (Mann–Whitney: p = 0.02474): males have more enriched values than females, suggesting that they may have consumed more animal products.
Since the δ18O values in both phases do not cluster along sex lines, herding was not associated with pastoral seasonal mobility, at least the kind ethnohistorically documented in the Mediterranean (Ortu 1988), which men mostly carried out. The livestock was kept in the territory surrounding the village, without long-distance transfers to locations with sharp elevation gaps. This may have been unnecessary due to the area being a mid-point between the Sardinian highlands and the lowlands. On a local scale, the site is also a mid-point between the bottom valley and the Pranu’e Siddi plateau, with about 200-m elevation difference within a few miles radius. This aligns with findings from Late Neolithic-Copper Age Italy, suggesting generally sedentary communities (De Angelis et al. 2021), with only a few outliers to be explained as outsiders. Bioapatite δ18O ratios show a statistically significant difference in δ18O between the two phases (ECA: − 3.4 ± 0.4‰; LCA: − 3.9 ± 0.3‰; p = 0.00318, Mann–Whitney test), which can be tentatively connected to drier and/or colder conditions in the former vs. the latter.
Discussion
Discussion can be articulated in different strands as follows: (i) estimating protein consumption; (ii) estimating overall diet and the source of animal protein; and (iii) overall interpretation.
(i) The proportion of animal protein in the human diet is best estimated from the δ15N interval between consumers and (likely) consumed animals, which for the ECA phase is 2.4‰ for all domestic animals, 2.7‰ for herbivores only. This appears to be the most solid basis, despite the many aspects that need to be clarified in this relationship (Hedges and Reynard 2007). Studies of Copper Age Mediterranean diets have mainly used linear mixing models between known potential protein sources, also using specific calculation tools (Fraser et al. 2013; Kellner and Schoeninger 2007), although the lack of faunal samples leads in some cases to comparisons with faunal or plant remains from the closest spatial and temporal approximation (De Angelis et al. 2019; Fontanals-Coll et al. 2015, 2017; Waterman et al. 2016b), introducing error in evaluating the different estimates, due to climate- and environment-derived variation (van Klinken et al. 1994). Based on the mass-balance linear model as used graphically by Fraser et al. (2013), with a 3‰ trophic level interval for δ15N, animal protein consumption at Scaba ’e Arriu, ECA phase could be estimated at approximately 90% (Fig. 7). However, recorded trophic level-related differences are far from consistent. Assuming a possible interval of up to 5.5‰ (Hedges and Reynard 2007; O’Connell et al. 2012; O’Connell and Hedges 1999), animal protein estimation turns out significantly lower (ca. 50%) (Fig. 7). Furthermore, a problem at the root of such procedure is the limitation of the linear mixing model itself. In fact, concentration-dependent models fit more accurately the variability of protein content of different foods (Phillips 2012; Phillips et al. 2014; Phillips and Koch 2002): foods richer in protein (and among them, those richer in Nitrogen) will affect isotopic values proportionally more than the same amount of foods that are poorer in protein. Whereas this is particularly true between fish and plant foods, it holds significance also for diets that include cereals, animal products, and legumes. Rather than calculating specific numbers without a baseline for plants and legumes, we only propose a correction of the estimate considering the reported proportion in the quantity of nitrogen in plants (1%) vs. terrestrial meat (14%) (Phillips and Koch 2002), which can be converted in a reduction between 5 and 20% of estimates based on the standard model: the values for Scaba ’e Arriu, ECA phase, would therefore be lowered to between 50 and 65% (from 70% estimate), or between 30 and 45% (from 50% estimate), numbers more in line with averages in the majority of ethnographically recorded populations, whose proteins are primarily of vegetal origin.
Estimation of the fraction of animal protein at Scaba ‘e Arriu, phase Filigosa, based on different fractionation models, using the graphic tool employed by Fraser et al. (2013). Human and herbivore averages are actual observed numbers
(ii) Estimating the origin of protein from δ15N is complicated for the reasons discussed above. This problem is magnified if we have the goal of inferring diet and the source of animal proteins on collagen data alone due to the vast and often overlapping range of values for different species potentially consumed. Here, we argue for the usefulness of using δ13Ccol-apa spacing to infer the origin of lipids, which is deemed to partially overlap with the degree of carnivory (Katzenberg and Weber 1999; Schwarcz 2000). At Scaba ’e Arriu, there is a large δ13Ccol-apa spacing in the ECA compared to LCA (7.7 ± 0.6‰ vs. 6.0 ± 0.3‰: p = 0.00005, Mann–Whitney test, without outlier n.5949) (Fig. 6). Similar enriched bioapatite δ13C values have been interpreted elsewhere as indicative of C4 plant consumption (Fontanals-Coll et al. 2015; Waterman et al. 2016a, 2014, 2016b), and elsewhere more explicit evidence for millet consumption has been highlighted at least in the Bronze Age (Masotti et al. 2017; Tafuri et al. 2009; Varalli et al. 2021, 2016a, 2016b), but no evidence of the presence of millet has been found so far on Sardinia through the Bronze Age (Ucchesu et al. 2018, 2014, 2017), which excludes any such source to explain enriched bioapatite δ13C. Based on work on lipid residues isotopic values (Evershed et al. 1999, 2002), a difference in lipid isotopic signature was recorded between porcine and ruminant fats, which allows for their characterization and distinction. δ13C values of porcine lipids are enriched and cause δ13Ccol-apa spacing to be wider since their signature does not affect collagen as much as bioapatite. Significantly δ13C-depleted lipids in ruminants, on the contrary, reduce the δ13Ccol-apa spacing. Considering how minimal the amount of lipids present in cereals is (relative proportion of 2–3% in wheat and barley, less than 4% in bread, compared to protein and carbohydrates) vs. meat (10–15% in deer, goat, and wild boar; 30–40% and more in beef and lamb) and especially milk and cheese (30 to 50%) (USDA ARS 2022), and how in Copper Age Sardinia collection and consumption of lipid-rich wild olives and hazelnuts in large amounts is unlikely and unsubstantiated by any evidence, it is lipids from ruminants, together with a diet rich in cereal grains, the best explanation for depleted bioapatite δ13C and low δ13Ccol-apa (see discussion in Herrscher et al. 2018).
In sum, consumption of dairy and ruminant meat must be considered limited in the ECA phase, whereas it appears to have been much higher in the LCA. While the importance of hunting cannot be estimated—only one faunal assemblage has been studied to date for the LCA (Carannante and Chilardi 2015), the most likely basis for such a significant difference across the two phases is the exploitation of milk. Since lactase persistence was still rare in the Mediterranean during this period (Leonardi et al. 2012; Plantinga et al. 2012), dairy products that enhance digestibility must be inferred. Such inferences based on bioapatite δ13C highlight the limits of collagen alone in understanding significant paleoeconomic facts in agropastoral C3-based ecosystems and reveal a radical change in subsistence focus.
In terms of the overall interpretation of the factors at play in the change in economic patterns suggested by stable isotopic values, besides tradition and praxis that may have been affected by heightened mobility, more intense contacts, and possibly even immigration, climate change must be considered. A phase of increased rainfall around the mid-3rd millennium BC, preceded by a drier period around 2900–2700 BC, was identified in Tunisia (Zielhofer et al. 2004) and is followed by the well-established 4.2 kya event. On such grounds, it appears possible that during the LCA group’s life, precipitation, if more intense than during the ECA period, could have provided greater agricultural outputs and possibly more profitable exploitation of land for pasture, including the expansion of pastoralism in lands previously unproductive. δ18O values do not contradict such a connection at Scaba ‘e Arriu, which are compatible with drier conditions in the ECA (more enriched) and wetter conditions in the LCA (more depleted). δ18O variation can be correlated with many variables with different intensities based on the circulation of air masses, seasonality, and other factors beyond simple amounts of rain (Longinelli and Selmo 2003; Rozanski et al. 1992) and could also derive from using different water sources and different mobility patterns. Therefore, while the data appear compatible with the scenario outlined here, the role of climate in the contexts examined requires further testing.
Conclusions
Isotopic analyses enabled the reconstruction of several aspects of the paleoeconomy at the Scaba ’e Arriu site and their evolution between the Early and Late Copper Age, concerning changes in both cultural and climatic patterns.
The ECA phase was characterized by a subsistence based on vegetal foods, with a small portion of the diet derived from animals. Whereas the protein component can be assessed through the interval of human and animal collagen δ15N values, the overall diet reflected in bioapatite δ13C indicates a limited amount of animal products. In the LCA phase, more depleted bioapatite δ13C values are interpreted in terms of lipid signature, indicating a substantial proportion of ruminant-derived foods, which is to be read as the marker of an intensified exploitation of dairy products.
Previous data concerning climate based on several proxies, with the identification of a drier phase in the twenty-ninth to twenty-seventh century BC (e.g., Jalut et al. 2000; Zielhofer et al. 2004) and milder, wetter conditions afterward, is an essential element to understand the possible factors tied to dietary and economic dynamics. δ18O values recorded in the two phases at Scaba ‘e Arriu are compatible with this possibility.
Whereas both groups appear largely sedentary, a few individuals may have been outsiders. Furthermore, in the ECA group, there is a statistically significant difference according to sex, possibly due to differential foods or food processing between males and females.
Finally, from the limited faunal sample, it appears that domestic species cluster separately, indicating distinct management practices and ecological niches for caprines, which grazed in the marginal areas, vs. tame cattle and pigs, more strictly controlled, which supports the finding of mature bovines (likely used for traction), and morphologically domestic pigs.
Data availability
All data are provided between tables in the text and in Supplementary Materials. There are no additional data.
Code availability
Not applicable.
References
Albarella U, Manconi F, Rowley-ConwyP, Vigne, JD (2006) Pigs of Corsica and Sardinia: a biometrical re-evaluation of their status and history. In: Tecchiati U, Sala B (eds) Archaeozoological studies in honour of Alfredo Riedel. Province of Bolzano, Bolzano, pp 285–302
Ambrose SH (1990) Preparation and characterization of bone and tooth collagen for isotopic analysis. J Archaeol Sci 17:431–451
Ambrose SH (2000) Controlled diet and climate experiments on nitrogen isotope ratios of rat bone collagen, hair and muscle. In: Ambrose SH, Katzenberg MA (eds) Biogeochemical approaches to paleodietary analysis. Kluwer Academic/Plenum, New York, pp 243–259
Amundson R, Austin AT, Schuur EAG, Yoo K, Matzek V, Kendall C, Uebersax A, Brenner D, Baisden WT (2003) Global patterns of the isotopic composition of soil and plant nitrogen. Global Biogeochemical Cycles 17, 31/31–31/10
Beck J (2020) The labor of building a community: exploring the divergent trajectories of complex sites in Copper Age Iberia. Am Anthropol 122:896–901
Berna F, Matthews A, Weiner S (2004) Solubilities of bone mineral from archaeological sites: the recrystallization window. J Archaeol Sci 31:867–882
Bogaard A, Heaton THE, Poulton P, Merbach I (2007) The impact of manuring on nitrogen isotope ratios in cereals: archaeological implications for reconstruction of diet and crop management practices. J Archaeol Sci 34:335–343
Cámara Serrano JA, Spanedda L (2002) Decoración, representaciones figuradas y areas rituales en la prehistoria reciente sarda: acumulación, control del territorio y jeraquización. In: Waldren WH, Ensenyat JA (Eds.), World islands in prehistory. International Insular Investigations. V Deyá Conference of Prehistory. Archaeopress, Oxford, pp 373–393
Carannante A, Chilardi S (2015) L’alimentazione e lo sfruttamento delle risorse animali nell’area di Mogoro tra il III e il II millennio a.C. I dati archeozoologici dai siti di Puisteris e Cuccurada. In: Cicilloni R (ed) Ricerche archeologiche a Cuccurada – Mogoro (Sardegna centro-occidentale). Morlacchi, Perugia, pp 422–447
Chesson LS, Beasley MM, Bartelink EJ, Jans MME, Berg GE (2021) Using bone bioapatite yield for quality control in stable isotope analysis applications. J Archaeol Sci Rep 35:1–7
Chilleri F, Pacciani E, Fonzo O (2012) Trapanazioni craniche nella Sardegna Prenuragica. Il caso di Scaba ‘e Arriu. In: Lugliè C, Cicilloni R (eds) Atti della XLIV Riunione Scientifica IIPP, La preistoria e la Protostoria della Sardegna, Cagliari, Barumini e Sassari 23–28 novembre 2009. Istituto Italiano di Preistoria e Protostoria, pp 1011–1017
Cocco D, Usai L (1988) Un monumento preistorico nel territorio di Cornus, Ampsicora e il territorio di Cornus. Atti del II Convegno sull’archeologia romana e altomedievale nell’oristanese, Cuglieri, 22 dicembre 1985. Scorpione, Taranto, pp 13–24
Cook GD (2001) Effects of frequent fires and grazing on stable nitrogen isotope ratios of vegetation in northern Australia. Austral Ecol 26:630–636
Costa Caramé ME, Diaz-Zorita Bonilla M, García Sanjuán L, Wheatley DW (2010) The Copper Age settlement of Valencina de la Concepción (Seville, Spain): demography, metallurgy and spatial organization. Trab Prehist 67:85–117
Craig O, Biazzo M, Tafuri MA (2006) Palaeodietary records of coastal Mediterranean populations. J Mediterr Stud 16:63–77
Dalfes HN, Kukla G, Weiss H (1997) Third millennium BC climate change and Old World collapse. In: NATO (ed) NATO Advanced Research Workshop on Third millennium BC climate change and Old World collapse. Springer, Kemer, Turkey, September 19–24, 1994
Damnati B (2000) Holocene lake records in the Northern Hemisphere of Africa. J Afr Earth Sc 31:253–262
De Angelis F, Scorrano G, Martínez-Labarga C, Giustini F, Brilli M, Pacciani E, Silvestrini M, Calattini M, Volante N, Martini F, Sarti L, Rickards O (2019) Eneolithic subsistence economy in Central Italy: first dietary reconstructions through stable isotopes. Archaeol Anthropol Sci 11:4171–4186
De Angelis F, Pellegrini M, Martinez-Labarga C, Anzivino L, Scorrano G, Brilli M, Giustini F, Angle M, Calattini M, Carboni G, Catalano P, Ceccaroni E, Cosentino S, Di Giannantonio S, Isola I, Martini F, Pacciani E, Radina F, Rolfo MF, Silvestrini M, Volante N, Zanchetta G, Sarti L, Rickards O (2021) Exploring mobility in Italian Neolithic and Copper Age communities. Nat Sci Rep 11:1–14
DeNiro MJ (1985) Post-mortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature 317:806–809
Evershed RP, Dudd SN, Charters S, Mottram H, Stott AW, Raven A, van Bergen PF, Bland HA, Jones M, Bada J (1999) Lipids as carriers of anthropogenic signals from prehistory [and Discussion]. Philos Trans: Biol Sci 354:19–31
Evershed RP, Payne S, Sherratt AG, Copley MS, Coolidge J, Urem-Kotsu D, Kotsakis K, Özdoğan M, Özdoğan AE, Nieuwenhuyse O, Akkermans PMMG, Bailey D, Andeescu R-R, Campbell S, Farid S, Hodder I, Yalman N, Özbaşaran M, Bıçakcı E, Garfinkel Y, Levy T, Burton MM (2008) Earliest date for milk use in the Near East and southeastern Europe linked to cattle herding. Nature Letters 455:428–431
Evershed RP, Dudd SN, Copley MS, Berstan R, Stott AW, Mottram H, Buckley SA, Crossman Z (2002) Chemistry of Archaeological Fats. Accounts Chem Res 35;660-668
Fahy GE, Deter C, Pitfield R, Miszkiewicz JJ, Mahoney P (2017) Bone deep: variation in stable isotope ratios and histomorphometric measurements of bone remodelling within adult humans. J Archaeol Sci 87:10–16
Ferrante C, Mattone A (2004) Le comunità rurali nella Sardegna medievale (secoli XI-XV). Diritto @ Storia 3. https://www.dirittoestoria.it/
Fontanals-Coll M, Díaz-Zorita Bonilla M, Subirà ME (2015) A paleodietary study of stable isotope analysis from a high-status burial in the Copper Age: the Montelirio megalithic structure at Valencina de la Concepción-Castilleja de Guzmán, Spain. Int J Osteoarchaeol 26:447–459
Fontanals-Coll M, Subirà ME, Diaz-Zorita Bonilla M, Gibaja JF (2017) First insight into the Neolithic subsistence economy in the north-east Iberian Peninsula: paleodietary reconstruction through stable isotopes. Am J Phys Anthropol 162:36–50
Fraser RA, Bogaard A, Schäfer M, Arbogast R, Heaton THE (2013) Integrating botanical, faunal and human stable carbon and nitrogen isotope values to reconstruct land use and paleodiet at LBK Vaihingen an der Enz, Baden-Württemberg. World Archaeol 45:492–517
Furholt M (2020) Mondes sociaux et communautés de pratique: un modèle culturel polythétique pour l’Europe du IIIe millénaire avant notre ère à la lumière des débats actuels sur la migration. Préhistoires Méditerranéennes 8:1–26
Garten CT (1993) Variation in foliar 15N abundance and the availability of soil nitrogen on Walker Branch watershed. Ecology 74:2098–2113
Garvie-Lok SJ, Varney TL, Katzenberg MA (2004) Preparation of bone carbonate for stable isotope analysis: the effects of treatment time and acid concentration. J Archaeol Sci 31:763–776
Giraudi C (2004) The Upper Pleistocene to Holocene sediments on the Mediterranean island of Lampedusa (Italy). J Quat Sci 19:537–545
Greenfield HJ (2010) The Secondary Products Revolution: the past, the present and the future. World Archaeol 42:29–54
Grimes V, Pellegrini M (2013) A comparison of pretreatment methods for the analysis of phosphate oxygen isotope ratios in bioapatite. Rapid Commun Mass Spectrom 27:375–390
Hayden C (1998) Public and domestic: the social background to the development of gender in prehistoric Sardinia. In: Whitehouse RD (ed) Gender and Italian Archaeology. Institute of Archaeology, University College London
Hedges REM, Reynard LM (2007) Nitrogen isotopes and the trophic level of humans in archaeology. J Archaeol Sci 34:1240–1251
Hedges REM, Millard AR, Pike AWG (1995) Measurements and relationships of diagenetic alteration of bone from three archaeological sites. J Archaeol Sci 22:201–209
Hedges REM, Clement JC, Thomas DL, O’Connell TC (2007) Collagen turnover in the adult femoral mid-shaft: modeled from anthropogenic radiocarbon tracer measurements. Am J Phys Anthropol 133:808–816
Jalut G, Esteban Amat A, Bonnet L, Gauquelin T, Fontugne M (2000) Holocene climatic changes in the Western Mediterranean, from south-east France to south-east Spain. Palaeogeogr Palaeoclimatol Palaeoecol 160:255–290
Jans MME, Nielsen-Marsh CM, Smith CI, Collins MJ, Kars H (2004) Characterisation of microbial attack on archeological bone. J Archaeol Sci 31:87–95
Jim S, Ambrose SH, Evershed RP (2004) Stable carbon isotopic evidence for differences in the dietary origin of bone cholesterol, collagen and apatite: implications for their use in palaeodietary reconstruction. Geochim Cosmochim Acta 68:61–72
Katzenberg MA, Weber A (1999) Stable isotope ecology and palaeodiet in the Lake Baikal region of Siberia. J Archaeol Sci 26:651–659
Kellner CM, Schoeninger MJ (2007) A simple carbon isotope model for reconstructing prehistoric human diet. Am J Phys Anthropol 133:1112–1127
Koch PL (1998) Isotopic reconstruction of past continental environments. Ann Rev Earth Planet Sci 26:573–613
Koch PL, Tuross N, Fogel ML (1997) The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. J Archaeol Sci 24:417–429
Koch PL, Behrensmeyer AK, Stott AW, Tuross N, Evershed RP, Fogel ML (2000) The effects of weathering on the stable isotope composition of bones. Anc Biomol 3:117–134
Koch PL, Fox-Dobbs K, Newsome SD (2009) The isotopic ecology of fossil vertebrates and conservation paleobiology. In: Dietl GP, Flessa KW (eds) Conservation paleobiology. Using the past to manage for the future, Paleontological Society Short Course, Oct. 17th, 2009, pp 95–112
Kohn MJ (1996) Predicting animal δ18O: accounting for diet and physiological adaptation. Geochim Cosmochim Acta 60:4811–4829
Kohn MJ, Cerling TE (2002) Stable isotope compositions of biological apatite. In: Kohn MJ, Rakovan J, Hughes JM (eds) Phosphates. Geochemical, geobiological, and materials importance. Reviews in Mineralogy and Geochemistry, Washington, DC, pp 455–488
Lai L (2008) The interplay of economic, climatic and cultural change investigated through isotopic analyses of bone tissue: the case of Sardinia 4000–1900 BC. University of South Florida, Tampa
Lai L (2009) Il clima nella Sardegna preistorica e protostorica: problemi e nuove prospettive. In: Lugliè C, Cicilloni R (eds) Atti della XLIV Riunione Scientifica dell’Istituto Italiano di Preistoria e Protostoria, “La preistoria e la protostoria della Sardegna”, Cagliari, Barumini, Sassari 23–28 novembre 2009. Istituto Italiano di Preistoria e Protostoria; Università degli Studi di Cagliari, Centro Interdipartimentale per la Preistoria e Protostoria del Mediterraneo, Firenze, pp 313–324
Lai L (2016) IV Lifeways and landscapes of Late Prehistoric Sardinia: a stable isotopic view on the age of the domus de janas. In: Tanda G (ed) Nuove tecniche di documentazione e di analisi per una ricostruzione delle società dalla fine del V al III millennio a.C., vol. II. Condaghes, pp 69–102
Lai L, Fonzo O, Tykot RH, Goddard E, Hollander D (2011) Le due comunità di Scaba ’e Arriu (Siddi). Risorse alimentari nella Sardegna del III millennio a.C. indagate tramite analisi isotopiche di tessuti ossei. Studio antropologico dei reperti umani, Atti della XLIII Riunione Scientifica Istituto Italiano di Preistoria e Protostoria, Bologna (Italy), 26–29 november 2008. Istituto Italiano di Preistoria e Protostoria, Firenze, pp 401–408
Lai L, Tykot RH, Beckett JF, Fonzo O, Usai E, Goddard E, Hollander D (2014) Diet in the Sardinian Bronze Age: models, isotopic data, issues and perspectives. Préhistoires Méditerranéennes 4(2013):1–19
Lai L, Fonzo O, Usai E, Medda L, Tykot RH, Goddard E, Hollander D, Tanda G (2017) Diet and ritual in the western Mediterranean Copper Age: human and animal stable isotopes from the collective burial at S. Caterina di Pittinuri (Sardinia, Italy). In: Tomé T, Díaz-Zorita Bonilla M, Silva AM, Cunha C, Boaventura R (eds) Current approaches to collective burials in the late European prehistory. Proceedings of the XVII World UISPP Congress, Burgos (Spagna), 1–7 September 2014, pp 67–77
Le Lannou M (1941) Pâtres et paysans de la Sardaigne. Arrault et C.ie, Tours, France
Lee-Thorp JA (2008) On isotopes and old bones. Archaeometry 50:925–950
Lee-Thorp JA, Sponheimer M (2003) Three case studies used to reassess the reliability of fossil bone and enamel isotope signals for paleodietary studies. J Anthropol Archaeol 22:208–216
Leonardi M, Gerbault P, Thomas MG, Burger J (2012) The evolution of lactase persistence in Europe. A synthesis of archaeological and genetic evidence. Int Dairy J 22:88–97
Liden K, Takahashi C, Nelson DE (1995) The effects of lipids in stable carbon isotope analysis and the effects of NaOH treatment on the composition of extracted bone collagen. J Archaeol Sci 22:321–326
Lilliu G (1988) La civiltà dei Sardi. Nuova Eri, Turin
Longinelli A, Selmo E (2003) Isotopic composition of precipitation in Italy: a first overall map. J Hydrol 270:75–88
Lumaret R, Mir C, Michaud H, Raynal V (2002) Phylogeographical variation of chloroplast DNA in holm oak (Quercus ilex L.). Mol Ecol 11(11):2327–2336
Magny MJ, Haas JN (2004) A major widespread climatic change around 5300 cal. yr BP at the time of the Alpine Iceman. J Quat Sci 19:423–430
Magny MJ, Miramont C, Sivan O (2002) Assessment of the impact of climate and anthropogenic factors on Holocene Mediterranean vegetation in Europe on the basis of palaeohydrological records. Palaeogeogr Palaeoclimatol Palaeoecol 186:47–59
Magri D (1997) Middle and Late Holocene vegetation and climate changes in Peninsular Italy. In: Dalfes HN, Kukla G, Weiss H (eds) Third millennium BC climate change and Old World collapse. Springer, Berlin, Heidelberg, pp 517–530
Magri D (1999) Late Quaternary vegetation history at Lagaccione near Lago di Bolsena (central Italy). Rev Palaeobot Palynol 106:171–208
Magri D, Sadori L (1995) Late-Pleistocene and Holocene pollen stratigraphy at Lago di Vico (central Italy). Vegetation History and Archaeobotany
Masotti S, Varalli A, Goude G, Moggi-Cecchi J, Gualdi-Russo E (2017) A combined analysis of dietary habits in the Bronze Age site of Ballabio (northern Italy). Archaeol Anthropol Sci 11:1029–1047
Melis MG (2000) L’età del rame in Sardegna: origine ed evoluzione degli aspetti autoctoni. Soter, Villanova Monteleone
Moravetti A (2009) La Cultura di Monte Claro e il Vaso campaniforme, Atti della XLIV riunione scientifica dell’Istituto Italiano di Preistoria e Protostoria, ‘La preistoria e protostoria della Sardegna’, Cagliari, Barumini, Sassari 23–28 november 2009. Istituto Italiano di Preistoria e Protostoria, Firenze, pp 97–109
Murgia ME, Murgia MS, Casula E, Casu C, Fonzo O, Garau A, Paglietti G, Orrù G (2020) Dental pathology in present-day and Copper age samples. Ariesdue 12:613–614
Murgia ME, Murgia MS, Casu C, Casula E, Fonzo O, Paglietti G, Garau V, Orrù G (2021) Morphological changes in dental surfaces suggest health status and alimentary habits in the subjects belonging to the Copper Age in Sardinia Island (III millennium BC). Biointerface Research in Applied Chemistry 11:12784–12795
Nielsen-Marsh CM, Hedges REM (2000a) Patterns of diagenesis in bone I: the effects of site environments. J Archaeol Sci 27:1139–1150
Nielsen-Marsh CM, Hedges REM (2000b) Patterns of diagenesis in bone II: effects of acetic acid treatment and the removal of diagenetic CO32-. J Archaeol Sci 27:1151–1159
Nielsen-Marsh CM, Smith CI, Jans MME, Nord A, Kars H, Collins MJ (2007) Bone diagenesis in the European Holocene II: taphonomic and environmental considerations. J Archaeol Sci 34:1523–1531
O’Connell TC, Hedges REM (1999) Isotopic comparison of hair and bone: archaeological analyses. J Archaeol Sci 26:661–665
O’Connell TC, Kneale CJ, Tasevska N, Kuhnle GGC (2012) The diet-body offset in human nitrogen isotopic values: a controlled dietary study. Am J Phys Anthropol 149:426–434
Olivieri A, Sidore C, Achilli A, Angius A, Posth C, Furtwängler A, Brandini S, Capodiferro MR, Gandini F, Zoledziewska M, Pitzalis M, Maschio A, Busonero F, Lai L, Skeates R, Gradoli MG, Beckett J, Marongiu M, Mazzarello V, Marongiu P, Rubino S, Rito T, Macaulay V, Semino O, Pala M, Abecasis GR, Schlessinger D, Conde-Sousa E, Soares P, Richards MB, Cucca F, Torroni A (2017) Mitogenome diversity in Sardinians: a genetic window onto an island’s past. Mol Biol Evol 34:1230–1239
Ortu GG (1988) La transumanza nella storia della Sardegna. Mélanges de l’Ecole francaise de Rome. Moyen Age, Temps Modernes 100:821–838
Parkinson EW (2019) Body size, skeletal biomechanics and habitual behaviour: a bioarchaeological approach to social and economic change in the Neolithic and Copper Age Central Mediterranean. University of Cambridge, UK, Cambridge, UK, Department of Archaeology
Parkinson EW, Stoddart S, Stock JT (2018) “Spatial patterns in physical activity and mobility behaviour in the Neolithic and Copper Age central Mediterranean.” 87th Annual Meeting of the American Association of Physical Anthropologists. Austin: Wiley. p. Vol. 199. 2018., 87th Annual Meeting of the American Association of Physical Anthropology, Austin: Wiley, TX (USA)
Pellegrini M, Snoeck C (2016) Comparing bioapatite carbonate pre-treatments for isotopic measurements: part 2 — impact on carbon and oxygen isotope compositions. Chem Geol 420:88–96
Person A, Bocherens H, Saliège J-F, Paris F, Zeitoun V, Gérard M (1995) Early diagenetic evolution of bone phosphate: an X-ray diffractometry analysis. J Archaeol Sci 22:211–221
Phillips DL (2012) Converting isotope values to diet composition: the use of mixing models. J Mammal 93:342–352
Phillips DL, Koch PL (2002) Incorporating concentration dependence in stable isotope mixing models. Oecologia 130:114–125
Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, Semmens BX, Ward EJ (2014) Best practices for use of stable isotope mixing models in food-web studies. Can J Zool 92:823–835
Plantinga TS, Alonso S, Izagirre N, Hervella M, Fregel R, van der Meer JWM, Netea MG, de la Rúa C (2012) Low prevalence of lactase persistence in Neolithic South-West Europe. Eur J Hum Genet 20:778–782
Price TD, Grupe G, Schröter P (1998) Migration in the Bell Beaker period of central Europe. Antiquity 72:405–411
Price TD, Knipper C, Grupe G, Smrcka V (2004) Strontium isotopes and prehistoric human migration: the Bell Beaker period in central Europe. Eur J Archaeol 7:9–40
Pucéat E, Reynard B, Lécuyer C (2004) Can crystallinity be used to determine the degree of chemical alteration of biogenic apatites? Chem Geol 205:83–97
Ragucci G, Usai E (1999) Nuovi contributi allo studio della Marmilla prenuragica: la tomba di Scaba ’e Arriu, Siddi (CA). Studi Sardi 1994–1998(31):111–196
Ramrath A, Negendank JF, Sadori L (2000) Sediments from Lago di Mezzano, central Italy: a record of Lateglacial/Holocene climatic variations and anthropogenic impact. The Holocene 10:87–95
Reid DJ, Dean MC (2006) Variation in modern human enamel formation times. J Hum Evol 50:329–346
Reille M (1992) New pollen-analytical researches in Corsica: the problem of Quercus ilex L. and Erica arborea L., the origin of Pinus halepensis Miller forests. New Phytol 122:359–378
Richards MP, Schulting RJ, Hedges REM (2003) Sharp shift in diet at onset of Neolithic. Nature 425:366
Richards M (2003) The Neolithic invasion of Europe. Annual Review of Anthropology 135–162
Rimbu N, Lohmann G, Lorenz SJ, Kim JH, Schneider RR (2004) Holocene climate variability as derived from alkenone sea surface temperature and coupled ocean-atmosphere model experiments. Clim Dyn 23:215–227
Robb JE (1994) Gender contradictions, moral coalitions and inequality in prehistoric Italy. J Eur Archaeol 2:20–49
Roberts SJ, Smith CI, Millard A, Collins MJ (2002) The taphonomy of cooked bone: characterizing boiling and its physico-chemical effects. Archaeometry 44:485–494
Rowley Conwy P, Albarella U, Dobney K (2012) Distinguishing wild boar from domestic pigs in prehistory: a review of approaches and recent results. J World Prehist 25:1–44
Rozanski K, Araguás-Araguás L, Gonfiantini R (1992) Relation between long-term trends of oxygen-18 isotope composition of precipitation and climate. Science 258:981–985
Saito L, Miller WW, Johnson DW, Qualls RG, Provencher L, Carroll E, Szameitat P (2007) Fire effects on stable isotopes in a Sierran forested watershed. J Environ Qual 36:91–100
Schoeninger MJ, DeNiro MJ (1982) Carbon isotope ratios of apatite from bone cannot be used to reconstruct diets of animals. Nature 297:577–578
Schwarcz HP (2000) Some biochemical aspects of carbon isotopic paleodiet studies. In: Ambrose SH, Katzenberg MA (eds) Biogeochemical approaches to paleodietary analysis. Kluwer Academic/Plenum, New York, pp 189–209
Shemesh A (1990) Crystallinity and diagenesis of sedimentary apatites. Geochim Cosmochim Acta 54:2433–2438
Sherratt AG (1983) The secondary exploitation of animals in the Old World. World Archaeol 15:90–104
Skippington J, Veth P, Manne T, Slack M (2019) Preanalytical processing of archaeological mammal enamel apatite carbonates for stable isotope investigations: a comparative analysis of the effect of acid treatment on samples from Northwest Australia. Int J Osteoarchaeol 29:760–771
Snoeck C, Pellegrini M (2015) Comparing bioapatite carbonate pre-treatments for isotopic measurements: part 1—impact on structure and chemical composition. Chem Geol 417:394–403
Sponheimer M, Lee-Thorp JA (1999) Oxygen isotopes in enamel carbonate and their ecological significance. J Archaeol Sci 26:723–728
Tafuri MA, Craig O, Canci A (2009) Stable isotope evidence for the consumption of millet and other plants in Bronze Age Italy. Am J Phys Anthropol 139:146–153
Tieszen LL, Fagre T (1993) Effect of diet quality and composition on the isotopic composition of respiratory CO2, bone collagen, bioapatite, and soft tissues. In: Lambert JB, Grupe G (eds) Prehistoric Human Bone-Archaeology at the Molecular Level. Springer-Verlag, Berlin, pp 121–155
Trueman CN, Chenery C, Eberth DA, Spiro B (2003) Diagenetic effects on the oxygen isotope composition of bones of dinosaurs and other vertebrates recovered from terrestrial and marine sediments. Journal of the Geological Society, London 160:895–901
Trueman CN, Privat K, Field J (2008) Why do crystallinity values fail to predict the extent of diagenetic alteration of bone mineral? Palaeogeogr Palaeoclimatol Palaeoecol 266:160–168
Tuccimei P, Borsato A, Forti P, Frisia S, Paladini M, Piccini L, Salzano R, Sauro U (2003) Ricostruzione climatica dell’Olocene-Pleistocene superiore da una stalagmite del sistema carsico “Grotta del Fiume - Grotta Grande del Vento” (Gola di Frasassi, Ancona, Italia). Studi Trentini Scienze Naturali, Acta Geologica 139–151
Tykot RH (2004) Stable isotopes and diet: you are what you eat. In: Martini M, Milazzo M, Piacentini M (eds) Physics methods in archaeometry. Proceedings of the International School of Physics “Enrico Fermi” Course CLIV. Società Italiana di Fisica, Bologna, Italy, pp 433–444
Ucchesu M, Pena-Chocarro L, Sabato D, Tanda G (2014) Bronze Age subsistence in Sardinia, Italy: cultivated plants and wild resources. Veg Hist Archaeobotany 24:343–355
Ucchesu M, Sau S, Lugliè C (2017) Crop and wild plant exploitation in Italy during the Neolithic period: new data from Su Mulinu Mannu Middle Neolithic site of Sardinia. J Archaeol Sci Rep 14:1–11
Ucchesu M, Manunza MR, Sabato D (2018) Agriculture and exploitation of wild plants at Chalcolithic (4th to 3rd millennium cal BC) sites in Sardinia (Italy). Archaeol Anthropol Sci 10:1693–1702
Usai E (1998) Le sequenze culturali e i riti funerari dell’ipogeo di Scaba ’e Arriu di Siddi (Cagliari). Quaderni Della Soprintendenza Archeologica per Le Provincie Di Cagliari e Oristano 15:31–58
Usai E, Fonzo O, Mascia F (2011) L’ipogeo di Scaba ’e Arriu in Siddi: i rituali funerari e cultuali e le offerte animali, Atti della XLIII Riunione Scientifica dell’IIPP, Bologna (Italy), 26–29 november 2008. Istituto Italiano di Preistoria e Protostoria, Bologna (Italy), pp 363–371
USDA ARS (2022) FoodData Central. United States Department of Agriculture. https://fdc.nal.usda.gov/. Accessed 2023
van Klinken GJ, van der Plicht H, Hedges REM (1994) Bond 13C/15N ratios reflect (palaeo-) climatic variations. Geophys Res Lett 6:445–448
Varalli A, Moggi-Cecchi J, Dori I, Boccone S, Bortoluzzi S, Salzani P, Tafuri MA (2016a) Dietary continuity vs. discontinuity in Bronze Age Italy. The isotopic evidence from Arano di Cellore (Illasi, Versona, Italy). J Archaeol Sci Rep 7:104–113
Varalli A, Moggi-Cecchi J, Moroni A, Goude G (2016b) Dietary variability during Bronze Age in Central Italy: first results. Int J Osteoarchaeol 26:431–446
Varalli A, Desideri J, David-Elbiali M, Goude G, Honegger M, Besse M (2021) Bronze Age innovations and impact on human diet: a multi-isotopic and multi-proxy study of western Switzerland. PLoS ONE 16:1–36
Wang Y, Cerling TE (1994) A model of fossil tooth and bone diagenesis: implications for paleodiet reconstruction from stable isotopes. Palaegeography Palaeoclimatol Palaeoecol 107:281–289
Waterman AJ, Silva AM, Tykot RH (2014) Stable isotopic indicators of diet from two Late Prehistoric burial sites in Portugal: an investigation of dietary evidence of social differentiation. Open J Archaeom 2(5258):22–27
Waterman AJ, Lillios KT, Tykot RH, Kunst M (2016a) Environmental change and economic practices between the third and second millennia BC using isotope analyses of ovicaprid remains from the archeological site of Zambujal (Torres Vedras), Portugal. J Archaeol Sci Rep 5:181–189
Waterman AJ, Tykot RH, Silva AM (2016b) Stable isotope analysis of diet-based social differentiation at Late prehistoric collective burials in South-western Portugal. Archaeometry 58:131–151
Webster GS (2016) The archaeology of nuragic Sardinia. Equinox Publishing, Sheffield
Webster G, Webster M (2017) Punctuated insularity: the archaeology of 4th and 3rd millennium Sardinia. Archaeopress, Oxford
Weiner S (2010) Microarchaeology. Cambridge University Press, New York, Beyond the visible archaeological record
Weiner S, Bar-Yosef O (1990) States of preservation of bones from prehistoric sites in the Near East: a Survey. J Archaeol Sci 17:187–196
Weiss H (1997) Late third millennium abrupt climate change and social collapse in west Asia and Egypt. In: Dalfes HN, Kukla G, Weiss H (eds) Third millennium BC climate change and old world collapse. Springer, Berlin, Heidelberg, pp 711–723
White CD, Spence MW, Stuart-Williams HLQ, Schwarcz HP (1998) Oxygen isotopes and the identification of geographical origins: the Valley of Oaxaca versus the Valley of Mexico. J Archaeol Sci 25:643–655
Wilkens B (2012) Archeozoologia. Il Mediterraneo, la storia, la Sardegna, Edes, Sassari, Italy
Wright LE, Schwarcz HP (1996) Infrared and isotopic evidence for diagenesis of bone apatite at Dos Pilas, Guatemala: palaeodietary implications. J Archaeol Sci 23:933–944
Zazzo A (2014) Bone and enamel carbonate diagenesis: a radiocarbon prospective. Palaeogeogr Palaeoclimatol Palaeoecol 416:168–178
Zazzo A, Saliège J-F (2011) Radiocarbon dating of biological apatites: a review. Palaeogeogr Palaeoclimatol Palaeoecol 310:52–61
Zielhofer C, Faust D, Baena Escudero R, Diaz del Olmo F, Kadereit A, Moldenhauer K-M, Porras A (2004) Centennial-scale late-Pleistocene to mid-Holocene synthetic profile of the Medjerda Valley, northern Tunisia. The Holocene 14:851–861
Acknowledgements
The local Soprintendenza Archeologica per le province di Cagliari e Oristano and U. Badas, then director of the Civic Museum ‘Genna Maria,’ Villanovaforru, granted and facilitated access in sampling. Marina Nurchi, then director of the Laboratory of Thermodynamics of Complex Equilibria in Solution, Dept. of Chemical Sciences, University of Cagliari, made available the facility for sample processing; Maura Monduzzi enabled the collaboration that led to the performance of FTIR analyses. L. Lai also thanks A. Lai, M. Melis, B. Sisay, and S. Watson for their support.
Funding
Open access funding provided by the Carolinas Consortium. The Sardinian Autonomous Region supported L.L.’s doctoral research leading to the isotopic analyses and the bulk of data in this article. The University of Cagliari, project “Cultura visuale preistorica: le Domus de Janas”—Area 10—Scienze dell’antichità, Filologico Letterarie e Storico Artistiche—Settore Concorsuale: 10/A1—Archeologia—SSD: L-ANT/01, supported L.L’ post-doctoral project of which FTIR measurements were a component. The Municipality of Siddi (VS, Sardinia) and the National Science Foundation grant BCS-0612858 provided funding for the isotopic analyses. Sardegna Ricerche made available the hardware and software used for the FTIR measurements.
Author information
Authors and Affiliations
Contributions
LL designed research, obtained funding, selected and obtained samples, processed samples to obtain analytes, assisted in FTIR analyses, interpreted results, wrote the main manuscript text, created charts, and prepared figures. OF provided all osteological data and contextual information, assisted in obtaining samples, and contributed to interpreting the results. RT supervised the isotopic section of the research design and lab procedures, obtained funding, and contributed to interpreting the results. EG and DH managed the mass spectrometry analytical procedures and were responsible for the quality of the analytical results, contributing to interpretation. LM performed the FTIR analyses and processed the resulting data from the spectra. GT supervised FTIR analyses within the overall project. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All sampling procedures and analyses have been approved by the (then) Soprintendenza Archeologica per le Provincie di Cagliari e Oristano, Italian Ministry for Archaeological Resources.
Consent to participate
Not applicable.
Consent for publication
Publication of Figures from the Quaderni della Soprintendenza Archeologica per le Provincie di Cagliari e Oristano is permitted under the condition of citing the source: Fig. 1.c is a merger with the graphic elaboration of: “Le sequenze culturali e i riti funerari dell’ipogeo di Scaba ’e Arriu di Siddi (Cagliari),” in Quaderni della Soprintendenza Archeologica per le Province di Cagliari e Oristano, 15 (1998): 31–58, p. 51 (plan in TAV. I), and p. 53 (section of the dolmenic corridor in the antechamber and section of the chamber, in TAV. III).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
David Hollander is deceased.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lai, L., Fonzo, O., Tykot, R.H. et al. Intensified exploitation of animal products in the Mediterranean Copper Age: isotopic evidence from Scaba ’e Arriu (Sardinia). Archaeol Anthropol Sci 16, 53 (2024). https://doi.org/10.1007/s12520-024-01943-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-024-01943-4