Abstract
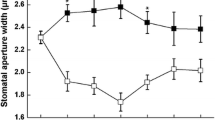
Stomatal guard cells are unique in that they have more mitochondria than chloroplasts. Several reports emphasized the importance of mitochondria as the major energy source during stomatal opening. We re-examined their role during stomatal closure. The marked sensitivity of stomata to both menadione (MD) and methyl viologen (MV) demonstrated that both mitochondria and chloroplasts helped to promote stomatal closure in Arabidopsis. As in the case of abscisic acid (ABA), a plant stress hormone, MD and MV induced stomatal closure at micromolar concentration. All three compounds generated superoxide and H2O2, as indicated by fluorescence probes, BES-So-AM and CM-H2DCFDA, respectively. Results from tiron (a superoxide scavenger) and catalase (an H2O2 scavenger) confirmed that both the superoxide and H2O2 were requisites for stomatal closure. Co-localization of the superoxide and H2O2 in mitochondria and chloroplasts using fluorescent probes revealed that exposure to MV initially triggered higher superoxide and H2O2 generation in mitochondria. In contrast, MD elevated superoxide/H2O2 levels in chloroplasts. However, with prolonged exposure, MD and MV induced ROS production in other organelles. We conclude that ROS production in mitochondria and chloroplasts leads to stomatal closure. We propose that stomatal guard cells can be good models for examining inter-organellar interactions.











Similar content being viewed by others
References
Adler J and Parmryd I 2010 Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander’s overlap coefficient. Cytometry A 77 733–742
Agurla S, Gayatri G and Raghavendra AS 2017 Signal transduction components in guard cells during stomatal closure by plant hormones and microbial elicitors; in Mechanism of plant hormone signaling under stress 1st edition (Ed.) G Pandey (USA: John Wiley & Sons) pp. 353–387
Agurla S, Gahir S, Munemasa S, et al. 2018 Mechanism of stomatal closure in plants exposed to drought and cold stress. Adv. Exp. Med. Biol. 1081 215–232
Arnaud D and Hwang I 2015 A sophisticated network of signaling pathways regulates stomatal defenses to bacterial pathogens. Mol. Plant. 8 566–581
Ashapkin VV, Kutueva LI, Aleksandrushkina NI, et al. 2020 Epigenetic mechanisms of plant adaptation to biotic and abiotic stresses. Int. J. Mol. Sci. 21 7457
Aswani V, Rajsheel P, Bapatla RB, et al. 2019 Oxidative stress induced in chloroplasts or mitochondria promotes proline accumulation in leaves of pea (Pisum sativum): another example of chloroplast-mitochondria interactions. Protoplasma 256 449–457
Bapatla RB, Saini D, Aswani V, et al. 2021 modulation of photorespiratory enzymes by oxidative and photo-oxidative stress induced by menadione in leaves of pea (Pisum sativum). Plants 10 987
Beyer WF Jr and Fridovich I 1987 Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal. Biochem. 161 559–566
Bharath P, Gahir S and Raghavendra AS 2021 Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 12 615114
Cui F, Brosché M, Shapiguzov A, He XQ, et al. 2019 Interaction of methyl viologen-induced chloroplast and mitochondrial signalling in Arabidopsis. Free Radic. Biol. Med. 134 555–566
Daloso DM, Medeiros DB, Dos Anjos L, et al. 2017 Metabolism within the specialized guard cells of plants. New Phytol. 216 1018–1033
Gallie DR and Chen Z 2019 Chloroplast-localized iron superoxide dismutases FSD2 and FSD3 are functionally distinct in Arabidopsis. PLoS One 14 e0220078
Gémes K, Kim YJ, Park KY, et al. 2016 An NADPH-oxidase/polyamine oxidase feedback loop controls oxidative burst under salinity. Plant Physiol. 172 1418–1431
Hedrich R and Shabala S 2018 Stomata in a saline world. Curr. Opin. Plant Biol. 46 87–95
Hu SH and Jinn TL 2022 Impacts of Mn, Fe, and oxidative stressors on MnSOD activation by AtMTM1 and AtMTM2 in Arabidopsis. Plants 11 619
Lawson T 2009 Guard cell photosynthesis and stomatal function. New Phytol. 181 13–34
Lawson T, Simkin AJ, Kelly G, et al. 2014 Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol. 203 1064–1081
Lehmann M, Schwarzländer M, Obata T, et al. 2009 The metabolic response of Arabidopsis roots to oxidative stress is distinct from that of heterotrophic cells in culture and highlights a complex relationship between the levels of transcripts, metabolites, and flux. Mol. Plant 2 390–406
Li M and Kim C 2021 Chloroplast ROS and stress signaling. Plant Commun. 3 100264
Lim J, Lim CW and Lee SC 2022a Core components of abscisic acid signaling and their post-translational modification. Front. Plant Sci. 13 895698
Lim SL, Flütsch S, Liu J, et al. 2022b Arabidopsis guard cell chloroplasts import cytosolic ATP for starch turnover and stomatal opening. Nat. Commun. 13 652
Lin ZF, Liu N, Lin GZ, et al. 2009 In situ localisation of superoxide generated in leaves of Alocasia macrorrhiza (L.) Shott under various stresses. J. Plant Biol. 52 340–347
Liu H, Song S, Zhang H, et al. 2022 Signaling transduction of ABA, ROS, and Ca2+ in plant stomatal closure in response to drought. Int. J. Mol. Sci. 23 14824
Livanos P, Galatis B, Quader H, et al. 2012 Disturbance of reactive oxygen species homeostasis induces atypical tubulin polymer formation and affects mitosis in root-tip cells of Triticum turgidum and Arabidopsis thaliana. Cytoskeleton 69 1–21
Martin MV, Fiol DF, Sundaresan V, et al. 2013 oiwa, a female gametophytic mutant impaired in a mitochondrial manganese-superoxide dismutase, reveals crucial roles for reactive oxygen species during embryo sac development and fertilization in Arabidopsis. Plant Cell 25 1573–1591
McAinsh MR, Clayton H, Mansfield TA, et al. 1996 Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol. 111 1031–1042
Morgan MJ, Lehmann M, Schwarzländer M, et al. 2008 Decrease in manganese superoxide dismutase leads to reduced root growth and affects tricarboxylic acid cycle flux and mitochondrial redox homeostasis. Plant Physiol. 147 101–114
Mori IC, Pinontoan R, Kawano T, et al. 2001 Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol. 42 1383–1388
Munemasa S, Oda K, Watanabe-Sugimoto M, et al. 2007 The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol. 143 1398–1407
Munns R and Millar AH 2023 Seven plant capacities to adapt to abiotic stress. J. Exp. Bot. 74 4308–4323
Myouga F, Hosoda C, Umezawa T, et al. 2008 A heterocomplex of iron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell 20 3148–3162
Obata T, Matthes A, Koszior S, et al. 2011 Alteration of mitochondrial protein complexes in relation to metabolic regulation under short-term oxidative stress in Arabidopsis seedlings. Phytochemistry 72 1081–1091
Parvathi K and Raghavendra AS 1995 Bioenergetic processes in guard cells related to stomatal function. Physiol. Plant. 93 146–154
Postiglione AE and Muday GK 2020 The role of ROS homeostasis in aba-induced guard cell signaling. Front. Plant Sci. 11 968
Postiglione AE and Muday GK 2023 Abscisic acid increases hydrogen peroxide in mitochondria to facilitate stomatal closure. Plant Physiol. 192 469–487
Qi J, Song CP, Wang B, Zhou J, et al. 2018 Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 60 805–826
Raghavendra AS 1981 Energy supply for stomatal opening in epidermal strips of Commelina benghalensis. Plant Physiol. 67 385–387
Reckmann U, Scheibe R and Raschke K 1990 Rubisco activity in guard cells compared with the solute requirement for stomatal opening. Plant Physiol. 92 246–253
Reis ADP, Carvalho RF, Costa IB, et al. 2022 Hydrogen peroxide is involved in drought stress long-distance signaling controlling early stomatal closure in tomato plants. Braz. J. Biol. 82 e267343
Rodrigues O and Shan L 2022 Stomata in a state of emergency: H2O2 is the target locked. Trends Plant Sci. 27 274–286
Saharan BS, Brar B, Duhan JS, et al. 2022 Molecular and physiological mechanisms to mitigate abiotic stress conditions in plants. Life 12 1634
Samuilov VD, Kiselevsky DB, Sinitsyn SV, et al. 2006 H2O2 intensifies CN-induced apoptosis in pea leaves. Biochemistry 71 384–394
Santelia D and Lawson T 2016 Rethinking guard cell metabolism. Plant Physiol. 172 1371–1392
Schwarzländer M, Fricker MD and Sweetlove LJ 2009 Monitoring the in vivo redox state of plant mitochondria: effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Biochim. Biophys. Acta 1787 468–475
Sierla M, Waszczak C, Vahisalu T, et al. 2016 Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 171 1569–1580
Sipari N, Lihavainen J, Shapiguzov A, et al. 2020 Primary metabolite responses to oxidative stress in early-senescing and paraquat resistant Arabidopsis thaliana rcd1 (radical-induced cell death1). Front. Plant Sci. 11 194
Sun YL, Zhu HZ, Zhou J, et al. 1999 Menadione-induced apoptosis and the degradation of lamin-like proteins in tobacco protoplasts. Cell Mol. Life Sci. 55 310–316
Suzuki N 2023 Fine tuning of ROS, redox and energy regulatory systems associated with the functions of chloroplasts and mitochondria in plants under heat stress. Int. J. Mol. Sci. 24 1356
Suzuki N, Koussevitzky S, Mittler R, et al. 2012 ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 35 259–270
Sweetlove LJ, Heazlewood JL, Herald V, et al. 2002 The impact of oxidative stress on Arabidopsis mitochondria. Plant J. 32 891–904
Ugalde JM, Fuchs P, Nietzel T, et al. 2021 Chloroplast-derived photo-oxidative stress causes changes in H2O2 and EGSH in other subcellular compartments. Plant Physiol. 186 125–141
Vani T and Raghavendra AS 1989 Tetrazolium reduction by guard cells in abaxial epidermis of Vicia faba: Blue light stimulation of a plasmalemma redox system. Plant Physiol. 90 59–62
Vavasseur A and Raghavendra AS 2005 Guard cell metabolism and CO2 sensing. New Phytol. 165 665–682
Wang B, Ding H, Chen Q, et al. 2019 Enhanced tolerance to methyl viologen-mediated oxidative stress via atgr2 expression from chloroplast genome. Front. Plant Sci. 10 1178
Willmer CM and Fricker M 1996 Stomata (London. UK: Chapman and Hall)
Yang J, Li C, Kong D, et al. 2020 Light-mediated signaling and metabolic changes coordinate stomatal opening and closure. Front. Plant Sci. 11 601478
Zinchuk V, Zinchuk O and Okada T 2007 Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena. Acta Histochem. Cytochem. 40 101–111
Acknowledgements
Our work was supported by research grants to ASR from the Institute of Eminence (IoE) Chair Professor and partly from DBT-SAHAJ/BUILDER (BT/INF/22/SP41176/2020). SG holds a Senior Research Fellowship from the University Grants Commission. PB was a BBL fellowship holder from our University. DS is an IoE-post doctoral fellow at our University. We appreciate the help of Mr. Dashrath in confocal microscopy.
Author information
Authors and Affiliations
Contributions
ASR designed the work. SG, PB, and DS performed the experiments. ASR and GP supervised the experiments and analyzed the results. SG and ASR wrote the first draft. All the authors read and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest
Additional information
Corresponding editor: Manoj Prasad
This article is part of the Topical Collection: Plant Mitochondria: Properties and Interactions with Other Organelles.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gahir, S., Bharath, P., Saini, D. et al. Role of mitochondria and chloroplasts during stomatal closure: Subcellular location of superoxide and H2O2 production in guard cells of Arabidopsis thaliana. J Biosci 49, 44 (2024). https://doi.org/10.1007/s12038-023-00418-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-023-00418-3