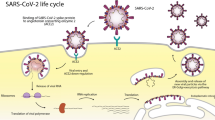
Abstract
AXL is the gene that encodes the Anexelekto (AXL) receptor tyrosine kinase that demonstrates significant roles in various cellular processes, including cell growth, survival, and migration. Anexelekto is a Greek word meaning excessive and uncontrolled, semantically implying the crucial involvement of AXL in cancer and immune biology, and in promoting cancer metastasis. AXL overexpression appears to drive epithelial to mesenchymal transition, tumor angiogenesis, decreased antitumor immune response, and resistance to therapeutic agents. Recently, AXL has been reported to play important roles in several viral infections, including SARS-CoV-2. We have previously outlined the importance of microRNAs (miRNAs, miRs) and especially miR-155 in SARS-CoV-2 pathophysiology through regulation of the Renin–Angiotensin Aldosterone System (RAAS) and influence on several aspects of host innate immunity. MiRNAs are negative regulators of gene expression, decreasing the stability of target RNAs or limiting their translation and, enthrallingly, miR-155 is also involved in AXL homeostasis—both endogenously and pharmaceutically using repurposed drugs (e.g., metformin)—highlighting thrifty evolutionary host innate immunity mechanisms that successfully can thwart viral entry and replication. Cancer, infections, and immune system disturbances will increasingly involve miRNA diagnostics and therapeutics in the future.

Similar content being viewed by others
Data availability
All data analysed during this narrative review are included in this published article.
References
Worldodometer. https://www.worldometers.info/coronavirus/. Accessed 2023–11–22 2023.
Zhang Q, Xiang R, Huo S, Zhou Y, Jiang S, Wang Q, et al. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct Target Ther. 2021;6(1):233. https://doi.org/10.1038/s41392-021-00653-w.
Lei M, Ma Y, Chen H, Huang P, Sun J, Wang X, et al. Emerging SARS-CoV-2 variants of concern potentially expand host range to chickens: insights from AXL, NRP1 and ACE2 receptors. Virol J. 2023;20(1):196. https://doi.org/10.1186/s12985-023-02123-x.
Papadopoulos KI, Papadopoulou A, Aw TC. A protective erythropoietin evolutionary landscape, NLRP3 inflammasome regulation, and multisystem inflammatory syndrome in children. Hum Cell. 2023;36(1):26–40. https://doi.org/10.1007/s13577-022-00819-w.
Papadopoulos KI, Papadopoulou A, Aw TC. Beauty and the beast: host microRNA-155 versus SARS-CoV-2. Hum Cell. 2023;36(3):908–22. https://doi.org/10.1007/s13577-023-00867-w.
Papadopoulos KI, Sutheesophon W, Manipalviratn S, Aw TC. Age and genotype dependent erythropoietin protection in COVID-19. World J Stem Cells. 2021;13(10):1513–29. https://doi.org/10.4252/wjsc.v13.i10.1513.
Wang S, Qiu Z, Hou Y, Deng X, Xu W, Zheng T, et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021;31(2):126–40. https://doi.org/10.1038/s41422-020-00460-y.
Ghosh RS. TAM receptors: A phosphatidylserine receptor family and its implications in viral infections. Int Rev Cell Mol Biol. 2020;357:81–122. https://doi.org/10.1016/bs.ircmb.2020.09.003.
Zhu C, Wei Y, Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. 2019;18(1):153. https://doi.org/10.1186/s12943-019-1090-3.
Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8(5):327–36. https://doi.org/10.1038/nri2303.
Goyette MA, Côté JF. AXL receptor tyrosine kinase as a promising therapeutic target directing multiple aspects of cancer progression and metastasis. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14030466.
Perera-Lecoin M, Meertens L, Carnec X, Amara A. Flavivirus entry receptors: an update. Viruses. 2013;6(1):69–88. https://doi.org/10.3390/v6010069.
Bhattacharyya S, Zagórska A, Lew ED, Shrestha B, Rothlin CV, Naughton J, et al. Enveloped viruses disable innate immune responses in dendritic cells by direct activation of TAM receptors. Cell Host Microbe. 2013;14(2):136–47. https://doi.org/10.1016/j.chom.2013.07.005.
Gay CM, Balaji K, Byers LA. Giving AXL the axe: targeting AXL in human malignancy. Br J Cancer. 2017;116(4):415–23. https://doi.org/10.1038/bjc.2016.428.
Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35(4):445–55. https://doi.org/10.1016/j.immuni.2011.09.004.
Shimojima M, Ikeda Y, Kawaoka Y. The mechanism of Axl-mediated Ebola virus infection. J Infect Dis. 2007;196(Suppl 2):S259–63. https://doi.org/10.1086/520594.
Shimojima M, Takada A, Ebihara H, Neumann G, Fujioka K, Irimura T, et al. Tyro3 family-mediated cell entry of Ebola and Marburg viruses. J Virol. 2006;80(20):10109–16. https://doi.org/10.1128/jvi.01157-06.
Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression analysis highlights axl as a candidate zika virus entry receptor in neural stem cells. Cell Stem Cell. 2016;18(5):591–6. https://doi.org/10.1016/j.stem.2016.03.012.
Bohan D, Van Ert H, Ruggio N, Rogers KJ, Badreddine M, Aguilar Briseño JA, et al. Phosphatidylserine receptors enhance SARS-CoV-2 infection. PLoS Pathog. 2021;17(11): e1009743. https://doi.org/10.1371/journal.ppat.1009743.
Meertens L, Carnec X, Lecoin MP, Ramdasi R, Guivel-Benhassine F, Lew E, et al. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe. 2012;12(4):544–57. https://doi.org/10.1016/j.chom.2012.08.009.
Ning K, Zou W, Xu P, Cheng F, Zhang EY, Zhang-Chen A, et al. Identification of AXL as a co-receptor for human parvovirus B19 infection of human erythroid progenitors. Sci Adv. 2023;9(2):eade0869. https://doi.org/10.1126/sciadv.ade0869.
Rizzi M, Tonello S, D’Onghia D, Sainaghi PP. Gas6/TAM Axis Involvement in modulating inflammation and fibrosis in covid-19 patients. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms24020951.
Boytz R, Słabicki M, Ramaswamy S, Patten JJ, Zou C, Meng C, et al. Anti-SARS-CoV-2 activity of targeted kinase inhibitors: repurposing clinically available drugs for COVID-19 therapy. J Med Virol. 2023;95(1): e28157. https://doi.org/10.1002/jmv.28157.
Papadopoulos KI, Wattanaarsakit P, Prasongchean W, Narain R. 10 - Gene therapies in clinical trials. In: Narain R, editor. polymers and nanomaterials for gene therapy. Woodhead Publishing; 2016. p. 231–56.
Plotnikova O, Baranova A, Skoblov M. Comprehensive analysis of human microrna-mrna interactome. Front Genet. 2019;10:933. https://doi.org/10.3389/fgene.2019.00933.
Mishra R, Kumar A, Ingle H, Kumar H. The interplay between viral-derived mirnas and host immunity during infection. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2019.03079.
Papadopoulos KI, Papadopoulou A, Aw TC. MicroRNA-155 mediates endogenous angiotensin II type 1 receptor regulation: implications for innovative type 2 diabetes mellitus management. World J Diabetes. 2023;14(9):1334–40. https://doi.org/10.4239/wjd.v14.i9.1334.
Masaki T, Arend KC, Li Y, Yamane D, McGivern DR, Kato T, et al. miR-122 stimulates hepatitis C virus RNA synthesis by altering the balance of viral RNAs engaged in replication versus translation. Cell Host Microbe. 2015;17(2):217–28. https://doi.org/10.1016/j.chom.2014.12.014.
Schult P, Roth H, Adams RL, Mas C, Imbert L, Orlik C, et al. microRNA-122 amplifies hepatitis C virus translation by shaping the structure of the internal ribosomal entry site. Nat Commun. 2018;9(1):2613. https://doi.org/10.1038/s41467-018-05053-3.
Kalkusova K, Taborska P, Stakheev D, Smrz D. The role of mir-155 in antitumor immunity. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14215414.
Chatzopoulou F, Kyritsis KA, Papagiannopoulos CI, Galatou E, Mittas N, Theodoroula NF, et al. Dissecting mirna-gene networks to map clinical utility roads of pharmacogenomics-guided therapeutic decisions in cardiovascular precision medicine. Cells. 2022;11(4):607.
Cortez MA, Anfossi S, Ramapriyan R, Menon H, Atalar SC, Aliru M, et al. Role of miRNAs in immune responses and immunotherapy in cancer. Genes Chromosom Cancer. 2019;58(4):244–53. https://doi.org/10.1002/gcc.22725.
Hou Y, Wang J, Wang X, Shi S, Wang W, Chen Z. Appraising microrna-155 as a noninvasive diagnostic biomarker for cancer detection: a meta-analysis. Medicine (Baltim). 2016;95(2): e2450. https://doi.org/10.1097/md.0000000000002450.
Dudda Jan C, Salaun B, Ji Y, Palmer Douglas C, Monnot Gwennaelle C, Merck E, et al. MicroRNA-155 is required for effector CD8<sup>+</sup> T Cell responses to virus infection and cancer. Immunity. 2013;38(4):742–53. https://doi.org/10.1016/j.immuni.2012.12.006.
Jafarzadeh A, Naseri A, Shojaie L, Nemati M, Jafarzadeh S, Bannazadeh Baghi H, et al. MicroRNA-155 and antiviral immune responses. Int Immunopharmacol. 2021;101(Pt A): 108188. https://doi.org/10.1016/j.intimp.2021.108188.
Papadopoulos KI, Sutheesophon W, Manipalviratn S, Aw TC. A southeast asian perspective on the COVID-19 Pandemic: Hemoglobin E (HbE)-trait confers resistance against COVID-19. Med Sci Monit Basic Res. 2021;27: e929207. https://doi.org/10.12659/MSMBR.929207.
Rai KR, Liao Y, Cai M, Qiu H, Wen F, Peng M, et al. MIR155HG plays a bivalent role in regulating innate antiviral immunity by encoding long noncoding RNA-155 and microRNA-155–5p. MBio. 2022;13(6):e0251022. https://doi.org/10.1128/mbio.02510-22.
de Miguel-Pérez D, Bayarri-Lara CI, Ortega FG, Russo A, Moyano Rodriguez MJ, Alvarez-Cubero MJ, et al. Post-surgery circulating tumor cells and AXL overexpression as new poor prognostic biomarkers in resected lung adenocarcinoma. Cancers (Basel). 2019. https://doi.org/10.3390/cancers11111750.
Kurowska-Stolarska M, Ballantine L, Stolarski B, Hunter J, Hueber A, Gracie A, et al. miR155 and miR34a regulate proinflammatory cytokine production by human monocytes. Ann of The Rheum Dis - ANN RHEUM DIS. 2010. https://doi.org/10.1136/ard.2010.129619g.
Yin Z, Herron S, Silveira S, Kleemann K, Gauthier C, Mallah D, et al. Identification of a protective microglial state mediated by miR-155 and interferon-γ signaling in a mouse model of Alzheimer’s disease. Nat Neurosci. 2023;26(7):1196–207. https://doi.org/10.1038/s41593-023-01355-y.
Sun CY, Zhang XP, Liu F, Wang W. Orchestration of lincRNA-p21 and miR-155 in modulating the adaptive dynamics of HIF-1α. Front Genet. 2020;11:871. https://doi.org/10.3389/fgene.2020.00871.
Liu C, Zhang J-W, Hu L, Song Y-C, Zhou L, Fan Y, et al. Activation of the AT1R/HIF-1a/ACE axis mediates angiotensin ii-induced VEGF synthesis in mesenchymal stem cells. Biomed Res Int. 2014;2014: 627380. https://doi.org/10.1155/2014/627380.
Shao S, Zhao L, An G, Zhang L, Jing X, Luo M, et al. Metformin suppresses HIF-1α expression in cancer-associated fibroblasts to prevent tumor-stromal cross talk in breast cancer. Faseb j. 2020;34(8):10860–70. https://doi.org/10.1096/fj.202000951RR.
Hart PC, Kenny HA, Grassl N, Watters KM, Litchfield LM, Coscia F, et al. Mesothelial cell HIF1α expression is metabolically downregulated by metformin to prevent oncogenic tumor-stromal crosstalk. Cell Rep. 2019;29(12):4086-98.e6. https://doi.org/10.1016/j.celrep.2019.11.079.
Ye J, Chen K, Qi L, Li R, Tang H, Zhou C, et al. Metformin suppresses hypoxia-induced migration via the HIF-1α/VEGF pathway in gallbladder cancer in vitro and in vivo. Oncol Rep. 2018;40(6):3501–10. https://doi.org/10.3892/or.2018.6751.
Zhang WN, Li XP, Wang PF, Zhu L, Xiao XH, Dai YJ. Comprehensive analysis of the novel omicron receptor AXL in cancers. Comput Struct Biotechnol J. 2022;20:3304–12. https://doi.org/10.1016/j.csbj.2022.06.051.
Patel B, Chapman SA, Neumann JT, Visaria A, Ogungbe O, Wen S, et al. Outcomes of patients with active cancers and pre-existing cardiovascular diseases infected with SARS-CoV-2. Cardiooncology. 2023;9(1):36. https://doi.org/10.1186/s40959-023-00187-w.
Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881–92. https://doi.org/10.1182/blood.2020008824.
San Martín-López JV, Mesa N, Bernal-Bello D, Morales-Ortega A, Rivilla M, Guerrero M, et al. Seven epidemic waves of COVID-19 in a hospital in madrid: analysis of severity and associated factors. Viruses. 2023. https://doi.org/10.3390/v15091839.
Ivanov N, Krastev B, Miteva DG, Batselova H, Alexandrova R, Velikova T. Effectiveness and safety of COVID-19 vaccines in patients with oncological diseases: State-of-the-art. World J Clin Oncol. 2023;14(9):343–56. https://doi.org/10.5306/wjco.v14.i9.343.
Harmonizome. 2023. https://maayanlab.cloud/Harmonizome/gene_set/hsa-miR-155-5p/MiRTarBase+microRNA+Targets. Accessed 2023–10–17 2023.
Hsu SD, Tseng YT, Shrestha S, Lin YL, Khaleel A, Chou CH, miRTarBase update, et al. An information resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2014;2014(42 (Database issue):D):78–85. https://doi.org/10.1093/nar/gkt1266.
Taylor CT, Scholz CC. The effect of HIF on metabolism and immunity. Nat Rev Nephrol. 2022;18(9):573–87. https://doi.org/10.1038/s41581-022-00587-8.
Kunej T. Integrative map of HIF-1A regulatory elements and variations. Genes (Basel). 2021. https://doi.org/10.3390/genes12101526.
Zhang R, Wu Y, Zhao M, Liu C, Zhou L, Shen S, et al. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;297(4):L631–40. https://doi.org/10.1152/ajplung.90415.2008.
Serocki M, Bartoszewska S, Janaszak-Jasiecka A, Ochocka RJ, Collawn JF, Bartoszewski R. miRNAs regulate the HIF switch during hypoxia: a novel therapeutic target. Angiogenesis. 2018;21(2):183–202. https://doi.org/10.1007/s10456-018-9600-2.
He B, Hu M, Liang Z, Ma Q, Zi Y, Dong Z, et al. Efficacy of shenqi pollen capsules for high-altitude deacclimatization syndrome via suppression of the reoxygenation injury and inflammatory response. J Immunol Res. 2019;2019:4521231. https://doi.org/10.1155/2019/4521231.
Yang D, Wang J, Xiao M, Zhou T, Shi X. Role of Mir-155 in controlling HIF-1α level and promoting endothelial cell maturation. Sci Rep. 2016;6:35316. https://doi.org/10.1038/srep35316.
Jelkmann W. Molecular biology of erythropoietin. Intern Med. 2004;43(8):649–59. https://doi.org/10.2169/internalmedicine.43.649.
Castillo-Uribe VA, Cucho-Vásquez BM, Contreras-León ZL, Accinelli RA, Huayanay-Falconi L, Chu-Rivera FR. Effect of altitude on COVID-19 mortality rate and case incidence in Peru, the country with the highest cumulative mortality rate worldwide. J Public Health. 2023. https://doi.org/10.1007/s10389-023-02104-y.
Rankin EB, Fuh KC, Castellini L, Viswanathan K, Finger EC, Diep AN, et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci U S A. 2014;111(37):13373–8. https://doi.org/10.1073/pnas.1404848111.
Goyette MA, Elkholi IE, Apcher C, Kuasne H, Rothlin CV, Muller WJ, et al. Targeting Axl favors an antitumorigenic microenvironment that enhances immunotherapy responses by decreasing Hif-1α levels. Proc Natl Acad Sci U S A. 2021. https://doi.org/10.1073/pnas.2023868118.
Yang LG, Cao MZ, Zhang J, Li XY, Sun QL. LncRNA XIST modulates HIF-1A/AXL signaling pathway by inhibiting miR-93-5p in colorectal cancer. Mol Genet Genomic Med. 2020;8(4): e1112. https://doi.org/10.1002/mgg3.1112.
Triggle CR, Mohammed I, Bshesh K, Marei I, Ye K, Ding H, et al. Metformin: is it a drug for all reasons and diseases? Metabolism. 2022;133: 155223. https://doi.org/10.1016/j.metabol.2022.155223.
Foretz M, Guigas B, Viollet B. Metformin: update on mechanisms of action and repurposing potential. Nat Rev Endocrinol. 2023;19(8):460–76. https://doi.org/10.1038/s41574-023-00833-4.
Papadopoulos KI, Sutheesophon W, Aw TC. Too hard to die: exercise training mediates specific and immediate SARS-CoV-2 protection. World J Virol. 2022;11(2):98–103. https://doi.org/10.5501/wjv.v11.i2.98.
Saito T, Itoh M, Tohda S. Metformin suppresses the growth of leukemia cells partly through downregulation of AXL receptor tyrosine kinase. Leuk Res. 2020;94: 106383. https://doi.org/10.1016/j.leukres.2020.106383.
Gayatri MB, Kancha RK, Patchva D, Velugonda N, Gundeti S, Reddy ABM. Metformin exerts antileukemic effects by modulating lactate metabolism and overcomes imatinib resistance in chronic myelogenous leukemia. FEBS J. 2023;290(18):4480–95. https://doi.org/10.1111/febs.16818.
Hong J, Maacha S, Pidkovka N, Bates A, Salaria SN, Washington MK, et al. AXL promotes metformin-induced apoptosis through mediation of autophagy by activating ROS-AMPK-ULK1 signaling in human esophageal adenocarcinoma. Front Oncol. 2022;12: 903874. https://doi.org/10.3389/fonc.2022.903874.
Kim NY, Lee HY, Lee C. Metformin targets Axl and Tyro3 receptor tyrosine kinases to inhibit cell proliferation and overcome chemoresistance in ovarian cancer cells. Int J Oncol. 2015;47(1):353–60. https://doi.org/10.3892/ijo.2015.3004.
Tossetta G. Metformin improves ovarian cancer sensitivity to paclitaxel and platinum-based drugs: a review of in vitro findings. Int J Mol Sci. 2022;23(21):12893.
Shi H, Sun Y, He M, Yang X, Hamada M, Fukunaga T, et al. Targeting the TR4 nuclear receptor-mediated lncTASR/AXL signaling with tretinoin increases the sunitinib sensitivity to better suppress the RCC progression. Oncog. 2020;39(3):530–45. https://doi.org/10.1038/s41388-019-0962-8.
Bansal N, Mishra PJ, Stein M, DiPaola RS, Bertino JR. Axl receptor tyrosine kinase is up-regulated in metformin resistant prostate cancer cells. Oncotarget. 2015;6(17):15321–31. https://doi.org/10.18632/oncotarget.4148.
Fujimori T, Kato K, Fujihara S, Iwama H, Yamashita T, Kobayashi K, et al. Antitumor effect of metformin on cholangiocarcinoma: In vitro and in vivo studies. Oncol Rep. 2015;34(6):2987–96. https://doi.org/10.3892/or.2015.4284.
Atici Y, Baskol G, Bayram F. A new approach for the pleiotropic effect of metformin use in type 2 diabetes mellitus. Turk J of Biochem. 2022;47(6):775–82. https://doi.org/10.1515/tjb-2022-0013.
Alimoradi N, Firouzabadi N, Fatehi R. How metformin affects various malignancies by means of microRNAs: a brief review. Cancer Cell Int. 2021;21(1):207. https://doi.org/10.1186/s12935-021-01921-z.
Petrovic AR, Jovanovic IP, Jurisevic MM, Jovanovic MZ, Jovanovic MM, Pavlovic SP, et al. Metformin promotes antitumor activity of NK cells via overexpression of miRNA-150 and miRNA-155. Am J Transl Res. 2023;15(4):2727–37.
Xia W, Qi X, Li M, Wu Y, Sun L, Fan X, et al. Metformin promotes anticancer activity of NK cells in a p38 MAPK dependent manner. Oncoimmunology. 2021;10(1):1995999. https://doi.org/10.1080/2162402x.2021.1995999.
Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, et al. miR-155 regulates IFN-γ production in natural killer cells. Blood. 2012;119(15):3478–85. https://doi.org/10.1182/blood-2011-12-398099.
Gou L, Liu G, Ma R, Regmi A, Zeng T, Zheng J, et al. High fat-induced inflammation in vascular endothelium can be improved by Abelmoschus esculentus and metformin via increasing the expressions of miR-146a and miR-155. Nutr Metab (Lond). 2020;17:35. https://doi.org/10.1186/s12986-020-00459-7.
Amara VR, Surapaneni SK, Tikoo K. Metformin attenuates cardiovascular and renal injury in uninephrectomized rats on DOCA-salt: Involvement of AMPK and miRNAs in cardioprotection. Toxicol Appl Pharmacol. 2019;362:95–104. https://doi.org/10.1016/j.taap.2018.10.004.
Petrelli F, Grappasonni I, Nguyen CTT, Tesauro M, Pantanetti P, Xhafa S, et al. Metformin and Covid-19: a systematic review of systematic reviews with meta-analysis. Acta Biomed. 2023;94(S3): e2023138. https://doi.org/10.23750/abm.v94iS3.14405.
Martin DE, Cadar AN, Bartley JM. Old drug, new tricks: the utility of metformin in infection and vaccination responses to influenza and SARS-CoV-2 in older adults. Front Aging. 2023;4:1272336. https://doi.org/10.3389/fragi.2023.1272336.
Behrooz M, Hajjarzadeh S, Kahroba H, Ostadrahimi A, Bastami M. Expression pattern of miR-193a, miR122, miR155, miR-15a, and miR146a in peripheral blood mononuclear cells of children with obesity and their relation to some metabolic and inflammatory biomarkers. BMC Pediatr. 2023;23(1):95. https://doi.org/10.1186/s12887-023-03867-9.
Dinesen S, El-Faitarouni A, Frisk NLS, Sørensen AE, Dalgaard LT. Circulating microRNA as biomarkers for gestational diabetes mellitus-a systematic review and meta-analysis. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms24076186.
Bramante CT, Ingraham NE, Murray TA, Marmor S, Hovertsen S, Gronski J, et al. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. Lancet Healthy Longev. 2021;2(1):e34–41. https://doi.org/10.1016/s2666-7568(20)30033-7.
Postler TS, Peng V, Bhatt DM, Ghosh S. Metformin selectively dampens the acute inflammatory response through an AMPK-dependent mechanism. Sci Rep. 2021;11(1):18721. https://doi.org/10.1038/s41598-021-97441-x.
Hsiao FC, Lin YF, Hsieh PS, Chu NF, Shieh YS, Hsieh CH, et al. Circulating growth arrest-specific 6 protein is associated with adiposity, systemic inflammation, and insulin resistance among overweight and obese adolescents. J Clin Endocrinol Metab. 2013;98(2):E267–74. https://doi.org/10.1210/jc.2012-3179.
McShane L, Tabas I, Lemke G, Kurowska-Stolarska M, Maffia P. TAM receptors in cardiovascular disease. Cardiovasc Res. 2019;115(8):1286–95. https://doi.org/10.1093/cvr/cvz100.
Lee CH, Changchien CY, Hung YJ. Targeting inflammation in type 2 diabetes by antibody-mediated Tyro-3, Axl. Mer receptor activation J Diabetes Investig. 2015;6(5):491–4. https://doi.org/10.1111/jdi.12351.
Wium M, Paccez JD, Zerbini LF. The dual role of TAM receptors in autoimmune diseases and cancer: an overview. Cells. 2018. https://doi.org/10.3390/cells7100166.
Funding
No financial support has been received in any form.
Author information
Authors and Affiliations
Contributions
Dr Konstantinos I. Papadopoulos conceived and conceptualized the argumentation, designed the layout, drafted the initial manuscript, and reviewed and revised the manuscript. Dr Alexandra Papadopoulou performed the literature search, extracted vital information, contributed to the synthesis of the work, and reviewed and revised the manuscript. Dr Tar-Choon Aw coordinated and supervised literature search, made substantial and direct intellectual contributions and critically reviewed the manuscript for important intellectual content. All authors approved the submitted final manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Papadopoulos, K.I., Papadopoulou, A. & Aw, T.C. Anexelekto (AXL) no more: microRNA-155 (miR-155) controls the “Uncontrolled” in SARS-CoV-2. Human Cell 37, 582–592 (2024). https://doi.org/10.1007/s13577-024-01041-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-024-01041-6