Abstract
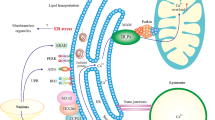
Alzheimer’s disease (AD) accounts for a major statistic among the class of neurodegenerative diseases. A number of mechanisms have been identified in its pathogenesis and progression which include the amyloid beta (Aβ) aggregation, hyperphosphorylation of tau protein, oxidative stress, endoplasmic reticulum (ER) stress and apoptosis. These processes are interconnected and contribute significantly to the loss of neurons, brain mass and consequential memory loss and other cognitive difficulties. Oxidative stress in AD appears to be caused by excess of oxygen free radicals and extracellular Aβ deposits that cause local inflammatory processes and activate microglia, another possible source of reactive oxygen species (ROS). ER Stress describes the accumulation of misfolded and unfolded proteins as a result of physiological and pathological stimuli including high protein demand, toxins, inflammatory cytokines, and mutant protein expression that disturbs ER homeostasis. When compared to age-matched controls, postmortem brain tissues from AD patients showed elevated levels of ER stress markers, such as PERK, eIF2α, IRE1α, the chaperone Grp78, and the downstream mediator of cell death CHOP. Apoptosis is in charge of eliminating unnecessary and undesired cells to maintain good health. However, it has been demonstrated that a malfunctioning apoptotic pathway is a major factor in the development of certain neurological and immunological problems and diseases in people, including neurodegenerative diseases. This article highlights and discussed some of the experimentally established mechanisms through which these processes lead to the development as well as the exacerbation of AD.


Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- ROS:
-
Reactive Oxygen Species
- DNA:
-
Deoxyribonucleic acid
- ATP:
-
Adenosine Triphosphate
- PUFA:
-
Polyunsaturated fatty acid
- RNA:
-
Ribonucleic acid
- CSF:
-
Cerebrospinal fluid
- APP:
-
Amyloid processing protein
- Aβ:
-
Amyliod beta
- NFT:
-
Neurofibrilliary tangles
- TBARS:
-
Thiobarbituric acid reactive substance
- HNE:
-
Trans-4-hydroxy-2-nonenal
- F2-IsoPs:
-
F2-isoprostanes
- MCI:
-
Mild cognitive impairment
- CSF:
-
Cerebrospinal fluid
- 3-NT:
-
3nitrotyrosine
- SOD:
-
Superoxide dismutase
- ABAD:
-
Aβ-binding alcohol dehydrogenase
- NMDAR:
-
N-methyl-D-aspartate receptors
- AMPA:
-
α-amino-3-hydroxy-5-methyl-4isoxa-zolepropionic acid
- ER:
-
Endoplasmic reticulum
- UPR:
-
Unfolded Protein Response
- VDAC:
-
Voltage-dependent anion channel
- LDH:
-
Lactate dehydrogenase
- MDH:
-
Malate dehydrogenase
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- BCL-2:
-
B Cell lymphoma2
- MAPK:
-
Mitogen activated protein kinase
- BAD:
-
BCL2 associated agonist of cell death
- APP/PS1:
-
amyloid precursor protein/presenilin 1
- 8OHdG:
-
8-hydroxy-2’-deoxyguanosine
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- IRE1:
-
Inositol-requiring enzyme-1
- PERK:
-
Protein kinase RNA-like endoplasmic reticulum kinase
- ATF6:
-
activating transcription factor 6
- XBP1:
-
X-box binding protein 1
- ASK1:
-
Apoptosis signal regulating kinase 1
- JNK:
-
c-Jun N-terminal protein kinase
- TNF:
-
Tumor necrosis factor
- TRAF2:
-
TNF Receptor Associated Factor 2
- eIF2α:
-
eukaryotic initiation factor 2
- GADD34:
-
growth arrest and DNA damage-inducible protein
- Atf-4:
-
Activating transcription factor 4
- uORF:
-
upstream open reading frames
- BZIP:
-
basic leucine-zipper (bZIP) proteins
- CHOP:
-
C/EBP homologous protein
- ATF6:
-
Activating transcription factor 6
- ERAD:
-
Endoplasmic reticulum–associated protein degradation
- BACE1:
-
β-site APP cleaving enzyme-1
- CREB:
-
cAMP response element-binding protein
- STZ:
-
Streptozotocin
- OHC:
-
Organotypic hippocampal slice cultures
- Bax:
-
Bcl-2-associated X protein
- PAR-4:
-
Prostate apoptosis response
- CARD:
-
Caspase recruitment domain
- DED:
-
Death effector domain
- SMAC/direct:
-
IAP binding protein with low pI (DIABLO),
- HTRA:
-
high temperature requirement A (serine peptidase 2)
- APAF-1:
-
Apoptotic protease-activating factor 1
- CAD:
-
Caspase-activated DNase
- AIF:
-
Apoptosis-inducing factor
- CTL:
-
Cytotoxic T lymphocytes
- BAK:
-
Bcl-2 antagonist killer 1
- BIM:
-
Bcl-2 Interacting Mediator of cell death
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
References
Breijyeh, Z., & Karaman, R. (2020). Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules, 25, 24 https://doi.org/10.3390/MOLECULES25245789.
López Ricardo, Y., Reyes Zamora, M. C., Perodin Hernández, J., & Rodríguez Martínez, C. (2022). Prevalence of Alzheimer′s disease in rural and urban areas in Cuba and factors influencing on its occurrence: epidemiological cross-sectional protocol. BMJ Open, 12(11), e052704. https://doi.org/10.1136/bmjopen-2021-052704.
McGirr, S., Venegas, C., & Swaminathan, A. (2020). Alzheimers disease: A brief review. Journal of Experimental Neurology, 1(3), 89–98.
Tahami Monfared, A. A., Byrnes, M. J., White, L. A., & Zhang, Q. (2022). Alzheimer’s disease: Epidemiology and clinical progression. Neurology and Therapy, 11(2), 553–569. https://doi.org/10.1007/s40120-022-00338-8.
Schilling, L. P., Balthazar, M. L. F., Radanovic, M., Forlenza, O. V., Silagi, M. L., Smid, J., Barbosa, B. J. A. P., Frota, N. A. F., Souza, L. Cde, Vale, F. A. C., Caramelli, P., Bertolucci, P. H. F., Chaves, M. L. F., Brucki, S. M. D., Damasceno, B. P., & Nitrini, R. (2022). Diagnosis of Alzheimer’s disease: recommendations of the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology. Dementia & Neuropsychologia, 16(3), 25–39. https://doi.org/10.1590/1980-5764-dn-2022-s102en.
Trejo-Lopez, J. A., Yachnis, A. T., & Prokop, S. (2022). Neuropathology of Alzheimer’s Disease. Neurotherapeutics, 19(1), 173–185. https://doi.org/10.1007/s13311-021-01146-y.
Srivastava, S., Ahmad, R., & Khare, S. K. (2021). Alzheimer’s disease and its treatment by different approaches: A review. European Journal of Medicinal Chemistry, 216, 113320. https://doi.org/10.1016/j.ejmech.2021.113320.
Ekundayo, B. E., Obafemi, T. O., Adewale, O. B., & Oyinloye, B. E. (2023). Donepezil-based combination therapy for Alzheimer’s disease and related neuropathies. Comparative Clinical Pathology, 32(4), 699–708. https://doi.org/10.1007/s00580-023-03487-w.
Moreira, P. I., Plácido, A. I., Pereira, C. M. F., Duarte, A. I., Candeias, E., Correia, S. C., Santos, R. X., Carvalho, C., Cardoso, S., & Oliveira, C. R. (2014). The role of endoplasmic reticulum in amyloid precursor protein processing and trafficking: Implications for Alzheimer’s disease. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1842(9), 1444–1453. https://doi.org/10.1016/jbbadis201405003.
Egbuna, C., & Ifemeje, J. C. (2017). Oxidative stress and nutrition. Tropical Journal of Applied Natural Sciences, 2(1), 110–116.
Manisha, W. H., Richa, R., & Deepali, J. (2017). Oxidative stress and antioxidants: an overview. International Journal of Advanced Research and Review, 110, 110–111.
Sharifi-Rad, M., Anil Kumar, N. V., Zucca, P., Varoni, E. M., Dini, L., Panzarini, E., & Sharifi-Rad, J. (2020). Lifestyle oxidative stress and antioxidants: back and forth in the pathophysiology of chronic diseases. Frontiers in Physiology, 11, 694. https://doi.org/10.3389/fphys202000694.
Salim, S. (2016). Oxidative stress: a potential link between emotional wellbeing and immune response. Current opinion in pharmacology, 29, 70–76.
Preiser, J. C. (2012). Oxidative stress. Journal of Parenteral and Enteral Nutrition, 36(2), 147–154. https://doi.org/10.1177/0148607111434963.
Sinenko, S. A., Starkova, T. Y., Kuzmin, A. A., & Tomilin, A. N. (2021). Physiological signalling functions of reactive oxygen species in stem cells: from flies to man. Front Cell Dev Biol, 9, 714370. https://doi.org/10.3389/fcell2021714370.
Gupta, R. K., Patel, A. K., Shah, N., Chaudhary, A. K., Jha, U. K., Yadav, U. C., & Pakuwal, U. (2014). Oxidative stress and antioxidants in disease and cancer: A review. Asian Pacific Journal of Cancer Prevention, 15, 4405–4409.
Adwas, A. A., Elsayed, A. S. I., Azab, A. E., & Quwaydir, F. A. (2019). Oxidative stress and antioxidant mechanisms in human body. Journal of Applied Biotechnology and Bioengineering, 6(1), 43–47.
Di Bona, D., Scapagnini, G., Candore, G., Castiglia, L., Colonna-Romano, G., Duro, G., & Vasto, S. (2010). Immune-inflammatory responses and oxidative stress in Alzheimer’s disease: therapeutic implications. Current Pharmaceutical Design, 16(6), 684–691.
Haque, R. (2018). Amyloid Beta (Aβ) and oxidative stress: Progression of Alzheimer’s disease. Advances in Biotechnology Microbiology 11(1). https://doi.org/10.19080/aibm201811555802.
Ahmad, W., Ijaz, B., Shabbiri, K., Ahmed, F., & Rehman, S. (2017). Oxidative toxicity in diabetes and Alzheimer’s disease: Mechanisms behind ROS/ RNS generation. Journal of Biomedical Science, 24(1), 76. https://doi.org/10.1186/s12929-017-0379-z.
Padurariu, M., Ciobica, A., Lefter, R., Lacramioara Serban, I., Stefanescu, C., & Chirita, R. (2013). The oxidative stress hypothesis in Alzheimer’s disease. Psychiatria Danubina, 25(4), 4–9.
Chen, Z., & Zhong, C. (2014). Oxidative stress in Alzheimer’s disease. Neuroscience Bulletin, 30(2), 271–281. https://doi.org/10.1007/s12264-013-1423-y.
Ferreiro, E., Baldeiras, I., Ferreira, I. L., Costa, R. O., Rego, A. C., Pereira, C. F., & Oliveira, C. R. (2012). Mitochondrial- and endoplasmic reticulum-associated oxidative stress in alzheimers disease: From pathogenesis to biomarkers. International Journal of Cell Biology, 2012, 735206. https://doi.org/10.1155/2012/735206.
Tönnies, E., & Trushina, E. (2017). Oxidative stress synaptic dysfunction and Alzheimer’s disease. Journal of Alzheimer’s Disease, 57(4), 1105–1121. https://doi.org/10.3233/JAD-161088.
Hajam, Y. A., Rani, R., Ganie, S. Y., Sheikh, T. A., Javaid, D., Qadri, S. S., Pramodh, S., Alsulimani, A., Alkhanani, M. F., Harakeh, S., Hussain, A., Haque, S., & Reshi, M. S. (2022). Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells, 11(3), 552. https://doi.org/10.3390/cells11030552.
Tamagno, E., Guglielmotto, M., Vasciaveo, V., & Tabaton, M. (2021). Oxidative stress and beta amyloid in Alzheimer’s disease which comes first: the chicken or the egg? Antioxidants (Basel), 10(9), 1479. https://doi.org/10.3390/antiox10091479.
Huang, W. J., Zhang, X., & Chen, W. W. (2016). Role of oxidative stress in Alzheimer’s disease (review). Biomedical Reports, 4(5), 519–522. https://doi.org/10.3892/br2016630.
Zhao, Z. (2019). Iron and oxidizing species in oxidative stress and Alzheimer’s disease. Aging Medicine, 2(2), 82–87. https://doi.org/10.1002/agm212074.
Misrani, A., Tabassum, S., & Yang, L. (2021). Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease. Front Aging Neurosci, 13, 617588. https://doi.org/10.3389/fnagi2021617588.
Alves da Costa, C. A., da Manaa, W., el Duplan, E., & Checler, F. (2020). The endoplasmic reticulum stress/unfolded protein response and their contributions to Parkinson’s disease physiopathology. Cells, 9(11), 2495. https://doi.org/10.3390/cells9112495.
Oslowski, C. M., & Urano, F. (2011). Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods in Enzymology, 490, 71–92. https://doi.org/10.1016/B978-0-12-385114-700004-0.
Manalo, R. V. M., & Medina, P. M. B. (2018). The endoplasmic reticulum stress response in disease pathogenesis and pathophysiology Egyptian. Journal of Medical Human Genetics, 19(2), 59–68. https://doi.org/10.1016/jejmhg201707004.
Lin, J. H., Walter, P., & Yen, T. S. B. (2008). Endoplasmic reticulum stress in disease pathogenesis. Annual Review of Pathology: Mechanisms of Disease, 3, 399–425. https://doi.org/10.1146/annurevpathmechdis3121806151434.
Hosoi, T., Nomura, J., Ozawa, K., Nishi, A., Nomura, Y. (2016) Possible involvement of endoplasmic reticulum stress in the pathogenesis of Alzheimer’s disease. Endoplasmic Reticulum Stress in Diseases 2(1). https://doi.org/10.1515/ersc-2015-0008
Sprenkle, N. T., Sims, S. G., Sánchez, C. L., & Meares, G. P. (2017). Endoplasmic reticulum stress and inflammation in the central nervous system. Molecular Neurodegeneration, 12(1), 1–18. https://doi.org/10.1186/s13024-017-0183-y.
Gerakis, Y., & Hetz, C. (2018). Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS Journal, 285(6), 995–1011. https://doi.org/10.1111/febs14332.
Gao, X., & Xu, Y. (2021). Therapeutic effects of natural compounds and small molecule inhibitors targeting endoplasmic reticulum stress in Alzheimer’s disease. Frontiers in Cell and Developmental Biology, 9, 745011. https://doi.org/10.3389/fcell2021745011.
Ghemrawi, R., & Khair, M. (2020). Endoplasmic reticulum stress and unfolded protein response in neurodegenerative diseases. International Journal of Molecular Sciences, 21(17), 1–25. https://doi.org/10.3390/ijms21176127.
Obulesu, M., & Lakshmi, M. J. (2014). Apoptosis in Alzheimer’s disease: An understanding of the physiology pathology and therapeutic avenues. Neurochemical Research, 39(12), 2301–2312. https://doi.org/10.1007/s11064-014-1454-4.
Islam, S. M., Sifat Rahi, M., Arif Jahangir, C., Mahmudul Hasan, M., Ahmed Sajib, S., Haque, A., Rashel Kabir, S., Md Faisal Hoque, K., Ali Moni, M., Abu Reza, M. (2021). Deciphering the molecular pathways of apoptosis using leaf extract of Basella alba against Ehrlichs Ascites Carcinoma (EAC) cell line in Swiss albino mice model. Mol Biol Rep, 48(1), 85–96. https://doi.org/10.1101/485078.
Joshi, A., Haque, N., Lateef, A., Patel, A., Patel, P. (2017) Apoptosis and Its Role in Physiology International. Journal of Livestock Research 1. https://doi.org/10.5455/ijlr20170624054330
Singh, V., Khurana, A., Navik, U., Allawadhi, P., Bharani, K.K., & Weiskirchen, R. (2022) Apoptosis and pharmacological therapies for targeting thereof for cancer therapeutics. Science 4(2). https://doi.org/10.3390/sci4020015
Chi, H., Chang, H. Y., & Sang, T. K. (2018). Neuronal cell death mechanisms in major neurodegenerative diseases. International Journal of Molecular Sciences, 19(10), 3082. https://doi.org/10.3390/ijms19103082.
Zhang, L., Qian, Y., Li, J., Zhou, X., Xu, H., Yan, J., Xiang, J., Yuan, X., Sun, B., Sisodia, S. S., Jiang, Y. H., Cao, X., Jing, N., & Lin, A. (2021). BAD-mediated neuronal apoptosis and neuroinflammation contribute to Alzheimer’s disease pathology. IScience, 24(9), 102942. https://doi.org/10.1016/jisci2021102942.
Moujalled, D., Strasser, A., & Liddell, J. R. (2021). Molecular mechanisms of cell death in neurological diseases. Cell Death and Differentiation, 28(7), 2029–2044. https://doi.org/10.1038/s41418-021-00814-y.
Teixeira, J. P., de Castro, A. A., Soares, F. V., da Cunha, E. F. F., & Ramalho, T. C. (2019). Future therapeutic perspectives into the Alzheimer’s disease targeting the oxidative stress hypothesis. Molecules, 24(23), 4410. https://doi.org/10.3390/molecules24234410.
Rekatsina, M., Paladini, A., Piroli, A., Zis, P., Pergolizzi, J. V., & Varrassi, G. (2020). Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: a narrative review. Advances in therapy, 37, 113–139.
Qu, Z., Sun, J., Zhang, W., Yu, J., & Zhuang, C. (2020). Transcription factor NRF2 as a promising therapeutic target for Alzheimer’s disease. Free Radical Biology and Medicine, 159, 87–102. https://doi.org/10.1016/j.freeradbiomed.2020.06.028.
Uddin, M. S., al Mamun, A., Kabir, M. T., Ahmad, J., Jeandet, P., Sarwar, M. S., Ashraf, G. M., & Aleya, L. (2020). Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. European Journal of Pharmacology, 886, 173412. https://doi.org/10.1016/j.ejphar.2020.173412.
Remondelli, P., & Renna, M. (2017). The endoplasmic reticulum unfolded protein response in neurodegenerative disorders and its potential therapeutic significance. Frontiers in Molecular Neuroscience, 10, 187. https://doi.org/10.3389/fnmol.2017.00187.
Ajoolabady, A., Lindholm, D., Ren, J., & Pratico, D. (2022). ER stress and UPR in Alzheimer’s disease: mechanisms, pathogenesis, treatments. In Cell Death and Disease, 13(8), 706. https://doi.org/10.1038/s41419-022-05153-5.
Hedskog, L., Pinho, C. M., Filadi, R., Rönnbäck, A., Hertwig, L., Wiehager, B., Larssen, P., Gellhaar, S., Sandebring, A., Westerlund, M., Graff, C., Winblad, B., Galter, D., Behbahani, H., Pizzo, P., Glaser, E., & Ankarcrona, M. (2013). Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proceedings of the National Academy of Sciences of the United States of America, 110(19), 7916–7921. https://doi.org/10.1073/pnas.1300677110.
Li, Z., Cao, Y., Pei, H., Ma, L., Yang, Y., & Li, H. (2023). The contribution of mitochondria-associated endoplasmic reticulum membranes (MAMs) dysfunction in Alzheimer’s disease and the potential countermeasure. Frontiers in Neuroscience, 17, 1158204. https://doi.org/10.3389/fnins.2023.1158204.
Schon, E. A., & Area-Gomez, E. (2013). Mitochondria-associated ER membranes in Alzheimer disease. Molecular and Cellular Neuroscience, 55, 26–36. https://doi.org/10.1016/j.mcn.2012.07.011.
Eysert, F., Kinoshita, P. F., Mary, A., Vaillant-Beuchot, L., Checler, F., & Chami, M. (2020). Molecular dysfunctions of mitochondria-associated membranes (Mams) in alzheimer’s disease. International Journal of Molecular Sciences, 21(24), 1–29. https://doi.org/10.3390/ijms21249521.
Fernandes, T., Resende, R., Silva, D. F., Marques, A. P., Santos, A. E., Cardoso, S. M., Rosário Domingues, M., Moreira, P. I., & Pereira, C. F. (2021). Structural and functional alterations in mitochondria-associated membranes (Mams) and in mitochondria activate stress response mechanisms in an in vitro model of alzheimer’s disease. Biomedicines, 9(8), 881. https://doi.org/10.3390/biomedicines9080881.
Yu, W., Jin, H., & Huang, Y. (2021). Mitochondria-associated membranes (MAMs): A potential therapeutic target for treating Alzheimer’s disease. Clinical Science, 135, 109–126. https://doi.org/10.1042/CS20200844.
Cassidy, L., Fernandez, F., Johnson, J. B., Naiker, M., Owoola, A. G., & Broszczak, D. A. (2020). Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complementary Therapies in Medicine, 49, 102294. https://doi.org/10.1016/j.ctim.2019.102294.
Hashimoto, S., & Saido, T. C. (2018). Critical review: Involvement of endoplasmic reticulum stress in the aetiology of Alzheimer’s disease. Open Biology, 8(4), 180024. https://doi.org/10.1098/rsob.180024.
Thenmozhi, A., William Raja, T. R., Manivasagam, T., Janakiraman, U., & Essa, M. M. (2017). Hesperidin ameliorates cognitive dysfunction oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer’s disease. Nutritional Neuroscience, 20(6), 360–368.
Ekundayo, B. E., Obafemi, T. O., Afolabi, B. A., Adewale, O. B., Onasanya, A., Osukoya, O. A., & Adu, I. A. (2022). Gallic acid and hesperidin elevate neurotransmitters level and protect against oxidative stress inflammation and apoptosis in aluminum chloride-induced Alzheimer’s disease in rats. Pharmacological Research-Modern Chinese Medicine, 5, 100193.
Promyo, K., Iqbal, F., Chaidee, N., & Chetsawang, B. (2020). Aluminum chloride-induced amyloid β accumulation and endoplasmic reticulum stress in rat brain are averted by melatonin. Food Chem Toxicol, 146, 111829. https://doi.org/10.1016/jfct2020111829.
Awad, H. H., Desouky, M. A., & Zidan, A. (2023). Neuromodulatory effect of vardenafil on aluminium chloride/D-galactose induced Alzheimer’s disease in rats: emphasis on amyloid-beta p-tau PI3K/Akt/p53 pathway endoplasmic reticulum stress and cellular senescence. Inflammopharmacol, 31, 2653–2673. https://doi.org/10.1007/s10787-023-01287-w.
Aboelwafa, H. R., El-kott, A. F., Abd-Ella, E. M., & Yousef, H. N. (2020). The possible neuroprotective effect of silymarin against aluminum chloride-prompted Alzheimer’s-like disease in rats brain. Sci, 10, 628. https://doi.org/10.3390/brainsci10090628.
Qusti, S. Y. (2017). Selenium and melatonin attenuates inflammation and oxidative stress in the brain of aged rats with aluminum chloride-induced Alzheimer. International Journal of Pharmaceutical Research Allied Sciences, 6(2), 277–289.
Abdel-Salam, O. M., Hamdy, S. M., Seadawy, S. A. M., Galal, A. F., Abouelfadl, D. M., & Atrees, S. S. (2016). Effect of piracetam vincamine vinpocetine and donepezil on oxidative stress and neurodegeneration induced by aluminum chloride in rats. Comparative Clinical Pathology, 25, 305–318.
Ahmad Rather, M., Justin-Thenmozhi, A., & Manivasagam, T. (2019). Asiatic acid attenuated aluminum chloride-induced tau pathology oxidative stress and apoptosis Via AKT/GSK-3β signalling pathway in wistar rats. Neurotox Res, 35, 955–968. https://doi.org/10.1007/s12640-019-9999-2.
Liu, B. Y., Lee, Y. J., Jen, H. C., & Hwang, D. F. (2019). Efficacy of taurine against aluminum maltolate-induced apoptosis in SH-SY5Y cells via reduction of oxidative stress endoplasmic reticulum stress and mitochondrial dysfunction. Journal of Marine Science and Technology, 27(1), 8.
Chen, X., Zhang, M., Ahmed, M., Surapaneni, K. M., Veeraraghavan, V. P., & Arulselvan, P. (2021). Neuroprotective effects of ononin against the aluminium chloride-induced Alzheimer’s disease in rats. Saudi Journal of Biological Sciences, 28(8), 4232–4239.
Zhao, Y., Dang, M., Zhang, W., Lei, Y., Ramesh, T., Veeraraghavan, V. P., & Hou, X. (2020). Neuroprotective effects of Syringic acid against aluminium chloride induced oxidative stress mediated neuroinflammation in rat model of Alzheimer’s disease. Journal of Functional Foods, 71, 104009.
Raj, K., Gupta, G. D., & Singh, S. (2021). Spermine protects aluminium chloride and iron-induced neurotoxicity in rat model of Alzheimer’s disease via attenuation of tau phosphorylation Amyloid-β (1–42) and NF-κB pathway. Inflammopharmacol, 29, 1777–1793. https://doi.org/10.1007/s10787-021-00883-y.
Chavali, V. D., Agarwal, M., & Vyas, V. K. (2020). Neuroprotective effects of ethyl pyruvate against aluminum chloride-induced Alzheimer’s disease in rats via inhibiting toll-like receptor 4. J Mol Neurosci, 70, 836–850. https://doi.org/10.1007/s12031-020-01489-9.
Attia, H., Albuhayri, S., Alaraidh, S., Alotaibi, A., Yacoub, H., Mohamad, R., & Al‐Amin, M. (2020). Biotin coenzyme Q10 and their combination ameliorate aluminium chloride‐induced Alzheimer’s disease via attenuating neuroinflammation and improving brain insulin signalling. Journal of Biochemical and Molecular Toxicology, 34(9), e22519.
Anand, A., Khurana, N., Kaur, S., Ali, N., AlAsmari, A. F., Waseem, M., & Sharma, N. (2023). The multifactorial role of vanillin in amelioration of aluminium chloride and D-galactose induced Alzheimer’s disease in mice. European Journal of Pharmacology, 954, 175832. https://doi.org/10.1016/j.ejphar.2023.175832.
Prakash, D., & Sudhandiran, G. (2015). Dietary flavonoid fisetin regulates aluminium chloride-induced neuronal apoptosis in cortex and hippocampus of mice brain. The Journal of nutritional biochemistry, 26(12), 1527–1539.
Author Contributions
All authors contributed equally to the writing, proof reading, correction and preparation of the draft and final copy of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Consent for Publication
Authors give their consent for publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ekundayo, B.E., Obafemi, T.O., Adewale, O.B. et al. Oxidative Stress, Endoplasmic Reticulum Stress and Apoptosis in the Pathology of Alzheimer’s Disease. Cell Biochem Biophys (2024). https://doi.org/10.1007/s12013-024-01248-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s12013-024-01248-2