Abstract
Coastal marshes mitigate allochthonous nitrogen (N) inputs to adjacent marine habitat; however, their extent is declining rapidly. As a result, marsh restoration and construction have become a major foci of wetland management. Constructed marshes can quickly reach similar plant biomass to natural marshes, but biogeochemical functions like N removal and retention can take decades to reach functional equivalency, often due to lags in organic matter (OM) pools development in newly constructed marshes. We compared denitrification and dissimilatory nitrate reduction to ammonium (DNRA) rates in a 32 year-old constructed marsh and adjacent reference marsh in the Northern Gulf of Mexico. Marsh sediments packed into 3 mm “thin discs” were subjected to three OM quality treatments (no OM addition, labile OM, or recalcitrant OM) and two N treatments (ambient nitrate or elevated nitrate) during a 13 day incubation. We found that OM addition, rather than marsh type or nitrate treatment, was the most important driver of nitrate reduction, increasing both denitrification and DNRA and promoting DNRA over denitrification in both marshes. Fungal and bacterial biomass were higher in the natural marsh across treatments, but recalcitrant OM increased fungal biomass in the constructed marsh, suggesting OM-limitation of fungal growth. We found that constructed marshes are capable of similar denitrification and DNRA as natural marshes after 30 years, and that labile OM addition promotes N retention in both natural and constructed marshes.
Graphical Abstract
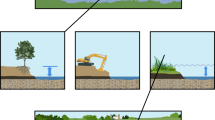
Conceptual figure highlighting the findings of this experiment. Under control treatment with no C addition (bottom panel), constructed and natural marshes have similar rates of both DNRA and denitrification. The natural marsh has higher fungal and bacterial biomass, while fungal biomass is not detectable in the constructed marsh. Under labile OM additions (upper left panel), rates of both DNRA and denitrification are increased and DNRA becomes favored over denitrification in both marshes. Recalcitrant OM additions (upper right) increase denitrification, but do not affect DNRA or % denitrification. The addition of recalcitrant OM also increases the detectability of fungal biomass in the constructed marsh.

Similar content being viewed by others
Introduction
Wetlands, with their unique hydrology, vegetation, and sediment chemistry, are globally important sites of nitrogen (N) and carbon (C) cycling. Coastal marshes are especially important in mitigating excess nutrient inputs and coastal eutrophication by removing or sequestering N before it reaches marine habitats (Jordan et al. 2011) where nutrient pollution can promote harmful algal blooms, marine hypoxia, and the degradation of commercial fisheries (Valiela and Cole 2002; Sousa et al. 2012). Despite their valuable ecosystem services, coastal wetlands are disappearing at an alarming pace, with further loss predicted into the future (Kennish 2001; Kirwan and Megonigal 2013; Li et al. 2018; Fluet-Choinard et al. 2023). As a result, attempts to restore or construct wetland habitat to recover lost ecosystem functions have become an important area of wetland management.
Despite restoration attempts over the last few decades, it is still unclear how effective marsh construction and restoration can be in restoring ecosystem functions. Much of the research that has assessed restoration in engineered wetlands has focused largely on structural restoration (i.e., plant biomass and species richness) and less on functional restoration like the return of N-removal capacity and C storage (Bruland et al. 2006; Moreno-Mateos et al. 2012). Above and below ground biomass in engineered or restored wetlands can quickly reach levels similar to that of references marshes (i.e., within the first decade post-construction), but the trajectory of ecosystem functional recovery is more variable and can take decades or longer to reach values similar to those in reference wetlands (Craft et al. 1999; Moreno-Mateos et al. 2012). Furthermore, some studies have observed that even after 25 years, restored marshes still have yet to recover N-removal capacity (Craft et al. 2003; Moreno-Mateos et al. 2012) while others have observed restored and constructed marshes to be functionally similar to reference marshes in terms of N-removal potential as early as 8–25 years after restoration (He et al. 2016; Kleinhuizen and Mortazavi 2018).
Soil C stocks in constructed salt marshes can also take decades to recover, as they rely on biomass turnover and burial over time (Craft 2001; Craft et al. 2003; Cornell et al. 2007; Moreno-Mateos et al. 2012). As constructed marshes mature, the quality, composition, and lability of C stocks changes over time (Baldock et al. 2004; He et al. 2016). Labile organic matter (OM) is more readily assimilated and mineralized than high molecular weight polyphenolic compounds like lignin, and is thus a preferable substrate for heterotrophic processes (Magill and Aber 2000; Strauss and Lamberti 2002; Dodla et al. 2008). OM quality, rather than total C content, has also been shown to directly affect N cycling in coastal wetlands, as well as in stream sediments (Magill and Aber 2000; Dodla et al. 2008; Stelzer et al. 2014). While young marshes may accumulate OM at rates much higher than that of reference wetlands (Krull and Craft 2009; Mitsch et al. 2013; Henry and Twilley 2013), this OM is primarily labile (Craft et al. 2003; Davis et al. 2015). As marshes age, the rate of OM accumulation slows (Krull and Craft 2009; He et al. 2016) and the ratio of labile to recalcitrant OM accumulation decreases with age (Craft et al. 2003; Baldock et al. 2004). One potential reason for the lag between recovery of N-removal capacity and vegetation return is the lower total OM content and potential differences in lability in constructed marshes, which often have accumulated less OM even decades after construction (Craft et al. 1999, 2003; Davis et al. 2015; He et al. 2016).
Denitrification and dissimilatory nitrate reduction to ammonium (DNRA) are two microbially-mediated pathways by which N is transformed in marsh sediments. Denitrification is the stepwise reduction of NO3− to nitrous oxide and dinitrogen gases and is the dominant pathway for permanent N removal (Seitzinger et al. 2006), whereas DNRA conserves N in the system as ammonium (Burgin and Hamilton 2007). Supply of C and differences in OM composition and quality can impact microbial community structure and biomass, and potentially influence rates of denitrification and DNRA (Cleveland et al. 2007; Grebliunas and Perry 2016; Lynum et al. 2020). Labile OM additions in particular have been shown to stimulate denitrification in salt marsh sediments (Dodla et al. 2008) and both denitrification and DNRA in stream sediments (Stelzer et al. 2014) and marine shelf sediments (Hardison et al. 2015). While labile C additions in terrestrial soils and freshwater wetland sediments increase bacterial growth, abundance, and productivity (Meidute et al. 2008; Grebliunas and Perry 2016; Lynum et al. 2020), increases in recalcitrant C can increase fungal biomass, abundance, and productivity (Cleveland et al. 2007; Meidute et al. 2008; Chigineva et al. 2009; Grebliunas and Perry 2016).
This study used experimental microcosms with six different combinations of OM and nitrate (NO3−) amendment treatments to assess whether constructed salt marshes can remove N as efficiently as natural salt marshes and whether N-removal is affected differently by the addition of either labile or recalcitrant OM in the presence of excess NO3− in each marsh type. Additionally, this study sought to determine how amendment with NO3− affected N removal under different OM amendment scenarios, to highlight potential management considerations in marsh construction to either preserve or enhance starting OM stocks. Additionally, we were interested in whether OM quality or NO3− concentrations had significant effects on microbial and fungal biomass in salt marsh sediments; specifically, whether limitations in constructed marsh OM stocks may limit microbial and fungal community recovery.
Methods
Site description
Our study system consists of a naturally occurring salt marsh and adjacent constructed salt marshes along the West Fowl River in Mobile County, Alabama, USA (Fig. 1). As part of a mitigation effort in compliance with the Clean Water Act, the constructed sites (30° 22′ 02.6′′ N, 88° 09′ 06.7′′ W) were built in 1987 by clearing six parallel sections of pine forest to a final elevation of + 0.3 m MSL to match historical mean high water. These newly cleared sections of marsh were connected to a naturally occurring tidal creek by means of drainage ditches excavated through the center of each constructed marsh. These marshes were originally planted with Spartina alterniflora and Juncus roemerianus transplants, however only J. roemerianus has persisted. This constructed marsh system borders an adjacent natural marsh (30° 22′ 03.0′′ N, 88° 09′ 38.1′′ W), which encompasses an area of approximately 6.1 hectares comprised of mixed stands of S. alterniflora, S. patens and J. roemerianus vegetation without the clear zonation observed in other northern Gulf Coast marshes (Battaglia et al. 2012). Salinity in the West Fowl River adjacent to both sites ranged from 9 to 11% during the course of this study. For this study, samples were collected from one of the constructed marshes and from the natural marsh. In the natural marsh, collection was limited to J. roemerianus stands to ensure comparability between sites, as the constructed marsh is dominated only by J. roemerianus.
Map of Northern Gulf Coast and bordering US States depicting site location in Coastal AL, USA. Inset map showing location of constructed (30° 22′ 02.6′′ N, 88°09′ 06.7′′ W) and natural (30° 22′ 03.0′′ N, 88° 09′ 38.1′′ W) marshes near Fowl River, Mobile County, AL. Inset georeferenced satellite image showing the three randomly selected plots in both marshes (indicated by stars)
Thin disc incubations
Sediments were collected from three representative J. roemerianus patches selected randomly between the bordering forest and the shore in both the reference and one of the parallel sections of the constructed marsh (Fig. 1). Using a 7.9 cm inner diameter corer, 5 cores were collected from each plot to a depth of 25 cm. All fifteen cores from each marsh were homogenized manually via sieving with a 1 mm mesh screen to create a homogenous sediment sample from each system. Subsets of these homogenized samples were mixed with OM substrates as detailed below then distributed into 3 mm deep PVC “thin discs” for incubation experiments (Hardison et al. 2015). Thin discs were constructed by slicing a 10 cm diameter PVC pipe into 3 mm thick rings, which were affixed with PVC cement to an acrylic block base, forming an open-top cylinder with a volume of 22.8 mL (Fig. 2). The discs were then submerged in commercial 151 L glass “breeder” aquaria (Aqueon, Franklin, WI, USA) containing ~ 149 L 10% artificial seawater and subjected to one of the following combinations of experimental conditions: (1) ambient NO3− (NO3−_A), no OM addition (OM_A) as a control; (2) elevated NO3− (NO3−_E), no OM addition (OM_A); (3) ambient NO3− (NO3−_A), labile OM additions (OM_L); (4) elevated NO3− (NO3−_E), labile OM additions (OM_L); (5) ambient NO3− (NO3−_A), recalcitrant OM additions (OM_R); and (6) elevated NO3− (NO3−_E), recalcitrant OM additions (OM_R). This resulted in a total of 12 experimental tanks (6 per marsh type, representing one combination of the described experimental conditions in each tank). Ground spirulina (Micro Ingredients, Montclair, CA, USA) was autoclaved and then reground to be used as the OM_L treatment. For the OM_R treatment, standing dead shoots of J. roemerianus were collected from the natural marsh in November 2018, dried, and ground into a fine powder. For both OM addition treatments, 20 mg of substrate (spirulina or J. roemerianus powder) per g of wet sediment were added to the sediment and homogenized by mixing immediately prior to filling discs. Each tank received 5 mL of 0.022 M ammonium sulfate ((NH4)2SO4) per day (i.e., 220 µmol NH4) to prevent N limitation during the incubation. The ambient NO3− treatment received a loading rate of 11.17 µmol NO3− and the elevated NO3− received a loading rate of 93.11 µmol NO3− per disc per day, dispensed gradually by a peristaltic pump over 24 h as potassium nitrate (KNO3) solution. The concentration selected for ambient NO3− treatments was based on previously measured NO3− concentrations in the Gulf of Mexico (Kleinhuizen and Mortazavi 2018; Domangue and Mortazavi 2018)) and the elevated rate was chosen following a similar increase in load as Hardison et al. 2015. Water column nutrients were sampled every other day during a 13 day incubation for monitoring and sediment discs were collected on day 1 for C:N analysis and on days 1, 5, and 13 for analysis of process rates and microbial biomass (19 discs total per tank, with two replicate discs for rates and biomass at each timepoint).
Experimental design. Homogenized sediment from either marsh were subjected to three OM treatments, with the treatments receiving no OM or OM substrate of either ground Spirulina or J. roemerianus litter mixed into the sediment, and placed into 3 mm-depth thin discs constructed from sliced PVC pipe and acrylic blocks. Nineteen discs were then submerged in each 151 L aquaria containing approximately 149 L of 10% artificial seawater bubbled intermittently with CO2 and constantly with N2 gas to maintain pH and anoxic conditions. Peristaltic pumps provided a constant inflow of solution from reservoirs containing the two NO3 treatments to appropriate treatment tanks. 5 mL of 0.022 M ammonium sulfate were added to each tank daily. Each experimental tank contains one combination of marsh type (natural or constructed), OM treatment, and NO3 treatment
The aquaria were bubbled constantly with N2 to maintain hypoxia and bubbled intermittently with CO2 to maintain pH at 7.37 ± 0.06. Over the 13 day incubation period, aquarium water was sampled every other day for PO43 and NH4+ (n = 84) to monitor the progress of the incubations. NO3− and NO2− analysis was abandoned due to COVID-19 pandemic-related shutdowns. Nutrient concentrations are available in Supplemental Fig. 1 and Supplemental Table 2 and will not be presented here. Sediment discs were removed for analysis of total C and N, bacterial biomass, and ergosterol at three timepoints over the incubation period (days 1, 5 and 13). The concentration of NO3− solution for each NO3− loading treatment was adjusted at each timepoint to maintain a constant tank dosing scaled to number of discs remaining in each tank.
Water column nutrients and sediment C and N
Water samples for nutrient monitoring were collected every other day from each aquaria and filtered through 0.45 µm acrylic syringe filters (VWR, Radnor, Pennsylvania, USA), and stored at – 20 °C until analysis. PO43− concentrations were measured using the molybdate method by UV–vis spectrophotometer following established methods (Grasshoff 1983; Schnetger and Lehners 2014). NH4+ concentrations were measured fluorometrically (Holmes et al. 1999). On day 1, one disc from each treatment was removed and dried for C and N analysis at the University of Southern Mississippi. Sediment %C and %N were analyzed using a Costech Elemental Analyzer (Costech Analytical Technologies, Valencia, CA, USA) and molar C:N ratios were calculated from these measurements.
Denitrification, anammox, and DNRA measurements
On each of three sediment sampling timepoints (days 1, 5 and 13), two discs from each aquarium were removed for the measurement of denitrification and DNRA rates via isotope pairing technique (Nielsen 1992; Koop-Jakobsen and Giblin 2009). Sediment from two discs was homogenized by slurrying with 10% artificial seawater while bubbling with N2 gas to induce anoxia. Three mL of sediment slurry were then transferred to 12 mL glass exetainers (Labco Limited, Lampeter, UK), topped off with N2 sparged artificial seawater, and pre-incubated overnight to remove residual nutrients and oxygen. Na15NO3 was then added to a final concentration of 50 µM before half of the samples were immediately preserved with 50% (w/v) ZnCl2 for time initial (T0) to determine initial 30N2. The remaining samples were incubated for six hours before being preserved with 50% (w/v) ZnCl2 for time final (Tf) to determine excess production compared to T0. All samples were then analyzed for dissolved 29N2 and 30N2 concentrations with a membrane inlet mass spectrometer (MIMS) equipped with a copper reduction column (Kana et al. 1994; Eyre et al. 2002).
Denitrification potentials were calculated using the ratio of 29N2 and 30N2 after incubation:
where D15 represents denitrification of the added 15NO3−, and p29 and p30 represent the rates of 29N2 and 30N2 production.
where D14 represents denitrification of ambient 14NO3−,
where Dt represents total denitrification.
Potential anammox rates in sediment slurries were determined according to methods described by (Thamdrup and Dalsgaard 2002):
where \({A}_{total}\) denotes production of N2 through annamox, \({F}_{N}\) is the fraction of 15N in NO3−, and \({P}_{29}\) and \({P}_{30}\) represent the total produced mass of 29N2 and 30N2, respectively. Anammox was typically undetectable and therefore will not be discussed further.
Following addition of ZnCl2, samples for DNRA rates were bubbled with N2 gas to remove 29N2 and 30N2 produced by denitrification and/or anammox. An alkaline sodium hypobromite solution was added to convert the 15NH4+ product of DNRA to 29N2 and 30N2 before being analyzed on a MIMS. Potential DNRA rates were determined using methods described by (Yin et al. 2014):
where RDNRA denotes the total measured 15N-based potential DNRA rates, [29+30N2]initial and [29+30N2] final represent concentrations of 15NH4+ in the initial and final samples of the slurry experiments, respectively, V is the volume (L) of the incubation vial, W denotes the dry weight (kg) of the sediment, and T is the duration of the incubation (h). All process rates are presented as µmol N kg dry sediment−1 h−1. The percent (%) N-removal by denitrification was calculated according to:
Microbial biomass quantification
At each sediment sampling timepoint (days 1, 5, and 13), two discs were removed from the aquaria for microbial biomass quantification. Approximately 5 g of sediment from each disc were immediately transferred into 20 mL glass scintillation vials and preserved with 5 mL of a solution of 2% (v/v) sodium pyrophosphate (0.1% w/v) buffered formalin and stored at 4 °C until analysis for bacterial biomass at the University of Southern Mississippi. These samples were sonicated on ice using a Branson 150 sonifier at setting 4 for 4 × 20 s intervals. Diluted samples were vortexed, and bacterial cells stained using the Invitrogen bacteria counting kit for flow cytometry (Thermo Fisher, Waltham, MA) and analyzed with a BD LSRFortessa Cell Analyzer (flow rate = 400 events s−1; fluorescence measured using a fluorescein (FITC) channel with a 530 nm bandpass filter). Bacterial cells were counted as those with fluorescence above microbeads (FITC < 103); we also excluded any cells larger than microbeads (diameter 6 µm; forward scatter > 2 × 102).
Approximately 5 g of sediment from each disc were transferred to 15 mL centrifuge tubes and stored at – 20 °C until analysis for ergosterol, a chemical proxy for fungal biomass, at the University of Southern Mississippi. Samples for ergosterol were lyophilized, extracted, and quantified by a Shimadzu HPLC system using a LichroSpher 100 RP-18 column (0.46 × 25 cm, mobile phase HPLC grade methanol, flow rate of 1.5 mL min−1) at 282 nm.
Statistical analysis
We assessed the effect of OM and N treatments and incubation time and their interactions on natural log-transformed DNRA, denitrification rates, bacterial biomass, and square root-transformed percent N-removal using linear mixed-effect (LME) models with Marsh, Treatment (OM × NO3−), and Sampling Day as main effects and Tank as a random effect. To increase statistical power, non-significant main effects were removed and the model was re-run; specifically, Marsh for process rates, Day in ergosterol concentration analysis, and NO3− in bacterial cell count analysis. Analysis of variance (ANOVA) was run on the LME to interpret significant effects. Differences in significant main effects and interactions were tested using Tukey’s HSD post-hoc tests. Tests of simple effects (TSE) were used to interpret interactions where one or both main effects were not significant. ANOVAs (OM × Marsh and NO3− × Marsh) were run on sediment % C, % N, C:N ratio in both marshes, and log-transformed sediment ergosterol in the natural marsh only (OM × NO3−). Differences in significant main effects and interactions were tested using Tukey’s HSD post-hoc tests. Statistical comparisons were made using R version 3.6.1 (2020). Results are reported in Tables 1 and 2.
Results
C:N and process rates
OM_A treatments
In the absence of added OM, sediment % C at the beginning of the incubation was 5 × higher in the natural marsh (11.8% ± 0.72%) than the constructed (2.34% ± 0.49%) but this difference was not statistically significant (ANOVA, p = 0.57). Sediment % N was more than threefold higher in the natural marsh (0.79% ± 0.17%) than in the constructed marsh (0.21% ± 0.11%; ANOVA, p < 0.005). However, C:N ratio was similar in both marshes (ANOVA, p = 0.57). Sediment % C and % N and C:N ratio in the NO3− addition treatments were similar in the absence of added OM (ANOVA, p = 0.98, p = 0.98, and p = 0.90 respectively).
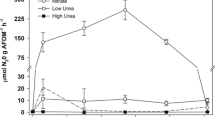
At ambient NO3− concentrations and in the absence of added OM (i.e., the control condition), denitrification rates were similar between the constructed (33.7 ± 26.6 µmol 15N kg dry sed−1 h−1) and natural (64.7 ± 68.3 µmol 15N kg dry sed−1 h−1) marshes (LME, p = 0.179). Across all treatments, time had a significant effect on denitrification rate (LME, p = 0.001), with 1.5-fold lower rates of denitrification on day 1 compared to day 5 (Tukey’s HSD, p = 0.040) and 4.2-fold higher rates of denitrification between day 1 and day 13 (Tukey’s HSD p < 0.001, Fig. 3A–C). DNRA rates were also similar between the constructed (80.0 ± 79.8 µmol 15N kg dry sed−1 h−1) and natural (81.1 ± 79.8 µmol 15N kg dry sed−1 h−1) marshes (LME, p = 0.339). Overall, DNRA was lowest on day 5 (LME, p = 0.003; Tukey’s HSD p < 0.010, Fig. 3D–F). The percent of N-reduction attributed to denitrification (% denitrification) was similar between the constructed (50.7 ± 22.8%) and natural (56.2 ± 19.4%) marshes (LME, p = 0.404).
Mean denitrification potential rates (first row), DNRA rates (second row), and % N reduction as denitrification (third row) in response to OM treatment over the course of the 13-day incubation. NO3− addition treatments are indicated by color. From left to right, each column represents OM_A, OM_L, and OM_R treatments, respectively
While time was not a significant effect on denitrification rates in ambient NO3− concentration treatments, (TSE, p = 0.635), time appeared to be an important factor in the NO3−_E treatment, with rates of denitrification increasing by threefold from day 1 to day 5, and by 3.2-between day 5 and day 13 (TSE, p < 0.005, Fig. 3A–C, Supplemental Fig. 2B). There were no differences in denitrification rates between NO3−_A and NO3−_E treatments on any day (TSE, p > 0.682). DNRA rates were similar in the NO3−_A and NO3−_E treatments (p = 0.776, Fig. 3D–F, Supplemental Fig. 2D). Similarly, there was also no main effect of NO3− treatment on % denitrification (LME, p = 0.582, Fig. 3G–I, Supplemental Fig. 2F).
OM addition treatments
Sediment %C was similar between OM_R and OM_L treatments (Tukey’s HSD p = 0.396) but sediment % C was about 50% higher than the control for both OM addition treatments (Tukey’s HSD p = 0.002). OM_L treatments increased sediment %N twofold relative to both the control and the OM_R treatments (ANOVA, p < 0.005; Tukey’s HSD p < 0.005), which had similar % N content (Tukey’s HSD, p = 0.641). C:N ratio was lower by 1.77-fold in the OM_L treatment (ANOVA, p < 0.005, Tukey’s HSD, p < 0.005) and by 1.3-fold in the OM_R treatment (Tukey’s HSD, p = 0.010).
While there was no main effect of either OM or NO3− treatments on denitrification rates, we observed significant interactions with time and OM addition in both OM addition treatments (TSE, p < 0.001 for both, Fig. 3B, C, Supplemental Fig. 2A). At ambient NO3− concentrations, denitrification in the OM_L increased 7.6-fold from day 1 to 5 and 3.8-fold from days 5–13. Similarly, the denitrification rates in the OM_R treatment increased 1.1-fold from day 1 to 5 and fourfold from days 5–13. Denitrification rates differed between OM treatments on each day (TSE, p < 0.001). Denitrification in the no OM addition NO3−_A control was higher than in either the OM_L or OM_R treatments on day 1 (TSE, p < 0.001), but OM additions increased denitrification rates compared to this control on the remaining days (TSE, p < 0.001 for each day). There was no statistically significant interaction between OM and NO3− treatments (LME, p = 0.705) on denitrification rates, rather, the effects of OM and NO3− treatments depended on time (LME, p = 0.006 for OM × Day and p = 0.001 for NO3− × Day). Although there were no significant marsh or treatment interactions, the highest denitrification rates were observed in natural marsh sediments in the OM_L treatment at elevated NO3− concentrations on Day 13 (Fig. 3B). This rate (393.1 µmol 15N kg dry sed−1 h−1) exceeded by the next highest rate measured in the OM_R at elevated NO3− concentrations on Day 13 by 1.6-fold (Fig. 3C).
At ambient NO3− concentrations, DNRA in the OM _L treatment increased by > 12-fold compared to the control and recalcitrant treatments across all dates (LME, p < 0.001; Tukey’s HSD, p < 0.031, Fig. 3E, Supplemental Fig. 2B). DNRA did not vary in the OM_R treatment. Although the different treatments appeared to show different patterns over time and there was a significant OM × Day interaction (LME, p = 0.010). The only change in rates for any OM treatment was a marginal increase in DNRA (Tukey’s HSD, p = 0.057) from day 5 to 13 in the OM_L treatment.
Within the OM_L treatment, % denitrification increased from day 1 to day 5 by approximately 15-fold (Tukey’s HSD, p < 0.005) but did not change between Days 5 and 13 (Tukey’s HSD, p = 0.650, Fig. 4H). Although there was no effect of recalcitrant OM on % denitrification (Tukey’s HSD, p = 0.172, Fig. 3i), N-reduction attributed to % denitrification was lower in the OM_L treated discs compared to discs with no OM addition (Tukey’s HSD, p < 0.005, Fig. 3H, Supplemental Fig. 2E). In the OM_L treatment % denitrification was lower compared to the highest average % denitrification observed in the OM_R treatment by 3.5-fold (75.5% ± 6.6%; Tukey’s HSD, p < 0.005). In OM_L tanks, % denitrification was > 22-fold lower (Tukey’s HSD, p < 0.005), 2-fold lower on day 5 (Tukey’s HSD, p = 0.009), and > 2-fold lower on day 13 (Tukey’s HSD, p = 0.001) compared to control tanks. There was no main effect of NO3− treatment on % denitrification (LME, p = 0.58179), instead, the effect of NO3− depended on OM treatment (LME, OM x NO3−, p = 0.005) and time (LME, NO3− n × Day, p = 0.005, Fig. 3G–I). At elevated NO3− concentraions, % denitrification was 2.5-fold higher in the OM_L treatment compared to the OM_L treatment at ambient NO3− concentrations (TSE, p = 0.014). However, % denitrification did not differ significantly between OM_R and control treatments regardless of NO3- treatment (TSE, p = 0.147, p = 0.999 respectively). On days 1 and 5, at elevated NO3− concentrations % denitrification was lower by 50% compared to rates at ambient NO3− concentrations (TSE, p = 0.021, p = 0.049 respectively) and did not differ at day 13 (TSE, p = 0.125).
Mean thin-disc sediment ergosterol concentrations. A Sediment ergosterol concentration in the natural (n = 35) and constructed marsh (n = 7). Due to the large number of samples below detection in the constructed marsh, no statistical comparison was made. The natural marsh is shown in black and the constructed marsh is shown in gray. B Sediment ergosterol between OM treatments in the natural marsh. From left to right, the treatments are None, Labile, and Recalcitrant. All bars are standard error
Microbial biomass
No OM addition treatments
Ergosterol in the constructed marsh was on average > 5-fold lower than in the natural marsh (LME, p = 0.0034) (Fig. 4), and was below detection in all treatments without OM addition. Only 7 out of 36 samples in the constructed marsh had detectable ergosterol with a mean value of 0.43 ug g dry sed−1 in these detectable samples compared to a mean of 2.47 ug g dry sed−1 in the natural marsh. No ergosterol was detectable in the constructed marsh control treatment. Ergosterol did not change over the course of the incubations in the natural marsh (LME, p = 0.399). Because of this disparity in detectable ergosterol between marshes, treatment effects on ergosterol were only analyzed on the natural marsh.
Bacterial cell count differed significantly between marsh types (LME, p < 0.005, Fig. 5), with the natural marsh exceeding more than 15-fold the number of bacterial cells than the constructed marsh on average. Elevated NO3− had no effect on ergosterol (in the natural marsh only; LME, p = 0.749) or bacterial biomass. Bacterial cell counts increased over time in the natural marsh (LME, p < 0.005). Bacterial cell count increased by > 6-fold between day 1 and 5 (Tukey’s HSD, p = 0.003, and increased by 5.9-fold between day 1 and 13 (Tukey’s HSD, p < 0.005). Cell counts were similar between day 5 and 13 (Tukey’s HSD, p = 0.682).
Mean sediment bacterial biomass. Bacterial biomass as measured by bacterial cell count over time in each marsh. From left to right, the OM treatments are control, labile OM, and recalcitrant OM. Significance (LME OM*Day*Marsh, Tukey’s HSD results) is indicated with an asterisk. Bars represent standard error
OM addition treatments
Of the 7 detectable ergosterol samples in the constructed marsh, 6 of these were observed in the OM_R treatment and 1 was observed in the OM_L treatment (Fig. 4). In the natural marsh, OM treatment did not have a significant effect on ergosterol concentrations (LME, p = 0.0970). For bacterial cell counts, there was no main effect of OM treatment; however, there was a significant OM x Day interaction (LME, p = 0.01590). In the OM_L treatment bacterial cell count increased by > 2 × compared to the control treatment on day 13 (Tukey’s HSD, p = 0.0137), whereas there was no difference in bacterial cell count between recalcitrant OM and the control on that day in the constructed marsh (Tukey’s HSD, p = 0.78). There was a marginally significant relationship between OM × Marsh (LME, p = 0.066) where bacterial cell counts in the natural marsh increased in both OM_R (Tukey’s HSD, p < 0.005) and OM_L treatments (Tukey’s HSD, p = 0.0002) by more than twofold. A simple effects test indicated that these different responses in bacterial cell counts to OM type by marsh type occurred on day 13 (TSE, p = 0.0146).
Discussion
N inputs to aquatic ecosystems are expected to increase over the next century as human population continues to increase (Bogard et al. 2020). Given the projection of continued nutrient increases and salt marsh degradation, we asked: do constructed marshes provide equivalent N removal as natural marshes? By removing vegetation effects, the thin discs used in this experiment isolated the potential of the microbial community alone to respond to these conditions. Our data demonstrate that constructed marshes have similar denitrification capacity to natural marshes, despite having lower % C, %N, and microbial biomass. Although some research has observed that biogeochemical equivalence can take more than a century to emerge (Moreno-Mateos et al. 2012), research on the Gulf Coast in particular has found that denitrification recovery can occur within two decades (Kleinhuizen and Mortazavi 2018). Our results indicate that microbial communities in constructed marshes are capable of similar capacity for both denitrification and DNRA in as early as 30 years (Fig. 1).
These results also show that denitrification and DNRA in both marsh types respond similarly to enhanced supply of OM and support previous observations that denitrification in natural and constructed wetlands was limited by labile OM availability (Kozub and Liehr 1999; Hamersley and Howes 2002, 2003; He et al. 2016). We found that recalcitrant OM had no significant effect on denitrification, neither increasing it as hypothesized in (Yang et al. 2015)) nor decreasing it as demonstrated in Dodla et al. (2008). Although there was no significant effect of OM additions on denitrification in either marsh, the patterns of higher denitrification under OM additions by the end of the incubation suggest that OM additions may sustain long-term denitrification capacity, as OM has been observed to sustain heterotrophic processes in other systems (Kozub and Liehr 1999; Babbin and Ward 2013; Hardison et al. 2015; Grebliunas and Perry 2016). Instead of being driven by OM quality, denitrification may simply be driven by the total availability of OM in a system (Ro et al. 2018), suggesting that as productivity increases in response to greater inputs of nutrients and the supply of OM to the heterotrophic community increases, denitrification will also increase.
Denitrification rates were not driven by higher inputs of NO3− alone in either the natural or constructed marsh. However, denitrification increased over time in elevated NO3− treatments in the presence of higher OM. The difference between denitrification in ambient and elevated NO3− treatments at any single time point was not significant, in contrast to prior investigations that showed denitrification to be limited by NO3− availability (Knowles 1982; Seitzinger et al. 2006; Dong et al. 2011; Algar and Vallino 2014; Hardison et al. 2015). This suggests that denitrification and DNRA in these marshes are OM limited rather than NO3− limited. However, porewater concentration of NO3− collected in marshes near these study sites suggest a much lower concentration than what was used here in the ambient NO3− addition treatment (Ledford et al. 2020). Although the NO3− concentration used in the ambient NO3− treatments was in line with nutrient data collected in the Gulf and historical USGS water quality data from freshwater streams near the study sites (Domangue and Mortazavi 2018; Kleinhuizen and Mortazavi 2018), USGS streamgages 02479945, 02479980, 02378300), it is possible that both NO3− treatments resulted in NO3− saturation, resulting in the observed absence of NO3− addition effect. However, even if this saturation led to the lack of response to NO3− inputs, these marshes might be unable to mitigate large pulses of NO3− into the system.
DNRA increased in response to increases in labile OM in both marshes and the addition of labile OM was the sole predictor of whether NO3− reduction was dominated by N removal or retention (Fig. 5). This is consistent with previous hypotheses and observations that higher ratios of OM to N favor N retention over removal (Hardison et al. 2015). Additionally, while the primary source of labile OM is viewed as originating as plant exudates in the rhizosphere, algae-derived OM (approximated in this study using Spirulina for the OM_L substate) rather than macrophyte-derived OM may support sediment metabolism in salt marshes as evidenced by sediment OM stoichiometry research (Hamersley and Howes 2003). Continued anthropogenic N inputs have the potential to proliferate inputs of algae-derived labile OM and create a feedback where greater algal growth pushes salt marshes to retain greater N, thus further supporting increased algal growth and contributing to salt marsh decline (Wasson et al. 2017; Ober and Martin 2018). Global climate change also has the potential to increase the rate of algal blooms in coastal waters (Gobler 2020), further contributing to this feedback between labile N inputs and N retention.
Furthermore, the observed lack of response in DNRA to higher NO3− inputs diverges from previous observations that NO3− availability drives partitioning of NO3− reduction in estuarine and marine sediments and further supports observations that the ratio of C/NO3− is more important in this partitioning than NO3− inputs alone (Dong et al. 2011; Bernard et al. 2015; Hardison et al. 2015; Peng et al. 2016). However, it is possible that our incubation experiment might not have continued long enough to capture NO3− related changes or that the ambient NO3− treatment might have resulted in NO3− saturation at both treatment levels. Partitioning of NO3− reduction from removal to retention might also be driven by sulfide production (Brunet and Garcia-Gil 1996; Murphy et al. 2020); however, the thin disc design allows for complete NO3− diffusion through the sediment and mitigates competitive sulfate competition (Aller and Mackin 1989).
Sediment C and N content were lower in the constructed marsh, even after 32 years. These findings are in line with other observational studies that have found that C and N can take many decades to recover (Craft et al. 2003; Moreno-Mateos et al. 2012). Critically, our C and N data and observed denitrification and DNRA rates demonstrate that functional recovery can occur in constructed marshes even with lower OM stocks. The similarity of denitrification and DNRA rates between marshes, despite lower C and N in the constructed marsh, may be explained by the similar C:N ratio between both sites. Experimental studies have demonstrated C:N ratio, rather than the stocks of neither C nor N independently, was a driving factor in N reduction processes (Kraft et al. 2014) with higher C:N ratios driving N reduction towards DNRA and low C:N ratios driving N reduction towards denitrification (Porubsky et al. 2009; Hardison et al. 2015; Peng et al. 2016). Thus, the similar process rates observed in this experiment might be attributable to similar C:N ratios between sites, despite differences in C and N stocks.
Despite decreased microbial biomass in the constructed marsh, denitrification and DNRA were similar to that observed in the natural marsh. This suggests the possibility that the microbial community in the constructed marsh is comprised of a larger proportion of denitrifiers, while the natural marsh likely has greater functional diversity or greater number of microbes competing for N (Kandeler et al. 2006; Dini-Andreote et al. 2016; Salles et al. 2017). However, this similarity may be attributable to potential alleviation of NO3− limitation as discussed above, and field measurements may show decreased process rates in the constructed marsh where microbial biomass and nutrient pools may be limiting. Additionally, measurements of denitrification rates in the same system using a different technique (Acetylene block technique versus IPT used in this study) found significant differences in denitrification rates between the natural and constructed marsh (Starr et al. 2022).
Neither OM quality nor NO3− availability influenced bacterial biomass in either marsh, although recalcitrant OM appeared to show a slight increase in bacterial biomass in the natural marsh over time. This is in contrast to other observations showing that OM quality differences and N substrate availability affects both bacterial and fungal growth (Meidute et al. 2008). Recalcitrant OM appeared to support increased bacterial growth over time, in contrast with the control and labile treatments, although this pattern is not statistically significant. The lack of fungal biomass response to NO3− is in contrast with other literature suggesting that fungi respond to NO3− during succession (Roy et al. 2017; Wang et al. 2019). Because our incubations were carried out in completely anoxic conditions, it is also possible that the oxygen requirements of fungal growth that are usually provided via rhizosphere interactions (Mansfield and Bärlocher 1993; Zhou et al. 2002; Daleo et al. 2007; Xu et al. 2016) were not met and contributed to the lack of response to either OM additions (specifically in the natural marsh) or N additions in this experiment. Nevertheless, the difference in fungal and bacterial biomass between marshes suggests that microbial community structure differs between natural and constructed marshes (Lynum et al. 2020).
This experiment simulated two scenarios of continued anthropogenic N inputs under different regimes of OM availability. We found that given enough bioavailable OM, rates of denitrification and DNRA increase in both natural and a 32 year-old constructed marsh sediments, despite lower microbial biomass and decreased stocks of sediment C and N in the constructed marsh. Additionally, OM quality did not influence bacterial biomass, but there was a difference between OM additions and other treatments on fungal biomass in the constructed marsh with an apparent increase in fungal biomass with increased recalcitrant OM inputs. However, the similar concentrations of water column nutrients, apparent promotion of fungal biomass with recalcitrant OM additions, and high rates of both denitrification and DNRA in response to the supply of labile OM additions suggests that at three decades post-construction, constructed marshes are capable of similar OM mineralization, denitrification, and DNRA as natural marshes.
Data availability
Data is available as a table in the supplemental information file.
References
Algar C, Vallino J (2014) Predicting microbial nitrate reduction pathways in coastal sediments. Aquat Microb Ecol 71:223–238. https://doi.org/10.3354/ame01678
Aller RC, Mackin JE (1989) Open-incubation, diffusion methods for measuring solute reaction rates in sediments. J Mar Res 47:411–440. https://doi.org/10.1357/002224089785076262
Babbin AR, Ward BB (2013) Controls on nitrogen loss processes in Chesapeake Bay sediments. Environ Sci Technol 47:4189–4196. https://doi.org/10.1021/es304842r
Baldock JA, Masiello CA, Gélinas Y, Hedges JI (2004) Cycling and composition of organic matter in terrestrial and marine ecosystems. Mar Chem 92:39–64. https://doi.org/10.1016/j.marchem.2004.06.016
Battaglia LL, Woodrey MS, Peterson MS, Dillon KS, Visser JM (2012) Wetlands of the northern Gulf Coast. Wetland habitats of North America: ecology and conservation concerns, pp 75–88
Bernard RJ, Mortazavi B, Kleinhuizen AA (2015) Dissimilatory nitrate reduction to ammonium (DNRA) seasonally dominates NO3—reduction pathways in an anthropogenically impacted sub-tropical coastal lagoon. Biogeochemistry 125:47–64. https://doi.org/10.1007/s10533-015-0111-6
Bogard MJ, Vogt RJ, Hayes NM, Leavitt PR (2020) Unabated nitrogen pollution favors growth of toxic cyanobacteria over chlorophytes in most hypereutrophic lakes. Environ Sci Technol 54:3219–3227. https://doi.org/10.1021/acs.est.9b06299
Bruland GL, Richardson CJ, Whalen SC (2006) Spatial variability of denitrification potential and related soil properties in created, restored, and paired natural wetlands. Wetlands 26(4):1042–1056
Brunet RC, Garcia-Gil LJ (1996) Sulfide-induced dissimilatory nitrate reduction to ammonia in anaerobic freshwater sediments. FEMS Microbiol Ecol 21:131–138. https://doi.org/10.1111/j.1574-6941.1996.tb00340.x
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5:89–96. https://doi.org/10.1890/1540-9295(2007)5[89:HWOTRO]2.0.CO;2
Chigineva NI, Aleksandrova AV, Tiunov AV (2009) The addition of labile carbon alters litter fungal communities and decreases litter decomposition rates. Appl Soil Ecol 42:264–270. https://doi.org/10.1016/j.apsoil.2009.05.001
Cleveland CC, Nemergut DR, Schmidt SK, Townsend AR (2007) Increases in soil respiration following labile carbon additions linked to rapid shifts in soil microbial community composition. Biogeochemistry 82:229–240. https://doi.org/10.1007/s10533-006-9065-z
Cornell JA, Craft CB, Megonigal JP (2007) Ecosystem gas exchange across a created salt marsh chronosequence. Wetlands 27:240–250
Craft C (2001) Soil organic carbon, nitrogen, and phosphorus as indicators of recovery in restored “Spartina” marshes. Ecol Restor 19:87–91
Craft C, Reader J, Sacco JN, Broome SW (1999) Twenty-Five years of ecosystem development of constructed Spartina alterniflora (Loisel) marshes. Ecol Appl 9:1405–1419
Craft C, Megonigal P, Broome S et al (2003) The pace of ecosystem development of constructed Spartina alterniflora marshes. Ecol Appl 13:1417–1432. https://doi.org/10.1890/02-5086
Daleo P, Fanjul E, Casariego AM et al (2007) Ecosystem engineers activate mycorrhizal mutualism in salt marshes. Ecol Lett 10:902–908. https://doi.org/10.1111/j.1461-0248.2007.01082.x
Davis JL, Currin CA, O’Brien C et al (2015) Living shorelines: Coastal resilience with a blue carbon benefit. PLoS ONE 10:1–18. https://doi.org/10.1371/journal.pone.0142595
Dini-Andreote F, de Brossi MJL, van Elsas JD, Salles JF (2016) Reconstructing the genetic potential of the microbially-mediated nitrogen cycle in a salt marsh ecosystem. Front Microbiol 7:1–13. https://doi.org/10.3389/fmicb.2016.00902
Dodla SK, Wang JJ, DeLaune RD, RobertL C (2008) Denitrification potential and its relation to organic carbon quality in three coastal wetland soils. Sci Total Environ 407:471–480. https://doi.org/10.1016/J.SCITOTENV.2008.08.022
Domangue RJ, Mortazavi B (2018) Nitrate reduction pathways in the presence of excess nitrogen in a shallow eutrophic estuary. Environ Pollut 238:599–606. https://doi.org/10.1016/j.envpol.2018.03.033
Dong LF, Sobey MN, Smith CJ et al (2011) Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries. Limnol Oceanogr 56:279–291. https://doi.org/10.4319/lo.2011.56.1.0279
Eyre BD, Rysgaard S, Dalsgaard T, Christensen PB (2002) Comparison of isotope pairing and N2: Ar methods for measuring sediment denitrification—assumptions, modifications, and implications. Estuaries 25:1077–1087. https://doi.org/10.1007/bf02692205
Fluet-Chouinard E, Stocker BD, Zhang Z, Malhotra A, Melton JR, Poulter B et al (2023) Extensive global wetland loss over the past three centuries. Nature 614(7947):281–286
Gobler CJ (2020) Climate change and harmful algal blooms: insights and perspective. Harmful Algae. https://doi.org/10.1016/J.HAL.2019.101731
Grasshoff K (1983) Determination of nitrite. Methods of seawater analysis. Wiley, Hoboken, pp 143–150
Grebliunas BD, Perry WL (2016) Carbon limitation of sediment bacterial production and denitrification in high nitrate low carbon systems. Environ Earth Sci 75:1–9. https://doi.org/10.1007/s12665-016-5464-1
Hamersley MR, Howes BL (2002) Control of denitrification in a septage-treating artificial wetland: the dual role of particulate organic carbon. Water Res 36:4415–4427
Hamersley M, Howes B (2003) Contribution of denitrification to nitrogen, carbon, and oxygen cycling in tidal creek sediments of a New England salt marsh. Mar Ecol Prog Ser 262:55–69. https://doi.org/10.3354/meps262055
Hardison AK, Algar CK, Giblin AE, Rich JJ (2015) Influence of organic carbon and nitrate loading on partitioning between dissimilatory nitrate reduction to ammonium (DNRA) and N2 production. Geochim Cosmochim Acta 164:146–160. https://doi.org/10.1016/J.GCA.2015.04.049
He Y, Widney S, Ruan M et al (2016) Accumulation of soil carbon drives denitrification potential and lab-incubated gas production along a chronosequence of salt marsh development. Estuar Coast Shelf Sci 172:72–80. https://doi.org/10.1016/j.ecss.2016.02.002
Henry KM, Twilley RR (2013) Soil development in a coastal Louisiana wetland during a climate-induced vegetation shift from salt marsh to mangrove. J Coast Res 29:1273–1283. https://doi.org/10.2112/JCOASTRES-D-12-00184.1
Holmes RM, Aminot A, Kerouel R et al (1999) A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can J Fish Aquat Sci 56:1801–1808
Jordan SJ, Stoffer J, Nestlerode JA (2011) Wetlands as sinks for reactive nitrogen at continental and global scales: a meta-analysis. Ecosystems 14:144–155. https://doi.org/10.1007/s10021-010-9400-z
Kana TM, Darkangelo C, Hunt MD et al (1994) Membrane inlet mass spectrometer for rapid high-precision determination of N2, 02, and Ar in environmental water samples. Global Biogeochem Cycles 66:597–601
Kandeler E, Deiglmayr K, Tscherko D et al (2006) Abundance of narG, nirS, nirK, and nosZ genes of denitrifying bacteria during primary successions of a glacier foreland. Appl Environ Microbiol 72:5957–5962. https://doi.org/10.1128/AEM.00439-06
Kennish MJ (2001) Coastal salt marsh systems in the U.S.: a review of anthropogenic impacts. Rev Anthropogenic Coast Res 1:731–748
Kirwan ML, Megonigal JP (2013) Tidal wetland stability in the face of human impacts and sea-level rise. Nature 504:53–60. https://doi.org/10.1038/nature12856
Kleinhuizen AA, Mortazavi B (2018) Denitrification capacity of a natural and a restored marsh in the Northern Gulf of Mexico. Environ Manage. https://doi.org/10.1007/s00267-018-1057-y
Knowles R (1982) Denitrification. Microbiol Rev 46:43–70. https://doi.org/10.1128/mmbr.46.1.43-70.1982
Koop-Jakobsen K, Giblin AE (2009) New approach for measuring denitrification in the rhizosphere of vegetated marsh sediments. Limnol Oceanogr Methods 7:626–637
Kozub DD, Liehr SK (1999) Assessing denitrification rate limiting factors in a constructed wetland receiving landfill leachate. Water Sci Technol 40:75–82. https://doi.org/10.1016/S0273-1223(99)00459-X
Kraft B, Tegetmeyer HE, Sharma R et al (2014) The environmental controls that govern the end product of bacterial nitrate respiration. Science 345:676–679. https://doi.org/10.1126/science.1254070
Krull K, Craft C (2009) Ecosystem development of a sandbar emergent tidal marsh, Altamaha River Estuary, Georgia, USA. Wetlands 29:314–322. https://doi.org/10.1672/06-178.1
Ledford TC, Mortazavi B, Tatariw C, Mason OU (2020) Elevated nutrient inputs to marshes differentially impact carbon and nitrogen cycling in two northern Gulf of Mexico saltmarsh plants. Biogeochemistry 149(1):1–16
Li X, Bellerby R, Craft C, Widney SE (2018) Coastal wetland loss, consequences, and challenges for restoration. Anthropocene Coasts 1:1–15. https://doi.org/10.1139/anc-2017-0001
Lynum CA, Bulseco AN, Dunphy CM et al (2020) Microbial community response to a passive salt marsh restoration. Estuar Coast 41:1439–1455. https://doi.org/10.1007/s12237-020-00719-y
Magill AH, Aber JD (2000) Variation in soil net mineralization rates with dissolved organic carbon additions. Soil Biol Biochem 32:597–601
Mansfield SD, Bärlocher F (1993) Seasonal variation of fungal biomass in the sediment of a salt marsh in New Brunswick. Microb Ecol 26:37–45. https://doi.org/10.1007/BF00166028
Meidute S, Demoling F, Bååth E (2008) Antagonistic and synergistic effects of fungal and bacterial growth in soil after adding different carbon and nitrogen sources. Soil Biol Biochem 40:2334–2343. https://doi.org/10.1016/j.soilbio.2008.05.011
Mitsch WJ, Bernal B, Nahlik AM et al (2013) Wetlands, carbon, and climate change. Landsc Ecol 28:583–597. https://doi.org/10.1007/s10980-012-9758-8
Moreno-Mateos D, Power ME, Comín FA, Yockteng R (2012) Structural and functional loss in restored wetland ecosystems. PLoS Biol 10:1001247. https://doi.org/10.1371/journal.pbio.1001247
Murphy AE, Bulseco AN, Ackerman R et al (2020) Sulphide addition favours respiratory ammonification (DNRA) over complete denitrification and alters the active microbial community in salt marsh sediments. Environ Microbiol 22:2124–2139. https://doi.org/10.1111/1462-2920.14969
Nielsen LP (1992) Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol Lett 86:357–362. https://doi.org/10.1111/j.1574-6968.1992.tb04828.x
Ober GT, Martin RM (2018) Sea-level rise and macroalgal blooms may combine to exacerbate decline in Spartina patens and Spartina alterniflora marshes. Hydrobiologia 823:13–26. https://doi.org/10.1007/s10750-018-3689-6
Peng X, Ji Q, Angell JH et al (2016) Long-term fertilization alters the relative importance of nitrate reduction pathways in salt marsh sediments. J Geophys Res Biogeosci 121:2082–2095. https://doi.org/10.1002/2016JG003484
Porubsky WP, Weston NB, Joye SB (2009) Benthic metabolism and the fate of dissolved inorganic nitrogen in intertidal sediments. Estuar Coast Shelf Sci 83:392–402. https://doi.org/10.1016/j.ecss.2009.04.012
Ro HM, Kim PG, Park JS et al (2018) Nitrogen removal through N cycling from sediments in a constructed coastal marsh as assessed by 15 N–isotope dilution. Mar Pollut Bull 129:275–283. https://doi.org/10.1016/j.marpolbul.2018.02.037
Roy J, Reichel R, Bruggemann N et al (2017) Succession of arbuscular mycorrhizal fungi along a 52-year agricultural recultivation chronosequence. FEMS Microbiol Ecol 93:102. https://doi.org/10.1093/femsec/fix102
Salles FJ, Pereira e Silva MC, Dini-Andreote F et al (2017) Successional patterns of key genes and processes involved in the microbial nitrogen cycle in a salt marsh chronosequence. Biogeochemistry 132:185–201. https://doi.org/10.1007/s10533-017-0296-y
Schnetger B, Lehners C (2014) Determination of nitrate plus nitrite in small volume marine water samples using vanadium(III)chloride as a reduction agent. Mar Chem 160:91–98. https://doi.org/10.1016/j.marchem.2014.01.010
Seitzinger S, Harrison JA, Böhlke JK et al (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16:2064–2090. https://doi.org/10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2
Sousa AI, Lillebø AI, Risgaard-Petersen N et al (2012) Denitrification: an ecosystem service provided by salt marshes. Mar Ecol Prog Ser. https://doi.org/10.3354/meps09526
Starr SF, Mortazavi B, Tatariw C et al (2022) Poor recovery of fungal denitrification limits nitrogen removal capacity in a constructed Gulf Coast marsh. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2022.108692
Stelzer RS, Scott JT, Bartsch LA et al (2014) Particulate organic matter quality influences nitrate retention and denitrification in stream sediments: evidence from a carbon burial experiment. Biogeochemistry 119:387–402. https://doi.org/10.1007/s10533-014-9975-0
Strauss EA, Lamberti GA (2002) Effect of dissolved organic carbon quality on microbial decomposition and nitrification rates in stream sediments. Freshw Biol 2:65–74
Thamdrup B, Dalsgaard T (2002) Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl Environ Microbiol 68:1312–1318. https://doi.org/10.1128/AEM.68.3.1312
Valiela I, Cole ML (2002) Comparative evidence that salt marshes and mangroves may protect seagrass meadows from land-derived nitrogen loads. Ecosystems 5:92–102. https://doi.org/10.1007/s10021-001-0058-4
Wang J, Liu G, Zhang C et al (2019) Higher temporal turnover of soil fungi than bacteria during long-term secondary succession in a semiarid abandoned farmland. Soil Tillage Res 194:104305. https://doi.org/10.1016/j.still.2019.104305
Wasson K, Jeppesen R, Endris C et al (2017) Eutrophication decreases salt marsh resilience through proliferation of algal mats. Biol Conserv 212:1–11. https://doi.org/10.1016/j.biocon.2017.05.019
Xu Z, Ban Y, Jiang Y et al (2016) Arbuscular mycorrhizal fungi in wetland habitats and their application in constructed wetland: a review. Pedosphere 26:592–617. https://doi.org/10.1016/S1002-0160(15)60067-4
Yang W, An SQ, Zhao H et al (2015) Labile and recalcitrant soil carbon and nitrogen pools in tidal salt marshes of the Eastern Chinese Coast as affected by short-Term C4 plant Spartina alterniflora invasion. Clean (weinh) 43:872–880. https://doi.org/10.1002/clen.201300846
Yin G, Hou L, Liu M et al (2014) A novel membrane inlet mass spectrometer method to measure 15NH4+ for isotope-enrichment experiments in aquatic ecosystems. Environ Sci Technol 48:9555–9562. https://doi.org/10.1021/es501261s
Zhou Z, Takaya N, Nakamura A et al (2002) Ammonia fermentation, a novel anoxic metabolism of nitrate by fungi. J Biol Chem 277:1892–1896. https://doi.org/10.1074/jbc.M109096200
Acknowledgements
We thank Dr. Loraé T. Simpson and Gabriella Crawford for their help. George Crozier inspired us to work at this site. Barry A. Vittor provided historical information about the study site. We are also grateful to George McKean and the Dauphin Island Sea Lab for their access to field sites and Jarred Miller from the UA Office of Council for his assistance.
Funding
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship, the Birmingham Audubon Society, and the National Science Foundation (CBET 1438092, 1643486), and College Academy for Research, Scholarship and Creative Activity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial or non-financial interests.
Additional information
Responsible Editor: Kate Lajtha.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Starr, S.F., Mortazavi, B., Tatariw, C. et al. Labile organic matter promotes nitrogen retention in natural and constructed gulf coast marshes. Biogeochemistry 167, 269–285 (2024). https://doi.org/10.1007/s10533-024-01128-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-024-01128-0