Abstract
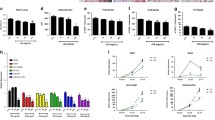
Autosomal Dominant Osteopetrosis type II (ADO2) is a rare bone disease of impaired osteoclastic bone resorption caused by heterozygous missense mutations in the chloride channel 7 (CLCN7). Adenylate cyclase, which catalyzes the formation of cAMP, is critical for lysosomal acidification in osteoclasts. We found reduced cAMP levels in ADO2 osteoclasts compared to wild-type (WT) osteoclasts, leading us to examine whether regulating cAMP would improve ADO2 osteoclast activity. Although forskolin, a known activator of adenylate cyclase and cAMP levels, negatively affected osteoclast number, it led to an overall increase in ADO2 and WT osteoclast resorption activity in vitro. Next, we examined cAMP hydrolysis by the phosphodiesterase 4 (PDE4) proteins in ADO2 versus WT osteoclasts. QPCR analysis revealed higher expression of the three major PDE4 subtypes (4a, 4b, 4d) in ADO2 osteoclasts compared in WT, consistent with reduced cAMP levels in ADO2 osteoclasts. In addition, we found that the PDE4 antagonists, rolipram and roflumilast, stimulated ADO2 and WT osteoclast formation in a dose-dependent manner. Importantly, roflumilast and rolipram displayed a concentration-dependent increase in osteoclast resorption activity which was greater in ADO2 than WT osteoclasts. Moreover, treatment with roflumilast rescued cAMP levels in ADO2 OCLs. The key findings from our studies demonstrate that osteoclasts from ADO2 mice exhibit reduced cAMP levels and PDE4 inhibition rescues cAMP levels and ADO2 osteoclast activity dysfunction in vitro. The mechanism of action of PDE4 inhibitors and their ability to reduce the high bone mass of ADO2 mice in vivo are currently under investigation. Importantly, these studies advance the understanding of the mechanisms underlying the ADO2 osteoclast dysfunction which is critical for the development of therapeutic approaches to treat clinically affected ADO2 patients.







Similar content being viewed by others
References
Stauber T, Weinert S, Jentsch TJ (2012) Cell biology and physiology of CLC chloride channels and transporters. Compr Physiol 2:1701–1744
Poroca DR, Pelis RM, Chappe VM (2017) ClC channels and transporters: structure, physiological functions, and implications in human chloride channelopathies. Front Pharmacol 8:151
Jentsch TJ, Pusch M (2018) CLC chloride channels and transporters: structure, function, physiology, and disease. Physiol Rev 98:1493–1590
Jentsch TJ, Stein V, Weinreich F, Zdebik AA (2002) Molecular structure and physiological function of chloride channels. Physiol Rev 82:503–568
Jentsch TJ, Poët M, Fuhrmann JC, Zdebik AA (2005) Physiological functions of CLC Cl- channels gleaned from human genetic disease and mouse models. Annu Rev Physiol 67:779–807
Graves AR, Curran PK, Smith CL, Mindell JA (2008) The Cl-/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 453:788–792
Guzman RE, Grieschat M, Fahlke C, Alekov AK (2013) ClC-3 is an intracellular chloride/proton exchanger with large voltage-dependent nonlinear capacitance. ACS Chem Neurosci 4:994–1003
Matsuda JJ, Filali MS, Volk KA, Collins MM, Moreland JG, Lamb FS (2008) Overexpression of CLC-3 in HEK293T cells yields novel currents that are pH dependent. Am J Physiol Cell Physiol 294:C251-262
Neagoe I, Stauber T, Fidzinski P, Bergsdorf EY, Jentsch TJ (2010) The late endosomal ClC-6 mediates proton/chloride countertransport in heterologous plasma membrane expression. J Biol Chem 285:21689–21697
Picollo A, Pusch M (2005) Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature 436:420–423
Scheel O, Zdebik AA, Lourdel S, Jentsch TJ (2005) Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436:424–427
Lange PF, Wartosch L, Jentsch TJ, Fuhrmann JC (2006) ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature 440:220–223
Meadows NA, Sharma SM, Faulkner GJ, Ostrowski MC, Hume DA, Cassady AI (2007) The expression of Clcn7 and Ostm1 in osteoclasts is coregulated by microphthalmia transcription factor. J Biol Chem 282:1891–1904
Leisle L, Ludwig CF, Wagner FA, Jentsch TJ, Stauber T (2011) ClC-7 is a slowly voltage-gated 2Cl(-)/1H(+)-exchanger and requires Ostm1 for transport activity. Embo J 30:2140–2152
Weinert S, Jabs S, Hohensee S, Chan WL, Kornak U, Jentsch TJ (2014) Transport activity and presence of ClC-7/Ostm1 complex account for different cellular functions. EMBO Rep 15:784–791
Brandt S, Jentsch TJ (1995) ClC-6 and ClC-7 are two novel broadly expressed members of the CLC chloride channel family. FEBS Lett 377:15–20
Kornak U, Kasper D, Bösl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ (2001) Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104:205–215
Schulz P, Werner J, Stauber T, Henriksen K, Fendler K (2010) The G215R mutation in the Cl-/H+-antiporter ClC-7 found in ADO II osteopetrosis does not abolish function but causes a severe trafficking defect. PLoS ONE 5:e12585
Henriksen K, Gram J, Schaller S, Dahl BH, Dziegiel MH, Bollerslev J, Karsdal MA (2004) Characterization of osteoclasts from patients harboring a G215R mutation in ClC-7 causing autosomal dominant osteopetrosis type II. Am J Pathol 164:1537–1545
Alam I, Gray AK, Chu K, Ichikawa S, Mohammad KS, Capannolo M, Capulli M, Maurizi A, Muraca M, Teti A, Econs MJ, Del Fattore A (2014) Generation of the first autosomal dominant osteopetrosis type II (ADO2) disease models. Bone 59:66–75
Chu K, Snyder R, Econs MJ (2006) Disease status in autosomal dominant osteopetrosis type 2 is determined by osteoclastic properties. J Bone Miner Res 21:1089–1097
Alam I, Gerard-O’Riley RL, Acton D, Hardman SL, Hong JM, Bruzzaniti A, Econs MJ (2021) Chloroquine increases osteoclast activity in vitro but does not improve the osteopetrotic bone phenotype of ADO2 mice. Bone 153:116160
Delaisse JM, Søe K, Andersen TL, Rojek AM, Marcussen N (2021) The mechanism switching the osteoclast from short to long duration bone resorption. Front Cell Dev Biol 9:644503
von Kleist L, Haucke V (2012) At the crossroads of chemistry and cell biology: inhibiting membrane traffic by small molecules. Traffic 13:495–504
Rahman N, Ramos-Espiritu L, Milner TA, Buck J, Levin LR (2016) Soluble adenylyl cyclase is essential for proper lysosomal acidification. J Gen Physiol 148:325–339
Tasken K, Aandahl EM (2004) Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev 84:137–167
Taylor SS, Buechler JA, Yonemoto W (1990) cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem 59:971–1005
Biel M, Zong X, Ludwig A, Sautter A, Hofmann F (1999) Structure and function of cyclic nucleotide-gated channels. Rev Physiol Biochem Pharmacol 135:151–171
Finn JT, Grunwald ME, Yau KW (1996) Cyclic nucleotide-gated ion channels: an extended family with diverse functions. Annu Rev Physiol 58:395–426
Bos JL (2003) Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol 4:733–738
Houslay MD, Adams DR (2003) PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J 370:1–18
Soderling SH, Beavo JA (2000) Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol 12:174–179
Inoue D, Watanabe R, Okazaki R (2016) COPD and osteoporosis: links, risks, and treatment challenges. Int J Chron Obstruct Pulmon Dis 11:637–648
Kawamatawong T (2017) Roles of roflumilast, a selective phosphodiesterase 4 inhibitor, in airway diseases. J Thorac Dis 9:1144–1154
Scuvee-Moreau J, Giesbers I, Dresse A (1987) Effect of rolipram, a phosphodiesterase inhibitor and potential antidepressant, on the firing rate of central monoaminergic neurons in the rat. Arch Int Pharmacodyn Ther 288:43–49
Huang S, Eleniste PP, Wayakanon K, Mandela P, Eipper BA, Mains RE, Allen MR, Bruzzaniti A (2014) The Rho-GEF Kalirin regulates bone mass and the function of osteoblasts and osteoclasts. Bone 60:235–245
Kang KS, Hong JM, Horan DJ, Lim KE, Bullock WA, Bruzzaniti A, Hann S, Warman ML, Robling AG (2019) Induction of Lrp5 HBM-causing mutations in Cathepsin-K expressing cells alters bone metabolism. Bone 120:166–175
Chen X, Zhang K, Hock J, Wang C, Yu X (2016) Enhanced but hypofunctional osteoclastogenesis in an autosomal dominant osteopetrosis type II case carrying a c.1856C>T mutation in CLCN7. Bone Res 4:16035
Alatalo SL, Ivaska KK, Waguespack SG, Econs MJ, Vaananen HK, Halleen JM (2004) Osteoclast-derived serum tartrate-resistant acid phosphatase 5b in Albers-Schonberg disease (type II autosomal dominant osteopetrosis). Clin Chem 50:883–890
Alam I, McQueen AK, Acton D, Reilly AM, Gerard-O’Riley RL, Oakes DK, Kasipathi C, Huffer A, Wright WB, Econs MJ (2017) Phenotypic severity of autosomal dominant osteopetrosis type II (ADO2) mice on different genetic backgrounds recapitulates the features of human disease. Bone 94:34–41
Murrills RJ, Dempster DW (1990) The effects of stimulators of intracellular cyclic AMP on rat and chick osteoclasts in vitro: validation of a simplified light microscope assay of bone resorption. Bone 11:333–344
Park YG, Kim YH, Kang SK, Kim CH (2006) cAMP-PKA signaling pathway regulates bone resorption mediated by processing of cathepsin K in cultured mouse osteoclasts. Int Immunopharmacol 6:947–956
Ramaswamy G, Kim H, Zhang D, Lounev V, Wu JY, Choi Y, Kaplan FS, Pignolo RJ, Shore EM (2017) Gsalpha controls cortical bone quality by regulating osteoclast differentiation via cAMP/PKA and beta-catenin pathways. Sci Rep 7:45140
McSorley T, Stefan E, Henn V, Wiesner B, Baillie GS, Houslay MD, Rosenthal W, Klussmann E (2006) Spatial organisation of AKAP18 and PDE4 isoforms in renal collecting duct principal cells. Eur J Cell Biol 85:673–678
Stefan E, Wiesner B, Baillie GS, Mollajew R, Henn V, Lorenz D, Furkert J, Santamaria K, Nedvetsky P, Hundsrucker C, Beyermann M, Krause E, Pohl P, Gall I, MacIntyre AN, Bachmann S, Houslay MD, Rosenthal W, Klussmann E (2007) Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J Am Soc Nephrol 18:199–212
Takami M, Cho ES, Lee SY, Kamijo R, Yim M (2005) Phosphodiesterase inhibitors stimulate osteoclast formation via TRANCE/RANKL expression in osteoblasts: possible involvement of ERK and p38 MAPK pathways. FEBS Lett 579:832–838
Park H, No AL, Lee JM, Chen L, Lee SY, Lee DS, Yim M (2010) PDE4 inhibitor upregulates PTH-induced osteoclast formation via CRE-mediated COX-2 expression in osteoblasts. FEBS Lett 584:173–180
Souness JE, Griffin M, Maslen C, Ebsworth K, Scott LC, Pollock K, Palfreyman MN, Karlsson JA (1996) Evidence that cyclic AMP phosphodiesterase inhibitors suppress TNF alpha generation from human monocytes by interacting with a “low-affinity” phosphodiesterase 4 conformer. Br J Pharmacol 118:649–658
Tenor H, Hatzelmann A, Church MK, Schudt C, Shute JK (1996) Effects of theophylline and rolipram on leukotriene C4 (LTC4) synthesis and chemotaxis of human eosinophils from normal and atopic subjects. Br J Pharmacol 118:1727–1735
Hatzelmann A, Morcillo EJ, Lungarella G, Adnot S, Sanjar S, Beume R, Schudt C, Tenor H (2010) The preclinical pharmacology of roflumilast—a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther 23:235–256
Rabe KF (2011) Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol 163:53–67
Stauber T, Wartosch L, Vishnolia S, Schulz A, Kornak U (2023) CLCN7, a gene shared by autosomal recessive and autosomal dominant osteopetrosis. Bone 168:116639
Waguespack SG, Koller DL, White KE, Fishburn T, Carn G, Buckwalter KA, Johnson M, Kocisko M, Evans WE, Foroud T, Econs MJ (2003) Chloride channel 7 (ClCN7) gene mutations and autosomal dominant osteopetrosis, type II. J Bone Miner Res 18:1513–1518
Henriksen K, Sørensen MG, Jensen VK, Dziegiel MH, Nosjean O, Karsdal MA (2008) Ion transporters involved in acidification of the resorption lacuna in osteoclasts. Calcif Tissue Int 83:230–242
Henriksen K, Sorensen MG, Nielsen RH, Gram J, Schaller S, Dziegiel MH, Everts V, Bollerslev J, Karsdal MA (2006) Degradation of the organic phase of bone by osteoclasts: a secondary role for lysosomal acidification. J Bone Miner Res 21:58–66
Al-Bari AA, Al Mamun A (2020) Current advances in regulation of bone homeostasis. FASEB Bioadv 2:668–679
Krogstad DJ, Schlesinger PH (1987) The basis of antimalarial action: non-weak base effects of chloroquine on acid vesicle pH. Am J Trop Med Hyg 36:213–220
Collins MP, Forgac M (2020) Regulation and function of V-ATPases in physiology and disease. Biochim Biophys Acta Biomembr 1862:183341
Nicholson GC, Moseley JM, Yates AJ, Martin TJ (1987) Control of cyclic adenosine 3’,5’-monophosphate production in osteoclasts: calcitonin-induced persistent activation and homologous desensitization of adenylate cyclase. Endocrinology 120:1902–1908
Doorn J, Siddappa R, van Blitterswijk CA, de Boer J (2012) Forskolin enhances in vivo bone formation by human mesenchymal stromal cells. Tissue Eng Part A 18:558–567
Siddappa R, Mulder W, Steeghs I, van de Klundert C, Fernandes H, Liu J, Arends R, van Blitterswijk C, de Boer J (2009) cAMP/PKA signaling inhibits osteogenic differentiation and bone formation in rodent models. Tissue Eng Part A 15:2135–2143
Siddappa R, Doorn J, Liu J, Langerwerf E, Arends R, van Blitterswijk C, de Boer J (2010) Timing, rather than the concentration of cyclic AMP, correlates to osteogenic differentiation of human mesenchymal stem cells. J Tissue Eng Regen Med 4:356–365
Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, Houslay MD, Zaccolo M (2004) Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ Res 95:67–75
Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ (2002) Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science 298:834–836
Ainatzoglou A, Stamoula E, Dardalas I, Siafis S, Papazisis G (2021) The effects of PDE inhibitors on multiple sclerosis: a review of in vitro and in vivo models. Curr Pharm Des 27:2387–2397
Bundschuh DS, Eltze M, Barsig J, Wollin L, Hatzelmann A, Beume R (2001) In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther 297:280–290
Cho ES, Yu JH, Kim MS, Yim M (2004) Rolipram, a phosphodiesterase 4 inhibitor, stimulates osteoclast formation by inducing TRANCE expression in mouse calvarial cells. Arch Pharmacal Res 27:1258–1262
Alam I, Hardman SL, Gerard-O'Riley RL, Acton D, Parker RS, Hong JM, Bruzzaniti A, Econs MJ (2024) Effect of Roflumilast, a Selective PDE4 Inhibitor, on Bone Phenotypes in ADO2 Mice. Calcif Tissue Int. https://doi.org/10.1007/s00223-023-01180-2. Epub ahead of print. PMID: 38300304
Acknowledgements
We thank previous student members of our respective laboratories for technical assistance with osteoclast assays. These studies were funded in part by funds from the school of dentistry to AB.
Funding
This work was supported by the US National Institutes of Health grant AR069583.
Author information
Authors and Affiliations
Contributions
Study design: JMH, RLG, DA, IA, MJE, and AB. Study conduct: JMH, RLG, DA, IA, and AB. Data analysis: JMH and AB. Data interpretation: JMH, RLG, DA, IA, MJE, and AB. Drafting manuscript: JMH and AB. Revising manuscript content: All authors approved of final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Jung Min Hong, Rita L. Gerard-O’Riley, Dena Acton, Imranul Alam, Michael J. Econs, and Angela Bruzzaniti declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
Animal studies were conducted following protocols approved by the Indiana University Institutional Animal Care and Use Committee (IACUC).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
223_2024_1191_MOESM1_ESM.tif
Supplementary Figure 1. Rolipram and roflumilast increase the size of ADO2 OCL. BMMs were cultured with the PDE4 inhibitors, rolipram or roflumilast, for 5-6 days to form mature multinucleated OCLs. The area (size) of TRAP-positive multi-nucleated OCLs were determined (shown as µm2 x 103) determined using ImagePro Software. OCLs were generated from 4 individual mice. OCLs in 4 replicate wells per mouse were scored, with the mean ± SEM shown in the graphs. Please see Figures 4A and 5A for comparison graphs and images of TRAP-positive OCLs in vehicle and rolipram or roflumilast treated groups, respectively. Supplementary file1 (TIF 542 kb)
223_2024_1191_MOESM2_ESM.tif
Supplementary Figure 2. Rolipram and roflumilast stimulate ADO2 OCL resorption activity. Mature multinucleated OCLs from WT and ADO2 BMMs were cultured on cortical bone slices in the presence of A) rolipram or B) roflumilast, two PDE4 antagonists. Conditioned media was assayed for CTX-I and the data normalized for the number of TRAP-positive OCLs. The data from 2-5 independent experiments was consolidated and analyzed together for statistical significance, with the 0 drug WT and ADO2 data representing 5 independent experiments. Please refer to Figures 4-5 for representative data graphs with their respective OCLs images. Graphs represent mean ± SD. Asterisks (*) indicates p < 0.05 for comparison to the vehicle control for each genotype, whereas hash (#) indicates statistical significance for comparison between genotypes at matching drug concentrations (p < 0.05). Supplementary file2 (TIF 535 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hong, J.M., Gerard-O’Riley, R.L., Acton, D. et al. The PDE4 Inhibitors Roflumilast and Rolipram Rescue ADO2 Osteoclast Resorption Dysfunction. Calcif Tissue Int 114, 430–443 (2024). https://doi.org/10.1007/s00223-024-01191-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-024-01191-7