Abstract
Polyester manufacturing industries produce highly polluted effluents, containing organics, nutrients, trace metals, and 1,4-dioxane, requiring a high degree of treatment before being discharged into the water bodies. This study focused on removing complex pollutants from a diluted polyester industrial effluent (DPIE) via a cost-efficient anaerobic/aerobic combined system, with biogas recovery. The integrated pilot-scale system was composed of an up-flow anaerobic multi-staged reactor (UASR; V = 41 L) followed by an auto-aerated immobilized biomass (AIB; Vsponge = 9.54 L) unit and operated at a total organic loading rate (OLR) of 0.75 ± 0.16 g COD/L/d and pH of 7.14 ± 0.14 at 25 °C. The UASR achieved removal efficiencies of 17.82 ± 3.14% and 15.90 ± 3.08% for chemical oxygen demand (COD, total and soluble) and 15.83 ± 4.68% for total Kjeldahl nitrogen (TKN), with bio-CH4 yield of 263.24 ± 31.98 mL/g COD. Adding the AIB unit improved the overall CODtotal, CODsoluble, and TKN to 93.94 ± 2.39%, 94.84 ± 2.23%, and 75.81 ± 3.66%, respectively. The NH4-N removal efficiency was 85.66 ± 2.90% due to the oxic/nitrification condition on the sponge’s outer surface. The entire system also achieved 73.26 ± 2.68%, 77.48 ± 5.74%, and 81.26 ± 6.17% removals for Fe (3.93 ± 0.95 ppm), Zn (5.92 ± 2.32 ppm), and 1,4 dioxane (2.50 ± 0.61 ppm). Moreover, the UASR-AIB maintained removal efficiencies of 76.53 ± 8.47% and 77.51 ± 7.38% for total suspended solids (TSS: 335.95 ± 42.84 mg/L) and volatile suspended solids (VSS: 263.50 ± 36.94 mg/L). Regarding the DPIE toxicity level, the EC50 value increased from 12.9 to 39.4% after UASR/AIB application. The UASR’s microbial community at the genus level demonstrated that the synergistic cooperation of solubilization, hydrolysis, acidogenesis, acetogenesis, and methanogenesis was responsible for the degradation of DPIE components.
Similar content being viewed by others
Introduction
Polyesters are manufactured through a polycondensation process, utilizing dicarboxylic acids, anhydrides, and a mixture of dihydroxy compounds (Osama et al. 2021; Soares et al. 2017). The effluents of this manufacturing industry contain various organic pollutants, which could be inert, dissolved, colloidal, and/or slowly biodegradable (Osama et al. 2021; Gar Alalm et al. 2017). These organic-based compounds are expressed by the soluble and particulate fractions of chemical oxygen demand (CODsoluble; CODparticulate), giving a total COD greater than 3000 mg/L (Sun et al. 2018). It also includes complex chemicals, heavy metals, solids, and trace elements. Nitrogen species, e.g., ammonia (NH4-N), nitrate (NO3-N), and total Kjeldahl nitrogen (TKN), could also be detected in polyester-related wastewater mixed with domestic sources (Mahmoud et al. 2017). Moreover, polyester wastewater includes 1,4-dioxane (molecular weight of 88.1 g/mol), which is assigned as a possible human carcinogen (Stepien et al. 2014; Tawfik et al. 2022a, b, c). Discharging this wastewater (e.g., 200–300 L/ton polyester resin) without proper treatment into the environment is of great concern to humans and ecosystems (Elsamadony et al. 2015). Therefore, removing these complex compounds/chemicals from the polyester resin wastewater creates an interesting point of research.
Recent researchers have attempted to treat polyester wastewater biologically due to its cost-effectiveness and simplicity of operation. For instance, Mahmoud et al. (2017) employed an anaerobic-based unit to perform a biological treatment of polyester resin wastewater laden with CODtotal (4275 mg/L), TKN (81.27 mg/L), 1,4-dioxane (4.21 mg/L), and heavy metals. Although their study succeeded in removing over 25% of 1,4-dioxane, 58% of Cr3+, 5% of Fe3+, 56% of Mn2+, and 14% of Ni2+, the post-treatment of anaerobic reactor effluent is still required. Another study employed an aerobic process for post-treatment of anaerobic effluent to treat polyester resin wastewater (Sun et al. 2018; Elnmer et al. 2019). While their study succeeded in reducing COD from 12,880 to 500 mg/L and the majority of phenols, alcohols, and acids, the amount of biogas that evolved from the anaerobic treatment phase remains to be quantified (Sun et al. 2018). Osama et al. (2021) employed a duckweed pond system to treat polyester resin wastewater and succeeded in eliminating 63.4% of 1,4-dioxane, 28.3% of Cd2+, 93.2% of Cu2+, 95.7% of Zn2+, and 93.6% of Ni2+. However, this phytoremediation process still requires a post-treatment step to have a final quality complying with the discharge standards. Photochemical oxidation was used as post-treatment of an aerobic unit effluent to remove organic compounds, nitrogen, and dyes from polyester-cotton wastewater (Soares et al. 2017). However, the intensive use of oxygen supply and expensive high energy requirements could hinder the application of this technology in the middle‐ and low‐income countries. Based on the aforementioned literature, there is a lack of studies treating polyester wastewater by a combined anaerobic/aerobic, with biogas recovery, which should be cheap and less energy-consuming.
A combined anaerobic/aerobic biological scheme has been widely employed for treating domestic and industrial wastewater sources (Tyagi et al. 2021; Farghaly et al. 2017), especially when containing complex pollutants. At the initial stage of this integrated system, anaerobic baffled reactors can partially treat wastewater under an oxygen-deprived condition (Liang et al. 2020). The anaerobic biodegradation of organic compounds considers multiple steps, viz. hydrolysis, acidogenesis, acetogenesis, and methanogenesis (Schmidt et al. 2018). The reactor’s configuration allows for the separation of the predominant microbial populations at each anaerobic phase, adding benefits to the treatment performance (Mahmoud et al. 2017). This technology is suitable for treating industrial effluents because it accepts high organic-loading and requires less installation procedures compared with the conventional aerobic-based bioprocesses (Barros et al. 2017). Moreover, the anaerobic baffled reactor requires lower operating costs than the activated sludge systems because it produces less amount of sludge after long retention times (Kong et al. 2019). Employing this anaerobic system as pretreatment to effectively degrade complex and recalcitrant organic compounds in polyester wastewater would offer more complete oxidation and stable treatment performance. As such, the organic residues in the anaerobic effluent could be removed by the second treatment step (aerobic unit). Because organic nitrogen is converted to ammonia under an anaerobic condition, a further nitrification step is also essential for nitrogen removal (Tawfik et al. 2010). To maintain a cost-effective wastewater treatment process, the subsequent aerobic process should save energy use and minimize the related expenses. In particular, an auto-aerated immobilized biomass (AIB) reactor has been broadly used to maintain organic oxidation and nitrification/denitrification using natural oxygen from the air (Kumar et al. 2020). This unit is occupied by sponge media having a proper oxygen concentration gradient along the sponge depth and in the attached biomass (Tanikawa et al. 2019). For instance, this oxygen profile variation supports the existence of (i) heterotrophs degrading organics and nitrifiers oxidizing ammonia on the sponge’s outer surface having oxic conditions and (ii) denitrifiers in the inner pores/voids of the sponge pieces (Okubo et al. 2016). Accordingly, this anaerobic baffled reactor/sponge-based unit scheme has been successfully used to treat complex wastes such as onion dehydration wastewater (El-Kamah et al. 2011), hazardous landfill leachate (Ismail et al. 2020), and dyeing wastewater (Nguyen et al. 2020). However, using this combined scheme to treat polyester-containing industrial effluents has not yet been investigated.
Hence, this study focused on the application of a combined anaerobic/aerobic system to treat the effluents of polyester manufacturing industries containing organics, nitrogen, 1,4-dioxane, and heavy metals. This objective was illustrated by understanding the degradation mechanisms and performances along the profiles of each treatment unit. The study also provided insights into the microbial community dynamics responsible for degrading the wastewater pollutants, with biogas recovery.
Materials and methods
Characteristics of wastewater
The influent wastewater was composed of a mixture of polyester manufacturing industry wastewater and domestic wastewater (about 70:30, v/v), giving a diluted polyester industrial effluent (DPIE; Table 1). Polyester wastewater was collected from a chemical industry located in Borg El Arab, Alexandria, Egypt, producing unsaturated polyester resins. Domestic wastewater was collected from hand basins, kitchen sinks, laundries, and sewerage systems situated in Borg El Arab, Alexandria, Egypt. This domestic wastewater was used to dilute the industrial effluent and adapt the pH value around 7.0, avoiding inhibition for microorganisms in the subsequent bio-treatment facilities (Nurliyana et al. 2015).
Inoculation process
Sludge samples were collected from an anaerobic digester located in Alexandria, Egypt, and inoculated into the UASR (Elsamadony and Tawfik 2018). The sludge volume (equivalent to 10 L) was 25% of the reactor’s capacity. This sludge had total solids (TS) of 47 g/L and volatile solids (VS) of 32 g/L, giving a VS/TS ratio of 0.68:1. The seed sludge was acclimatized with DPIE for 30 days under the same operational condition of the UASR unit. For the AIB unit, the sponge pieces were immersed in waste activated sludge (VS/TS ratio = 0.62). The AIB facility was randomly filled by these biomass-loaded sponge elements. The sludge inside the packing medium developed rapidly within approximately 2 weeks, where the voids became saturated by about 18 g VSS/L of sponge volume. This step was used to obtain the steady-state condition before starting the experimental assays, following previous studies (Ismail et al. 2020; Nguyen et al. 2020; Tanikawa et al. 2019).
Experimental set-up of pilot plant
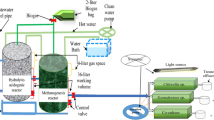
Figure 1 shows the anaerobic/aerobic (UASR/AIB) combined system used in this investigation to treat DPIE. The UASR unit had a height of 90 cm, length of 22.5 cm, and width of 22.5 cm and was made of Perspex material. These dimensions corresponded to a working volume of 41 L. The UASR had a pyramid shape at the bottom (wastewater inlet) and a gas–solid separator at the top. A number of baffles were fixed along the reactor’s height and inclined by an angle of 45 with the horizontal, prolonging the contact time between substrate and biomass. Ports were arranged along the reactor’s height for sample collection. A wet gas meter was connected to the UASR’s top to measure the volume of biogas evolved.
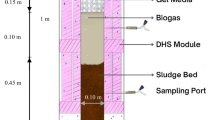
The AIB unit was manufactured from polyvinyl chloride and occupied by sponge pieces. This unit had an internal diameter of 25 cm and a height of 180 cm, giving a sponge working volume of 9.54 L. The reactor consisted of three similar segments connected in a vertical alignment. The segments were separated from each other by openings 10 cm in height, allowing for oxygen transfer from the surrounding air. The reactor’s inlet (at the top) included a rotary distributor to spread the feed homogeneously on the sponge’s surface. Each sponge element (3 cm in diameter and 3 cm in length) was made of polyurethane foam warped with a net-like cylindrical polyvinyl chloride ring. The packed media (specific surface area 256 m2/m3, density 30 kg/m3, and pore size 63 mm for each sponge) were randomly distributed in each segment, as reported earlier (Ismail and Tawfik 2016). A sedimentation zone with a volume of 4 L was installed at the bottom to receive the final effluent and enable sludge collection.
Operational condition
The polyester wastewater was fed continuously into the UASR unit using a peristaltic pump with a flow rate of 11.5 L/d. The influent wastewater moved from the bottom to the top of UASR with an up-flow velocity 0.0095 of m/h. Then, the UASR’s effluent was transferred to the top of the AIB reactor by gravity. The UASR unit was operated at a hydraulic retention time (HRT) of 85.6 h and an organic loading rate (OLR) of 0.92 g/L/d (estimated at CODfeed ≈ 3290 mg/L). This condition was associated with a food-to-microorganisms (F/M) ratio of 0.154 d–1 calculated using an average sludge mass of 245 g VS in the UASR. Moreover, the sludge retention time, with an excess sludge of 2.5 g/d, was about 98 days. The AIB unit was operated at a HRT of about 20 h, calculated based on the sponge volume. Using the amount of retained sludge (18 g VSS/L × 9.54 L = 172 g) and the excess sludge (Qwaste = 0.046 L/d with Xwaste = 29.2 g VSS/L), and by ignoring the sludge eluted in the effluent, the calculated SRT of AIB ranged from 125 to 130 days. The operational temperature of the bioreactors was 25 ± 5 °C, respectively, and the influent pH values were not controlled.
Analytical methods
Wastewater samples were collected from the bioreactors three times per week and analyzed for pH, COD (total, soluble, and particulate), NH4-N, NO3-N, total Kjeldahl nitrogen (TKN), total suspended solids (TSS), volatile suspended solids (VSS), and volatile fatty acids (VFAs). The soluble fractions were determined by passing the wastewater sample through 0.45 μm filter paper. All analytical procedures were performed according to the APHA standard method (APHA 2005). Carbohydrate concentration was measured by the phenol–sulfuric acid method, with the glucose standard solution (Dubois et al. 1956). The concentration of 1,4-dioxane was measured by the Shimadzu GCMS-QP2010 Ultra system using the gas chromatographic-mass spectrometer (GC/MS) method, as reported previously (Ismail et al. 2021). The sampling locations were at the influent, UASR’s effluent, and AIB’s effluent. The toxicity tests were performed using a Microtox® Model 500 Toxicity Analyzer, as reported earlier (Johnson 2005). The EC50 values obtained after 5 and 15 min were converted into toxic units and the statistical analysis was conducted using the Omni 4.1 software (Butarewicz et al. 2019). The solution pH was measured using a pH meter (HACH Hq40d multi) according to the PN-EN 12176 standard. The surface of sponge carriers, with the attached microorganism species, of the AIB reactor was analyzed by scanning electron microscope (SEM), as discussed earlier (Ismail et al. 2020; Tawfik et al. 2010; Allam et al. 2015).
Microbial community analysis
For total DNA extraction, sludge samples were collected from the UASR and pelleted by centrifugation (8000 g, 10 min). The FastDNA® SPIN kit for soil was used for extracting total microbial DNA, following the manufacturer’s instructions. DNA integrity was checked using 1% agarose gel electrophoresis, capturing the image by a gel documentation system (Gel Doc. BioRad). Epoch2 microplate reader (Bio-Tek Instruments, Thermo Scientific, USA) was used to determine the concentration and purity of purified DNA. The A260:A280 ratio was used to determine the purity of DNA. Purified DNA samples were sequenced using 16S rRNA (Macrogen Inc. company, South Korea). The bacterial and archaeal hypervariable region V4–V5 of the 16S rRNA gene with primers 515F (5’-GTGYCAGCMGCCGCGGTA-3’) and 928R (5’-CCCCGYCAATTCMTTTRAGT-3’) was targeted for Metagenome 16S amplicon sequencing (Poirier et al. 2016). The amplified libraries of V4-V5 region amplicons were pooled and sequenced using the Illumina MiSeq platform (2000 sequences), including 250-bp paired-end reads generated with a 7-cycle index read. (Sequences with an overlap longer than 10 bp without any mismatch were assembled.) After removing the barcode and primer sequences, the resultant overlapping paired-end reads were stitched and quality-filtered using Microsynth. Sequences were analyzed using the Quantitative Insights into Microbial Ecology (QIIME) software (Wu et al. 2010). Quality-filtered sequence data were exported as a FastQ file (15,000 to 90,000 reads for each sample). DADA2 plugin was employed to filter out non-biological sequences (e.g., primers, sequencing adapters, PCR spacers, etc.), remove noisy sequences, correct errors in marginal sequences, and eliminate chimeric sequences and singletons. Dereplication is the simplest clustering method, producing Amplicon sequence variants (ASVs), representing 100% operational taxonomic units (OTUs). Taxonomic classification and identification were performed using Microbiome Analyst tools (Dhariwal et al. 2017).
Results and discussion
Performance assessment of up-flow multi-staged anaerobic reactor (UASR)/auto-aerated immobilized biomass reactor (AIB) combined system
Organic matter removal
Figure 2(a&b) shows the variation of organic matter, expressed by the removal of COD fractions, in the proposed treatment systems. The CODsoluble, CODparticulate, and CODtotal were partially reduced through UASR, giving removal efficiencies of 12.24 ± 4.24%, 39.54 ± 17.83%, and 15.49 ± 4.21%, respectively. Removing the particulate fraction of COD was higher than other portions (e.g., CODsoluble) due to the entrapment of particulate organic matter in the UASR’s sludge bed. The particulate organics could also be removed via settling and adsorption in sludge bed. This particulate matter (e.g., lipids and proteins) could be further degraded and partially hydrolyzed into soluble compounds, justifying the increased CODsoluble fraction in the UASR’s effluent. The insufficient CODsoluble removal by UASR could also be ascribed to the decay of biomass accumulated at the reactor’s lower part. As such, the insufficient CODtotal removal efficiency by UASR could be because the majority of the influent COD was in the soluble form (CODsoluble = 0.88 CODtotal; see Table 1). Hence, the first treatment stage could be suitable to remove particulate COD and reduce the solids load imposed on the subsequent AIB unit.
Adding AIB to the treatment system (in the second stage) improved the organics removal performance, recording 94.84 ± 2.23% CODsoluble, 85.64 ± 10.37% CODparticulate, and 93.94 ± 2.39% CODtotal by the combined treatment scheme. The sponge medium in AIB was occupied by a high density of microbial biomass that utilized the soluble organic forms. Biomass located either on the surface or inside the sponge pieces, mainly in the AIB middle zone (see Fig. 1), tended to consume the soluble organic matter.
The TOC removal pattern was equivalent to that of COD (Fig. 2c). For instance, the TOC removal efficiency was only 10.29 ± 6.99% by UASR, whereas it reached 94.72 ± 2.67% by the combined system. Under the AIB’s aerobic condition, the organic compounds given by COHNS (carbon, oxygen, hydrogen, nitrogen, and sulfur) are converted to CO2, H2O, NH3, energy, and other end products. Because the final TOC concentration became below 80 mg/L (compared with influent TOC of 1021.13 ± 269.17 mg/L), AIB was appropriately used to polish the UASR’s effluent before final disposal.
The results presented in Fig. 2(d&e) also depicted that the UASR system achieved removal efficiencies of 14.67 ± 6.59% and 24.78 ± 15.60% for total and soluble carbohydrates, respectively. The carbohydrate-degrading bacterium, such as Clostridium, is usually responsible for carbohydrate conversion under anaerobic conditions (Tanikawa et al. 2020). The overall removal efficiencies of these carbohydrates increased up to 62.19 ± 18.79% and 69.01 ± 12.30%, respectively, via the integrated UASR/AIB system. Because the growth rate of the aerobes is relatively fast, they attempt to consume more organic components (e.g., carbohydrates) than the anaerobic community in UASR.
The pH of the UASR’s effluent remained approximately unchanged within the 6.8–7.2 range, suggesting the conversion of VFA to biogas. As such, a pH range of 6.5–7.5 tends to provide a suitable condition for methanogenic population growth (Kim et al. 2019). The balanced metabolic processes among various microbial species, which are involved in both acidogenic and methanogenic processes, could endorse stable pH values. The pH of the DHS’s effluent was stable in the range of 6.9–7.2, which did not fluctuate greatly during the study. This pH range provided a suitable condition for enhancing the synergistic effects of different microorganisms, maintaining proper COD and N removal efficiencies. Similarly, a practical-scale sponge-based reactor was operated at a pH range of 7.01–7.19, providing proper nitrification–denitrification reactions and maintaining a final effluent that complied with the water quality standards except for fecal coliform (Okubo et al. 2016).
Nitrogen removal
The results in Fig. 3(a-c) show that the AIB reactor was efficient in removing TKN and other nitrogen species. There was no noticeable enhancement in ammonia removal through UASR (Fig. 3a), probably due to the ammonification reactions associated with the hydrolysis and acidification of organic and particulate nitrogen (Rozaik et al. 2016). The nitrogen removal performance was improved to 75.81 ± 3.66% for TKN and 85.66 ± 2.89% for ammonia by adding the AIB unit (giving maximum removal efficiencies of 83.45% and 90.42%, respectively). The residual concentrations of these nitrogen species were 8.36 ± 1.90 mg/L and 2.24 ± 0.34 mg/L, respectively. The concentration of nitrate in the treated effluent was 2.26 ± 0.54 mg/L, compared with 0.49 ± 0.07 mg/L in raw wastewater. Apparently, a part of ammonia was converted to nitrate under the nitrification process in the AIB unit, as previously demonstrated (Tanikawa et al. 2019). Ammonia-oxidizing (i.e., Nitrosomonas) and nitrite-oxidizing (Nitrospira and Nitrospina) bacteria were dominated in the sponge media (Oshiki et al. 2020), supporting the nitrogen removal capability. The transformation of ammonium into nitrate by nitrifiers (slow-growing bacteria) in a sponge-based system has also been reported, achieving removal efficiencies of 72 ± 6% for TKN and 99 ± 1.3% for NH4-N (El-Kamah et al. 2011). Nitrate produced after nitrification could be used as a substrate of denitrification to produce nitrogen gas, where the simultaneous heterotrophic nitrification and aerobic denitrification process in AIB has been reported (Tanikawa et al. 2019). The reduction of TKN by the entire system could also be supported by the anaerobic conversion of organic nitrogen into ammonium in UASR followed by ammonium removal via heterotrophic nitrification in the AIB step.
Solids removal
The UASR was able to remove only 37.33 ± 17.03% of TSS and 35.43 ± 16.74% of VSS. Adding the AIB unit to the treatment process improved these removal efficiencies to 76.53 ± 8.47% and 77.51 ± 7.38%, respectively (Fig. 4a&b). Most of the coarse suspended solids and colloidal organic matter remained after UASR was removed in the AIB’s upper segment. The sponge medium in this segment is responsible for wastewater bio-filtration, maintaining biodegradation of organic matter by the attached biomass and physical retention of suspended particles by filtration.
Removal of trace elements and 1,4-dioxane
Table 2 lists the concentrations of trace elements in the influent and effluent of each stage of the proposed treatment system. Ca showed the highest overall removal efficiency of 82.15%, followed by Zn (77.5%), Fe (73.16%), Mn (3.48%), and Mg (2.4%). These trace elements were removed by anaerobes to enhance the UASR process stability and VFAs degradation. As such, these elements could be removed via either bacterial metabolism or sorption onto sludge/inert particulate matter. A previous study (Ismail et al. 2020) also found that the combined anaerobic/aerobic system could remove Fe2+ (85.6 ± 4.8%), Mn2+ (80.6 ± 4.5%), and Zn2+ (73.6 ± 5.4%) at a total HRT of 40 h. Their study depicted that the metal ions tended to form physico-chemical interaction with the extracellular polymeric substances and/or complexation with intermediate compounds in UASR (Ismail et al. 2020). The adsorption of metals onto organic matter was a major pathway for trace metal reduction by an anaerobic/aerobic treatment scheme (Kumar et al. 2020). In another study, precipitation in the form of sulfides was found as one of the main mechanisms of heavy metal removal in anaerobic bio-systems (Shi et al. 2017). This route depends on the hydrogen sulfide generated by sulfate-reducing bacteria (2CH2O + SO42– → 2HCO3– + H2S) to react with heavy metals (M2+ cation), forming metal sulfide precipitates (H2S + M2+ → MS↓ + 2H+).
The 1,4-dioxane concentration was 3.35 ± 1.21 mg/L in the feed and reduced to 2.76 ± 0.86 mg/L in the UASR’s effluent (Table 2). Apparently, about 18% of 1,4-dioxane was removed under the anaerobic degradation process. This insufficient removal efficiency could be because 1,4-dioxane does not contain functional groups that are accessible to hydrolysis (Sei et al. 2013). The final effluent contained 1,4-dioxane concentration below 1 mg/L, giving an overall removal efficiency of 79.34 ± 8.04%. This finding suggested that the UASR’s effluent contained a suitable electron donor that stimulated the aerobic biodegradation of 1,4-dioxane, as previously reported (Luo et al. 2021). Shen et al. (2008) found that the Fe(III)-reducing bacterium could utilize 1,4-dioxane as a carbon substrate for its growth, where > 50% of the carbon from 1,4-dioxane was transformed to CO2. Pseudonocardia dioxanivorans CB1190 was found to utilize 1,4-dioxane as a sole carbon and energy source (Sei et al. 2013). However, 1,4-dioxane degradation depends on the presence of an extra carbon source and microbial community structure. For instance, Tawfik et al. (2022a, b, c) used acetate as an electron donor to promote the production of the monooxygenase enzymes required to degrade 1,4-dioxane, leading to the release of some metabolites such as oxalic acid, ethylene glycol, and glycolic acid. The monooxygenase-expressing strains and their related enzymes mainly used for the hydroxylation of the 1,4-dioxane ring have also been reported (Tawfik 2020).
Toxicity test derived from the UASR/AIB combined system
The toxicity degree of raw DPIE was represented by an EC50 value of 12.9%. This toxicity was approximately comparable to the UASR effluent, which could be due to the presence of heavy metals, 1,4-dioxane, and organic matter. These pollutants were removed by the AIB unit, increasing the EC50 value to 39.4%. These results depicted that the final effluent must be diluted more than 61% to avoid adverse effects on the growth of aquatic species and to be harmless to the environment. A 1,4-dioxane concentration of 3.125 mg/L inhibited > 75% of sulfur-oxidizing bacteria, and the estimated EC50 value was 13% at a 1,4-dioxane dosage of 2.831 mg/L (Gurung et al. 2012). Their study also depicted that EC50 increased to 30% for lower 1,4-dioxane dosages (around 0.5 mg/L). Although the EC50 level increased after the AIB treatment step (reflecting less toxicity of the effluent), an additional wastewater treatment step would be required to reduce the toxicity-causing substances. Some of these post-treatment facilities include advanced oxidation processes and disinfection, as previously reported (Tang and Mao 2023). For example, Paździor et al. (2017) denoted that an ozonation step was required after the biological treatment process to increase the EC50 value from 9.75% to 51.18%.
Assessment of up-flow multi-staged anaerobic reactor (UASR) performance
Biogas production and volatile fatty acids (VFAs) of UASR unit
Figure 5(a) shows biogas production from polyester wastewater diluted with gray water via UASR. The reactor provided a maximum biogas production of 17.86 L/d, with an average value of 13.59 ± 2.50 L/d. This biogas contained a high portion of methane (72% v/v) followed by carbon dioxide (13% v/v) and hydrogen (5% v/v). For instance, the methane yield was 263.24 ± 31.98 mL/g COD, representing 75.2% of the maximum theoretical value (350 mL/g COD). It is suggested that a portion of influent organics were difficult to be biodegraded.
The results in Fig. 5(b-f) show the conversion efficiency of the volatile fatty acids (VFAs) under anaerobic conditions. The acetate (HAc) concentration decreased from 2007 ± 58 to 1853 ± 510 mg/L (Fig. 5b), suggesting its conversion into methane by archaeal communities (Pekyavas and Yangin-Gomec 2019). Acetate could also be utilized by Methanosaeta in the anaerobic reactors for methane production (Zhang et al. 2019). HPr was reduced from 462.11 ± 88.48 to 316.03 ± 92.99 mg/L (Fig. 5c), suggesting its slower degradation performance than other organic acids found in UASR. Propionate-oxidizing bacteria convert propionate to acetate and hydrogen as intermediate substances. These intermediates are further utilized by acetotrophic and hydrogenotrophic methanogenic archaea to generate methane (Barros et al. 2017). Propionate degradation could be enhanced by adding macro- and micronutrients (e.g., N, P, Fe, and Zn) to the anaerobic medium. HLa was also reduced from 433.68 ± 91.36 to 348.05 ± 81.32 mg/L during biogas production in UASR (Fig. 5d). Acetate (1 mol), bicarbonate (1 mol), and hydrogen (2 mol) are associated with the degradation of 1 mol of lactate (Wu et al. 2016). Et-OH was decreased from 1768 ± 262 to 1126 ± 245 mg/L (Fig. 5e). The conversion of ethanol to acetate by sulfate-reducing bacteria has been reported, where acetate is further utilized by methane-producing archaea for CH4 production (Wu et al. 2018). Hence, the general reduction in VFAs (propionate, lactate, butyrate, valerate, etc.) and ethanol suggested their conversion to acetate, H2, and CO2. These final products are further utilized by methanogens to generate CH4. Decreasing the VFAs from 2887 ± 658 to 2237 ± 498 mg/L (Fig. 5f) was accompanied by a slight decrease in the pH values from 7.14 (DPIE) to 6.8, respectively. This pH range has been considered suitable for maintaining a stable methanogenic reactor (Kamyab and Zilouei 2021).
Effect of C/N ratio and heavy metal on biogas production
The effect of varying the carbon-to-nitrogen (C/N) between 78/1 and 105/1 on the anaerobic UASR performance was investigated. There was a linear and positive correlation between C/N and biogas production (Fig. 6a-f). This finding could be because, at a lower C/N ratio, denitrifying bacteria tend to outcompete methanogens for the available organic matter (COD) as the carbon source (Kong et al. 2019). This action would reduce nitrate into nitrogen gas, representing an opposite pathway to CH4 production. Under an unfavorable C/N condition (either low or high), the UASR unit could also suffer from ammonia inhibition. Kodera et al. (2017) also reported that a sufficient C/N ratio would support the consumption of a high COD portion by methanogens rather than denitrifying bacteria. Increasing nitrate concentration in the anaerobic UASR medium would enable the formation of oxidation–reduction potential, creating toxicity against methanogens. In a similar study (Nurliyana et al. 2015), methane recovery from the facultative co-digestion of agricultural wastes was accelerated by incrementing the C/N ratio from 25 to 55. Their study demonstrated that the co-digestion system suffered from the formation of large quantities of ammonia at a low C/N ratio, representing a toxic condition to the methanogens. Moreover, higher C/N ratios negatively impact protein formation and deteriorate the energy and structural metabolism of the methanogens.
Figure 6(a-f) also displays the biogas production rate at different concentrations of minerals (Ca, Mg) and trace elements (Fe, Mn, Zn). These mineral and trace elements showed a positive impact on the methanogenic activity, increasing biogas production by more than 25%. Adding these elements as micro-nutrients to anaerobic digesters has been reported for maintaining a stable and effective process, especially in terms of methane yields (Demirel and Scherer 2011). Trace elements are used in enzymes/co-enzymes and co-factors involved in methanogenesis, whereas their deficiency has been associated with lower degradation performance and process stability (Schmidt et al. 2018). However, exceeding the concentrations of these elements over the recommended values reported in Choong et al. (2016) would adversely affect methanogenesis. As such, microbes impose metal efflux mechanisms to maintain various defense routes, forming microbe–metal interactions (e.g., accumulation, sorption, mineralization, and transformation). Liang et al. (2020) showed a 37.3% enhancement in biogas production from pig slurry anaerobic digestion due to adding attapulgite, which adsorbed ammonia nitrogen and released trace elements (Ca2+, Mg2+, K+, and Fe3+). Their study revealed that trace elements were essential to stimulate the methanogenic archaea and improve their enzyme activities. However, an overdose of attapulgite (≥ 30 g/L) increased the free ammonia and metal ions in the medium, causing detrimental effects to hydrolytic, acetogenic, and methanogenic microorganisms. Hence, it is essential to determine the optimum doses of the trace nutrients/elements that could support the metabolic activities and biomass yields.
Profile of UASR (pH, CODsoluble, and VFA compositions)
The variations of CODsoluble, pH, and VFA compositions along the UASR height are summarized in Table 3. These profiles are required to understand the methanogenic pathway and anaerobic medium composition as the influent moved upward, following Tawfik et al. (2022a, b, c). CODsoluble was reduced from 5326 ± 281 mg/L (at port-1) to 2441 ± 235 mg/L (at the last sampling port) as the influent wastewater flowed in an upward direction. The CODsoluble concentration showed its highest value in the first compartments, probably due to the dominance of hydrolysis and acetogenesis/acidogenesis that increased the soluble metabolites. The CODsoluble concentration was reduced mainly in the final compartments, where the intermediate products were metabolized by methanogens. Fernández-Palacios et al. (2019) also demonstrated that the acidogenic and acetogenic populations converted the organic matter into large amounts of VFAs, which were utilized by methanogens. This route was confirmed by increasing acetate (HAc) from 1876 ± 276 to 3141 ± 329 mg/L and lactate (HLa) from 371 ± 54 to 542 ± 49 mg/L with moving wastewater from influent to the first compartment. These VFA species were further decreased to 1514 ± 294 and 285 ± 32 mg/L in the final compartment, respectively. A similar profile was depicted for the propionate concentrations. The rapid release and degradation of intermediates during an anaerobic digestion of sugarcane bagasse have also been demonstrated (Tawfik et al. 2023). Ethanol (EtOH) showed a maximum value of 2991 ± 500 mg/L at compartment-1 and then reduced to 1091 ± 243 mg/L in the final compartment. A compartment-wise profile of an up-flow anaerobic unit (Mahmoud et al. 2017) also revealed that the maximum EtOH and CH4 productions were found in the 1st compartment (at HRT of 19.2 h) and the last compartment (at HRT of 96 h), respectively. Increasing the acidification process in the 1st compartment was also validated by maintaining the lowest pH of 5.92 ± 0.01, which was further elevated to 6.83 ± 0.13 at the final compartment. Gopala Krishna et al. (2009) also found that the VFAs species were consumed while moving wastewater from the first to final compartment in an anaerobic unit treating low-strength soluble wastewater. In parallel, the pH condition was very low in the 1st zone because acidogenesis and acetogenesis were dominant. Substrate-specific cellular enzymatic activities were responsible for complex organic acids generation, decreasing the medium pH (Kumar et al. 2020).
Assessment of auto-aerated immobilized biomass (AIB) reactor performance
AIB profile results (analysis of the different segments)
The primary treated wastewater was analyzed along the AIB’s height, regarding the concentrations of dissolved oxygen, COD, TKN, and ammonia (Table 4). The lowest DO value of 0.64 ± 0.20 mg/L was found directly after the anaerobic treatment process, i.e., at the AIB top (1st segment). As wastewater trickles downward, oxygen is diffused from the surrounding atmosphere into the sponge media. This pattern would facilitate the aerobic conditions in the lower AIB zones (DO = 3.20 ± 0.32 mg/L). Moreover, the amount of DO available at the upper segments is utilized for the degradation and/or mineralization of organic compounds to CO2, water, and simpler inorganic species. In particular, the COD fractions were mainly reduced in the upper portion of AIB, followed by slight removals in the subsequent segments. As such, the 1st segment reduced CODtotal from 2790 ± 655 mg/L to 992 ± 159 mg/L, getting a final concentration of 189 ± 49 mg/L (Segment 3). It has been reported that the AIB’s upper part reactor could remove a major portion of carbonaceous organic matter, as expressed by COD. Accordingly, the organic loading rate decreased in the last zone to 1.26 g COD/L/d compared with 9.78 g COD/L/d at the entrance. The remaining amount of oxygen was then utilized for ammonia oxidation, reducing NH4-N from 18.53 ± 2.97 mg/L (inlet) to 2.24 ± 0.34 mg/L (segment 3). In this nitrification process, nitrite is formed due to ammonia oxidation by the ammonia-oxidizing bacteria (AOB). This step is followed by the conversion of nitrite to nitrate by the nitrite-oxidizing bacteria (NOB). This hypothesis was confirmed by increasing NO3-N from 0.38 ± 0.07 mg/L (influent) to 2.26 ± 0.54 mg/L (segment 3). A portion of nitrate present in the inner zone of the sponge media could be subjected to denitrification under anoxic and anaerobic conditions. Although the organics/microorganisms enriched in the sponge’s inner surface could be utilized as a carbon source by denitrifiers, Tanikawa et al. (2019) used sodium acetate solution to accomplish the denitrification pathway. Concurrently, TKN was also utilized along the AIB profile, recording 29.07 ± 5.48 mg/L in influent and 8.36 ± 1.89 mg/L in the last segment. This reduction in TKN along with increased NO3-N suggested that the biofilm attached to and inside the sponge media contributed to the simultaneous nitrification/denitrification process (Tyagi et al. 2021). These findings could be used to illustrate the organic conversion and nitrification/denitrification biodegradation processes occurring along the AIB’s segments.
Scanning electronic microscope (SEM) of sponge and excess sludge
The sponge surface morphology indicated the immobilization and retention of biomass in the carrier media voids (Fig. 7a & b). For instance, the original sponge was not occupied by sludge and biodegradation byproducts. During wastewater treatment, the sponge media started to capture organics and particulate matter, in addition to the inorganic elements resulting from organic bioconversion. Biomass immobilization in the sponge pores is one of the main reasons for prolonging SRT and stabilizing the excess sludge. The VS/TS ratio of the excess sludge generated from the AIB system was around 0.5, indicating that the residual biomass had low degradable organics (volatile solids fraction). This result is also linked to the long SRT of about 130 d, providing a suitable condition for sludge stabilization. This long SRT compared with conventional activated sludge systems (SRT around 20 days) could be because no substantial amount of retained sludge was withdrawn from the AIB reactor during the course of the experiment. Accordingly, the management of sludge would be a flexible and cost-efficient process because it contained inert organic, inorganic matter, and other precipitates, requiring no biological-based stabilization step. Also, the sludge volume index (SVI) was 10.99 ± 0.32 mL/g, indicating that the generated sludge had good settling characteristics throughout the monitoring period.
Composition of the microbial community in UASR
The synergistic cooperation of solubilization, hydrolysis, acidogenesis, acetogenesis, and methanogenesis was responsible for the degradation of polyester wastewater components (Fig. 8). The detected genera in the inoculum included Hyphomicrobium, showing a relative abundance of (2.10%). This dehalogenation-related bacterium has been recognized for chloramphenicol removal (Li et al. 2021), and accumulation of polyhydroxyalkanoates (PHAs) under nitrogen limitation (Cattaneo et al. 2022). This heterotroph bacterium (Hyphomicrobium) could also grow optimally at a pH of about 7.0 and mesophilic temperature (Sekine et al. 2020). The bacterial genera also included Bosea (3.15%) that have been involved in the degradations of various organic wastes (Raut et al. 2021), aromatic compounds, and azo dye (Wang et al. 2023). The microbial culture contained Rhodoplanes (5.32%), assisting in the degradation of phenols, aromatic pollutants, and PAHs (Tang et al. 2021). The genus Pseudomonas, which has been responsible for the degradation of organic matter (Yu et al. 2023) and colored and toxic compounds (Kumkaew et al. 2023), was detected with a relative abundance of 1.21%. The genus Acinetobacter (6.02%) was also detected, and it has been observed in manures of biogas plants (Pulami et al. 2020).
The enriched taxa included Marinobacterium (3.55%), showing high performance in degrading organic matter (Yu et al. 2023). Mycobacterium, which was considered for generating biogas from a farm-scale biogas facility containing manure (Slana et al. 2011; Tawfik et al. 2004), exhibited a relative abundance of 1.05%. Proteiniclasticum belonging to the phylum Firmicutes was also detected (7.15%). Alkaliphilus were also observed (5.40%), and these species were responsible for generating biogas from digesters subjected to high ammonia concentrations (Westerholm et al. 2018). Planomicrobium was detected (3.24%) and it was dominant in the hydrolysis and acidogenesis phases (Khanthong et al. 2021), performing enzyme secretion responsible for converting complex substrates to acids.
The Firmicutes genus Syntrophomonas, which could decompose cellulose, protein, and other polysaccharides (Solli et al. 2014), was detected with a relative abundance of 0.34%. A high abundance of Clostridium (3.50%) could contribute positively to the hydrolysis of the polyester wastewater component, where this hydrolytic bacterium has been recognized for enhancing the hydrolysis, and acidogenesis/acetogenesis performances (Kim et al. 2019). Longilinea (1.91% relative abundance), belonging to phylum Chloroflexi, tends to generate acetate, lactate, and hydrogen further required for bio-CH4 formation (Fernando Herrera Adarme et al. 2022).
The methanogenic archaeal genera recovered were Methanobrevibacter (1.00%), and Methanobacterium (3.80%), suggesting the involvement of the hydrogenotrophic pathway in methane formation in the UASR (Ventorino et al. 2018). All Methanobacterium species could utilize CO2 and H2 as substrates for bio-methanation from organic compounds (methanogenesis) (Tejerizo et al. 2017). The members of the genus Methanobacterium became dominant in anaerobic habitats, such as bioreactors (Tejerizo et al. 2017). These hydrogenotrophic methanogens also included Methanosphaera (0.60%), and their integration with acetotrophic methanogen genera (mainly Methanosaeta 1.90%) could participate largely in methane production, as previously demonstrated (Ros et al. 2017; Meky et al. 2020). In particular, this synergistic microbial interaction suggested the role of archaea and bacteria in performing biodegradation and methane routes toward polyester wastewater treatment.
Conclusions
Applying an up-flow anaerobic multi-staged reactor (UASR) followed by an auto-aerated immobilized biomass (AIB) unit could properly treat the effluents of polyester manufacturing industries mixed with domestic wastewater, with biogas recovery. The concentrations of CODtotal, CODsoluble, and TKN were reduced from 3291 ± 733 to 2705 ± 605 mg/L, from 2899 ± 471 to 2441 ± 235 mg/L, and from 34 ± 6 to 29 ± 5 mg/L through the UASR unit, respectively. These pollutants were significantly dropped to 189 ± 49, 141 ± 42, and 8 ± 2 mg/L, respectively, due to using AIB for post-treatment of anaerobic effluent. The UASR/AIB integrated system also depicted promising removals of Fe2+, Zn2+, and 1,4-dioxane, causing a decrease in the toxicity level of the final water quality (expressed by EC50). Profile analysis of the UASR suggested the entrapment of colloidal/particulate organics into the sludge bed, followed by hydrolysis, acidogenesis, and methanogenesis. This finding was validated by evaluating the synergistic microbial interaction at the genus level. Profile analysis of the AIB unit demonstrated that the first segments (upper portion) were suitable for removing the residual organics, whereas the nitrification of ammonia to nitrate was accomplished through the subsequent segments (lower parts). Further studies are required to assess the inhibitory effects of different 1,4-dioxane concentrations on the hydrolysis, acetogenesis, and methanogenesis phases using batch tests. Moreover, it is recommended to investigate the practical-scale UASR/AIB system and the associated techno-economic feasibility for the end-of-pipe treatment of industrial effluents.
Data Availability
The data that support the findings of this study are available within the article.
Code availability
Not applicable.
References
Allam A, Fleifle A, Tawfik A et al (2015) A simulation-based suitability index of the quality and quantity of agricultural drainage water for reuse in irrigation. Sci Total Environ 536:79–90. https://doi.org/10.1016/j.scitotenv.2015.07.029
APHA, AWWA, WEF (2005). Standards methods for the examination of water and wastewater, XX Edn. Washington, DC: American Public Health Association 2005.
Barros V, Duda R, Vantini J, Omori W, Ferro M, Oliveira R (2017) Improved methane production from sugarcane vinasse with filter cake in thermophilic UASB reactors, with predominance of Methanothermobacter and Methanosarcina archaea and Thermotogae bacteria. Bioresour Technol 244:371–381. https://doi.org/10.1016/j.biortech.2017.07.106
Butarewicz A, Rosochacki S, Wrzaszcz E (2019) Toxicity of sewage from industrial wastewater tratment plants. J Ecol Eng 20(2):191–199. https://doi.org/10.12911/22998993/99060
Cattaneo C, Rodríguez Y, Rene E, García-Depraect O, Muñoz R (2022) Biogas bioconversion into poly(3-hydroxybutyrate) by a mixed microbial culture in a novel Taylor flow bioreactor. Waste Manag 150:364–372. https://doi.org/10.1016/j.wasman.2022.07.017
Choong YY, Norli I, Abdullah AZ, Yhaya MF (2016) Impacts of trace element supplementation on the performance of anaerobic digestion process: a critical review. Bioresour Technol 209:369–379. https://doi.org/10.1016/j.biortech.2016.03.028
Demirel B, Scherer P (2011) Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 35(3):992–998. https://doi.org/10.1016/j.biombioe.2010.12.022
Dhariwal A, Chong J, Habib S, King I, Agellon L, Xia J (2017) Microbiome analyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res 45(W1):W180–W188. https://doi.org/10.1093/nar/gkx295
Dubois M, Gilles K, Hamilton J, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
El-Kamah H, Mahmoud M, Tawfik A (2011) Performance of down-flow hanging sponge (DHS) reactor coupled with up-flow anaerobic sludge blanket (UASB) reactor for treatment of onion dehydration wastewater. Bioresour Technol 102(14):7029–7035. https://doi.org/10.1016/j.biortech.2011.04.017
Elnmer A, Khadr M, Kanae S, Tawfik A (2019) Mapping daily and seasonally evapotranspiration using remote sensing techniques over the Nile delta. Agric Water Manag 213:682–692. https://doi.org/10.1016/j.agwat.2018.11.009
Elsamadony M, Tawfik A (2018) Maximization of hydrogen fermentative process from delignified water hyacinth using sodium chlorite. Energy Convers Manag 157:1023. https://doi.org/10.1016/j.enconman.2017.12.013
Elsamadony M, Tawfik A, Danial A, Suzuki M (2015) Optimization of hydrogen production from organic fraction of municipal solid waste (OFMSW) dry anaerobic digestion with analysis of microbial community. Int J Energy Res 39:329. https://doi.org/10.1002/er.3297
Farghaly A, Elsamadony M, Ookawara S, Tawfik A (2017) Bioethanol production from paperboard mill sludge using acid-catalyzed bio-derived choline acetate ionic liquid pretreatment followed by fermentation process. Energy Convers Manag 145:255–264. https://doi.org/10.1016/j.enconman.2017.05.004
Fernández-Palacios E, Lafuente J, Mora M, Gabriel D (2019) Exploring the performance limits of a sulfidogenic UASB during the long-term use of crude glycerol as electron donor. Sci Total Environ 688:1184–1192. https://doi.org/10.1016/j.scitotenv.2019.06.371
Fernando Herrera Adarme O, Eduardo Lobo Baêta B, Cardoso Torres M, Camilo Otalora Tapiero F, Vinicius Alves Gurgel L, de Queiroz SS, Francisco de Aquino S (2022) Biogas production by anaerobic co-digestion of sugarcane biorefinery byproducts: Comparative analyses of performance and microbial community in novel single-and two-stage systems. Bioresour Technol 354:127185. https://doi.org/10.1016/j.biortech.2022.127185
Gar Alalm M, Tawfik A, Ookawara S (2017) Investigation of optimum conditions and costs estimation for degradation of phenol by solar photo-Fenton process. Appl Water Sci 7:375–382. https://doi.org/10.1007/s13201-014-0252-0
Gopala Krishna G, Kumar P, Kumar P (2009) Treatment of low-strength soluble wastewater using an anaerobic baffled reactor (ABR). J Environ Manag 90(1):166–176. https://doi.org/10.1016/j.jenvman.2007.08.017
Gurung A, Kim S, Joo J, Jang M, Oh S (2012) Assessing toxicities of industrial effluents and 1,4-dioxane using sulphur-oxidising bacteria in a batch test. Water Environ J 26:224–234. https://doi.org/10.1111/j.1747-6593.2011.00280.x
Ismail S, Tawfik A (2016) Performance of passive aerated immobilized biomass reactor coupled with Fenton process for treatment of landfill leachate. Int Biodeterior Biodegrad 111:22–30. https://doi.org/10.1016/j.ibiod.2016.04.010
Ismail S, Nasr M, Abdelrazek E, Awad H, Zhaof S, Meng F, Tawfik A (2020) Techno-economic feasibility of energy-saving self-aerated sponge tower combined with up-flow anaerobic sludge blanket reactor for treatment of hazardous landfill leachate. J Water Process Eng 37:101415. https://doi.org/10.1016/j.jwpe.2020.101415
Ismail S, Elreedy A, Fujii M, Ni S-Q, Tawfik A, Elsamadony M (2021) Fatigue of anammox consortia under long-term 1,4-dioxane exposure and recovery potential: N-kinetics and microbial dynamics. J Hazard Mater 414:125533. https://doi.org/10.1016/j.jhazmat.2021.125533
Johnson B (2005) Microtox® Acute Toxicity Test. In: Blaise, C., Férard, JF. (eds) Small-scale Freshwater Toxicity Investigations. Springer, Dordrecht, doi https://doi.org/10.1007/1-4020-3120-3_2.
Kamyab B, Zilouei H (2021) Investigating the efficiency of biogas production using modelling anaerobic digestion of baker’s yeast wastewater on two-stage mixed-UASB reactor. Fuel 285:119198. https://doi.org/10.1016/j.fuel.2020.119198
Khanthong K, Purnomo C, Daosud W, Laoong-u-thai Y (2021) Microbial diversity of marine shrimp pond sediment and its variability due to the effect of immobilized media in biohydrogen and biohythane production. J Environ Chem Eng 9(5):106166. https://doi.org/10.1016/j.jece.2021.106166
Kim M, Abdulazeez M, Haroun B, Nakhla G, Keleman M (2019) Microbial communities in co-digestion of food wastes and wastewater biosolids. Bioresour Technol 289:121580. https://doi.org/10.1016/j.biortech.2019.121580
Kodera T, Akizuki S, Toda T (2017) Formation of simultaneous denitrification and methanogenesis granules in biological wastewater treatment. Process Biochem 58:252–257. https://doi.org/10.1016/j.procbio.2017.04.038
Kong Z, Li L, Xue Y, Yang M, Li YY (2019) Challenges and prospects for the anaerobic treatment of chemical-industrial organic wastewater: a review. J Clean Prod 231:913–927. https://doi.org/10.1016/j.jclepro.2019.05.233
Kumar M, Gogoi A, Mukherjee S (2020) Metal removal, partitioning and phase distributions in the wastewater and sludge: Performance evaluation of conventional, upflow anaerobic sludge blanket and downflow hanging sponge treatment systems. J Clean Prod 249:119426. https://doi.org/10.1016/j.jclepro.2019.119426
Kumkaew P, Suaisom P, Mukkata K, Koonaphapdeelert S, Sawatdeenarunat C, Nitayavardhana S (2023) Biodecolorization of biogas plant effluent derived from anaerobically digested distillery wastewater by naturally selected Pseudomonas putida. Environ Res 236:116807. https://doi.org/10.1016/j.envres.2023.116807
Li J, Guo N, Zhao S, Xu J, Wang Y (2021) Mechanisms of metabolic performance enhancement and ARGs attenuation during nZVI-assisted anaerobic chloramphenicol wastewater treatment. J Hazard Mater 419:126508. https://doi.org/10.1016/j.jhazmat.2021.126508
Liang YG, Xu L, Bao J, Firmin K, Zong W (2020) Attapulgite enhances methane production from anaerobic digestion of pig slurry by changing enzyme activities and microbial community. Renew Energ 145:222–232. https://doi.org/10.1016/j.renene.2019.06.037
Luo YH, Long X, Wang B, Zhou C, Krajmalnik-Brown R, Rittmann B (2021) A synergistic platform for continuous co-removal of 1,1,1-trichloroethane, trichloroethene, and 1,4-dioxane via catalytic dechlorination followed by biodegradation. Environ Sci Technol 55(9):6363–6372. https://doi.org/10.1021/acs.est.1c00542
Mahmoud M, Elreedy A, Pascal P, Sophie L, Tawfik A (2017) Hythane (H2 and CH4) production from unsaturated polyester resin wastewater contaminated by 1,4-dioxane and heavy metals via up-flow anaerobic self-separation gases reactor. Energy Convers Manag 152:342–353. https://doi.org/10.1016/j.enconman.2017.09.060
Meky N, Ibrahim MG, Fujii M et al (2020) Integrated dark-photo fermentative hydrogen production from synthetic gelatinaceous wastewater via cost-effective hybrid reactor at ambient temperature. Energy Convers Manag 203:112250. https://doi.org/10.1016/j.enconman.2019.112250
Nguyen T, Watari T, Hatamoto M, Sutani D, Setiadi T, Yamaguchi T (2020) Evaluation of a combined anaerobic baffled reactor–downflow hanging sponge biosystem for treatment of synthetic dyeing wastewater. Environ Technol Innov 19:100913. https://doi.org/10.1016/j.eti.2020.100913
Nurliyana M, Hng P, Rasmina H, Kalsom M, Chin K, Lee S, Khoo G (2015) Effect of C/N ratio in methane productivity and biodegradability during facultative co-digestion of palm oil mill effluent and empty fruit bunch. Ind Crops Prod 76:409–415. https://doi.org/10.1016/j.indcrop.2015.04.047
Okubo T, Kubota K, Yamaguchi T, Uemura S, Harada H (2016) Development of a new non-aeration-based sewage treatment technology: Performance evaluation of a full-scale down-flow hanging sponge reactor employing third-generation sponge carriers. Water Res 102:138–146. https://doi.org/10.1016/j.watres.2016.06.035
Osama R, Awad H, Zha S, Meng F, Tawfik A (2021) Greenhouse gases emissions from duckweed pond system treating polyester resin wastewater containing 1,4-dioxane and heavy metals. Ecotoxicol Environ Saf 207:111253. https://doi.org/10.1016/j.ecoenv.2020.111253
Oshiki M, Aizuka T, Netsu H, Oomori S, Nagano A, Yamaguchi T, Araki N (2020) Total ammonia nitrogen (TAN) removal performance of a recirculating down-hanging sponge (DHS) reactor operated at 10 to 20 °C with activated carbon. Aquaculture 520:734963. https://doi.org/10.1016/j.aquaculture.2020.734963
Paździor K, Wrębiak J, Klepacz-Smółka A, Gmurek M, Bilińska L, Kos L, Ledakowicz S (2017) Influence of ozonation and biodegradation on toxicity of industrial textile wastewater. J Environ Manag 195:166–173. https://doi.org/10.1016/j.jenvman.2016.06.055
Pekyavas G, Yangin-Gomec C (2019) Response of Anammox bacteria to elevated nitrogen and organic matter in pre-digested chicken waste at a long-term operated UASB reactor initially seeded by methanogenic granules. Bioresour Technol Rep 7:100222. https://doi.org/10.1016/j.biteb.2019.100222
Poirier S, Desmond-Le Quéméner E, Madigou C, Bouchez T, Chapleur O (2016) Anaerobic digestion of biowaste under extreme ammonia concentration: Identification of key microbial phylotypes. Bioresour Technol 207:92–101. https://doi.org/10.1016/j.biortech.2016.01.124
Pulami D, Schauss T, Eisenberg T, Wilharm G, Blom J, Goesmann A, Glaeser S (2020) Acinetobacter baumannii in manure and anaerobic digestates of German biogas plants. FEMS Microbiol Ecol 96(10):fiaa176. https://doi.org/10.1093/femsec/fiaa176
Raut M, Pandhal J, Wright P (2021) Effective pretreatment of lignocellulosic co-substrates using barley straw-adapted microbial consortia to enhanced biomethanation by anaerobic digestion. Bioresour Technol 321:124437. https://doi.org/10.1016/j.biortech.2020.124437
Ros M, de Souza OFJ, Perez Murcia M, Bustamante M, Moral R, Coll M, Pascual J (2017) Mesophilic anaerobic digestion of pig slurry and fruit and vegetable waste: Dissection of the microbial community structure. J Clean Prod 156:757–765. https://doi.org/10.1016/j.jclepro.2017.04.110
Rozaik E, Abdelhalim H, Moharram M (2016) Anaerobic up flow fluidized bed reactor performance as a primary treatment unit in domestic wastewater treatment. HBRC J 12(1):99–105. https://doi.org/10.1016/j.hbrcj.2014.09.003
Schmidt T, McCabe B, Harris P, Lee S (2018) Effect of trace element addition and increasing organic loading rates on the anaerobic digestion of cattle slaughterhouse wastewater. Bioresour Technol 264:51–57. https://doi.org/10.1016/j.biortech.2018.05.050
Sei K, Miyagaki K, Kakinoki T, Fukugasako K, Inoue D, Ike M (2013) Isolation and characterization of bacterial strains that have high ability to degrade 1,4-dioxane as a sole carbon and energy source. Biodegradation 24(5):665–674. https://doi.org/10.1007/s10532-012-9614-1
Sekine M, Akizuki S, Kishi M, Kurosawa N, Toda T (2020) Simultaneous biological nitrification and desulfurization treatment of ammonium and sulfide-rich wastewater: effectiveness of a sequential batch operation. Chemosphere 244:125381. https://doi.org/10.1016/j.chemosphere.2019.125381
Shen W, Chen H, Pan S (2008) Anaerobic biodegradation of 1,4-dioxane by sludge enriched with iron-reducing microorganisms. Bioresour Technol 99(7):2483–2487. https://doi.org/10.1016/j.biortech.2007.04.054
Shi X, Ng K, Fu C, Low S, Ng H (2017) Removal of toxic component of wastewater by anaerobic processes. In: current developments in biotechnology and bioengineering: biological treatment of industrial effluents, pp 443–467. https://doi.org/10.1016/B978-0-444-63665-2.00017-5
Slana I, Pribylova R, Kralova A, Pavlik I (2011) Persistence of Mycobacterium avium subsp. paratuberculosis at a farm-scale biogas plant supplied with manure from paratuberculosis-affected dairy cattle. Appl Environ Microbiol 77(9):3115–3119. https://doi.org/10.1128/AEM.02407-10
Soares P, Souza R, Soler J, Silva T, Souza S, Boaventura R, Vilar V (2017) Remediation of a synthetic textile wastewater from polyester-cotton dyeing combining biological and photochemical oxidation processes. Sep Purif Technol 172:450–462. https://doi.org/10.1016/j.seppur.2016.08.036
Solli L, Håvelsrud O, Horn S, Rike A (2014) A metagenomic study of the microbial communities in four parallel biogas reactors. Biotechnol Biofuels 146(7):1–5. https://doi.org/10.1186/s13068-014-0146-2
Stepien D, Diehl P, Helm J, Thoms A, Püttmann W (2014) Fate of 1,4-dioxane in the aquatic environment: from sewage to drinking water. Water Res 48(1):406–419. https://doi.org/10.1016/j.watres.2013.09.057
Sun F, Hu J, Zhou Y, Mei R, Wang C, He Y, Wu W (2018) High efficient alternating anaerobic/aerobic process for polyester resin wastewater treatment: Performance and microbial community structure. Biochem Eng J 138:121–130. https://doi.org/10.1016/j.bej.2018.07.005
Tang Y, Mao X (2023) Recent advances in 1,4-dioxane removal technologies for water and wastewater treatment. Water 15:1535. https://doi.org/10.3390/w15081535
Tang M, Wang H, Tang Y, Dai B, Zhang X, Li Z, Yuan G (2021) Overall performance and microbial community analysis of a full-scale aerobic cold-rolling emulsion wastewater (CREW) treatment system. J Environ Chem Eng 9(5):106272. https://doi.org/10.1016/j.jece.2021.106272
Tanikawa D, Yamashita S, Kataoka T, Sonaka H, Hirakata Y, Hatamoto M, Yamaguchi T (2019) Non-aerated single-stage nitrogen removal using a down-flow hanging sponge reactor as post-treatment for nitrogen-rich wastewater treatment. Chemosphere 233:645–651. https://doi.org/10.1016/j.chemosphere.2019.06.012
Tanikawa D, Seo S, Motokawa D (2020) Development of a molasses wastewater treatment system equipped with a biological desulfurization process. Environ Sci Pollut Res 27(20):24738–24748. https://doi.org/10.1007/s11356-019-07077-8
Tawfik A (2020) Degradation pathways of 1,4-dioxane in biological and advanced oxidation processes. Desalin Water Treat 178:360–386. https://doi.org/10.5004/dwt.2020.24970
Tawfik A, Klapwijk B, Van Buuren J et al (2004) Physico-chemical factors affecting the E. coli removal in a rotating biological contactor (RBC) treating UASB effluent. Water Res 38:1081. https://doi.org/10.1016/S0043-1354(03)00345-2
Tawfik A, Ohashi A, Harada H (2010) Effect of sponge volume on the performance of down-flow hanging sponge system treating UASB reactor effluent. Bioproc Biosyst Eng 33(7):779–785. https://doi.org/10.1007/s00449-009-0399-5
Tawfik A, Al-sayed A, Hassan G, Nasr M, El-Shafai S, Alhajeri N, Sanz J (2022a) Electron donor addition for stimulating the microbial degradation of 1,4 dioxane by sequential batch membrane bioreactor: a techno-economic approach. Chemosphere 306:135580. https://doi.org/10.1016/j.chemosphere.2022.135580
Tawfik A, Bakr M, Nasr M, Haider J, Mesfer M, Lim H, Lam S (2022b) Economic and environmental sustainability for anaerobic biological treatment of wastewater from paper and cardboard manufacturing industry. Chemosphere 289:133166. https://doi.org/10.1016/j.chemosphere.2021.133166
Tawfik A, Alalm MG, Awad HM et al (2022c) Solar photo-oxidation of recalcitrant industrial wastewater: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-022-01390-4
Tawfik A, Azzam A, El-Dissouky A, Ibrahim A, Nasr M (2023) Synergistic effects of paper mill sludge and sulfonated graphene catalyst for maximizing bio-hydrogen harvesting from sugarcane bagasse de-polymerization. J Environ Manag 326:116724. https://doi.org/10.1016/j.jenvman.2022.116724
Tejerizo G, Kim Y, Maus I, Wibberg D, Winkler A, Off S, Schlüter A (2017) Genome sequence of Methanobacterium congolense strain Buetzberg, a hydrogenotrophic, methanogenic archaeon, isolated from a mesophilic industrial-scale biogas plant utilizing bio-waste. J Biotech 247:1–5. https://doi.org/10.1016/j.jbiotec.2017.02.015
Tyagi V, Ali M, Tawfik A, Maharjan N, Kazmi A, Okubo T, Harada H (2021) Future perspectives of energy saving down-flow hanging sponge (DHS) technology for wastewater valorization—a review. Rev Environ Sci Biotechnol 20(2):389–418. https://doi.org/10.1007/s11157-021-09573-1
Ventorino V, Romano I, Pagliano G, Robertiello A, Pepe OMW (2018) Pre-treatment and inoculum affect the microbial community structure and enhance the biogas reactor performance in a pilot-scale biodigestion of municipal solid waste. Waste Manag 73:69–77. https://doi.org/10.1016/j.wasman.2017.12.005
Wang K, Zhang H, Shen Y, Li J, Zhou W, Song H, Wang H (2023) Impact of salinity on anaerobic ceramic membrane bioreactor for textile wastewater treatment: process performance, membrane fouling and machine learning models. J Environ Manage 345:118717. https://doi.org/10.1016/j.jenvman.2023.118717
Westerholm M, Müller B, Singh A, Karlsson Lindsjö O, Schnürer A (2018) Detection of novel syntrophic acetate-oxidizing bacteria from biogas processes by continuous acetate enrichment approaches. Microb Biotechnol 11(4):680–693. https://doi.org/10.1111/1751-7915.13035
Wu H, Irizarry R, Bravo H (2010) Intensity normalization improves color calling in SOLiD sequencing. Nat Methods 7:336–337. https://doi.org/10.1038/nmeth0510-336
Wu Y, Wang C, Liu X, Ma H, Wu J, Zuo J, Wang K (2016) A new method of two-phase anaerobic digestion for fruit and vegetable waste treatment. Bioresour Technol 211:16–23. https://doi.org/10.1016/j.biortech.2016.03.050
Wu J, Niu Q, Li L, Hu Y, Mribet C, Hojo T, Li YY (2018) A gradual change between methanogenesis and sulfidogenesis during a long-term UASB treatment of sulfate-rich chemical wastewater. Sci Total Environ 636:168–176. https://doi.org/10.1016/j.scitotenv.2018.04.172
Yu H, Ko D, Lee C (2023) Continuous cultivation of mixed-culture microalgae using anaerobic digestion effluent in photobioreactors with different strategies for adjusting nitrogen loading rate. Bioresour Technol 387:129650. https://doi.org/10.1016/j.biortech.2023.129650
Zhang L, Ban Q, Li J, Wan C (2019) Functional bacterial and archaeal dynamics dictated by pH stress during sugar refinery wastewater in a UASB. Bioresour Technol 288:121464. https://doi.org/10.1016/j.biortech.2019.121464
Acknowledgements
The Ministry of Higher Education in Egypt and Aswan University are acknowledged. The last author acknowledges Kuwait University, college of life sciences.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
RH contributed to methodology, conceptualization, writing–review and editing; KK contributed to conceptualization, visualization, review and editing; AW contributed to supervision, methodology, conceptualization, writing—review and editing; ME contributed to supervision, methodology, conceptualization, writing—review and editing; MM contributed to methodology, formal analysis, writing—original draft; MN contributed to visualization, formal analysis, writing—review and editing; AT contributed to supervision, conceptualization, visualization, writing–review and editing.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Informed Consent Statement
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, R., Kriaa, K., Wahaballa, A.M. et al. Performance assessment of up-flow anaerobic multi-staged reactor followed by auto-aerated immobilized biomass unit for treating polyester wastewater, with biogas production. Appl Water Sci 14, 74 (2024). https://doi.org/10.1007/s13201-024-02129-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02129-y