Abstract
The River Nile is the artery of Egypt, as it presents more than 96% of the municipal, industrial, and irrigation necessities of Egypt. This study was dedicated to providing data about the effect of pollution at six stations on the River Nile at the Rosetta branch during the period from August 2019 to April 2020, using heavy metals analysis and zooplankton as biological indicators. It was found that the average of heavy metals concentration follows the descending order Al > Fe > Mn > Zn > Ni > Co. Most of the heavy metals recorded its highest values at El-Rahawy station. Zooplankton community was represented by 32 species in addition to 4 Meroplanktons. Five groups of zooplankton were recorded, viz. Rotifera (1717 org./L), Protozoa (552 org./L), Cladocera (54 org./L), Nematoda (46 org./L), and other Meroplankton (44 org./L), dominated by Rotifera followed by Protozoa, Cladocera, Nematoda, and other Meroplanktons contributing 71%, 23%, 2%, 2%, and 2%, respectively. The highest average density of total zooplankton was recorded during spring while the lowest was recorded during summer. The highest similarity of (79.12%) was observed between (Site 1) and (Site 5). Almost all diversity indices were conducted and showed its highest values in site 6. The principal component analysis conducted between heavy metals, and zooplankton showed a significant negative correlation was shown for the dominant zooplankton with the heavy metals except with cobalt. Nematoda and the rotifer Brachionus angularis recorded a positive correlation with heavy metals except cobalt.
Similar content being viewed by others
Introduction
Nile River is the longest river on Earth; its physical setup together with the high population density of the surrounding nations makes the Nile River system one of the highly vulnerable water sources in Africa (Dumont 2009). Particularly, Egypt is considered the most populous country in the Nile River riparian, observing the highest water budget-deficit in Africa. The Nile River represents the main source of freshwater (i.e., more than 97%) for more than 100 million Egyptians (Nikiel and Eltahir 2021).
The Nile Delta, approximately 22,000 km 2 in area, is among the largest deltas in the world (Dumont 2009), hosting more than 50% of Egypt’s population, although it represents only 2% of total Egypt’s area, showing one of the top densely populated areas on Earth (Hegazy et al. 2020).
Irrigation in Egypt mainly depends on River Nile water through a system of main canals and rayahs, secondary canals, third order, and meskas (Radwan and El-Sadek 2008). These irrigation canals are widespread over the Nile Delta area and run toward the Mediterranean coastal plain and discharge their water into the northern lakes or the sea (Elewa 2010). The main use of irrigations canals and rayahs is drinking, irrigation, navigation, and fishing (Khalifa and Bendary 2016).
At the north of Cairo, the Nile divides into two branches, the Rosetta branch to the west and the Damietta to the east (Helal 1981). Rosetta branch represents the area of investigation, and its length is about 225 km. The width of the branch varies from 150 to 200 m, and its average depth varies from 2 to 3.5 m. Recently, the Rosetta branch has been suffering from several environmental problems. It receives pollutants from three main sources: The first source is El-Rahawy drain which receives domestic and agriculture wastes from Giza city and pours more than 1,900,000 m3 per day of its effluents into Rosetta branch, the second source results from Kafr El-Zayat industrial area, and the third source of pollution is several small agricultural drains that discharge their wastes into the branch in addition to sewage discharged from several cities (El Bouraie et al. 2011). Consequently, several ecological effects have been detected in the Nile River environment (Goher et al. 2019). The serious health consequences, environmental degradation, and global life quality issues are results of water pollution that have an expensive cost to all humanity as well as increasing the severity of water deficiency problems (El-Amier et al. 2015). Hence, increasing water pollution not only causes the impairment of water quality, biodiversity, and the balance of aquatic ecosystems but also threatens human health, economic development, and social prosperity (Hassan et al. 2017).
Although several adverse health effects of metals have been known for a long time, exposure to heavy metals is continuous and even increasing in some parts of the world, particularly in developing countries (Badr et al. 2011). Heavy metals contamination is a very serious threat due to their toxicity, bioaccumulation, and biomagnifications in the food web (Eisler 1988). Also, metals are regarded as dangerous pollutants in the aquatic ecosystem due to their toxicity impact on living organisms (Ali et al. 2013; Khalil et al. 2007). Many countries with arid or semiarid climates that are sensitive to climate change (Mohammed et al. 2022; Abdel-Fatteh et al. 2020) face a delicate and crucial issue with surface water quality. Water pollution has a direct effect on human health, economy, and water resources availability (Brkic et al. 2019). About 80% of infections in low-income and developing countries are directly linked to contaminated drinking water and unhygienic settings (Das and Nag 2015). In order to decrease pollution and enhance water quality, researchers must examine and control the fluctuations in the amounts of heavy metals and other substances in both surface and groundwater (Gabr and Sousaa 2023).
Zooplanktons are a diverse group of heterotrophic organisms that consume phytoplankton, regenerate nutrients via their metabolism, and transfer energy to higher trophic levels (Steinberg and Condon 2009). They are major components in the trophic dynamics of freshwater ecosystems as they occupy an intermediate position in the food chain and indicate the environmental status. They respond to a wide variety of disturbances including nutrient loading, (Dodson 1992), acidification (Armorek and Kormann 1993), fish densities (Carpenter and Kitchell 1993), contamination, (Yan et al. 1996), and sediment inputs, (Cuker 1997). So, they could be used as a bioindicator for the physical, chemical, and biological processes in freshwater ecosystems, and safety of water. Moreover, zooplankton populations are considered bioindicators of eutrophication, as they are related to environmental conditions, responding more rapidly to changes than fish, and are easier to identify than phytoplanktons (Murugan et al. 1998; Sladecek 1983). Changes in zooplankton community composition can affect the degree of up and down regulations of phytoplankton communities, influence the amount of nutrient availability, processing, and determine the capacity of aquatic ecosystems to uptake carbon dioxide (Brucet et al. 2010).
This work aimed to study heavy metals levels and zooplanktons’ distribution seasonally in waters of different locations on Rossetta branch of the River Nile in Egypt, and their co-relation to assess to conclude and evaluate the extent of water quality in these areas.
Materials and methods
Samples collection
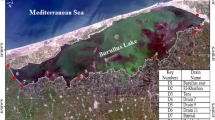
Seasonal samples were collected from six main stations from August 2019 to April 2020 in the Nile River. (Table 1, Fig. 1) summarize the monitoring sites.
Heavy metals analysis
Six elements including aluminum (Al), cobalt (Co), iron (Fe), manganese (Mn), nickel (Ni), and zinc (Zn) were measured in water samples collected seasonally from the investigated area. The metal concentrations were determined after the digestion by nitric acid as follows in (APHA 2005). Atomic absorption spectrophotometer (ICP-MS QCAP Thermo USA) was used for measuring portions of digested solutions.
where A is the Conc. of metal in digested solution mg L−1, B the final volume of digested solution, ml, C the Sample size, ml.
Collection and analysis of zooplankton
Zooplankton samples were collected from 30 L of water using a 55-µm-mesh-size plankton net. Samples were preserved in 5% neutral 33 formaldehyde solution. Each sample was shaken well, and then, the content was poured into a standard 150-ml total volume cup after washing the bottle with pure distilled water. One milliliter was dropped in a plastic counting grade with 2 mm sides’ height and then completed with pure distilled water for counting, using Carl Zeiss’s binocular stereomicroscope. This process was repeated three times. Zooplankton species were identified according to (Dang et al. 2015; Foissner and Berger 1996; Shiel and Koste 1992; Pennak 1978; Koste 1978; Ruttner-Kolisko 1974).
In the laboratory, three subsamples (one ml each) of the homogenized plankton samples were transferred into a counting cell, and zooplankton species were identified. The subsamples were examined under a binocular research microscope with magnification varied from 100 to 400X.
Zooplankton population density was then calculated as the number of individuals per liter from the equation conducted by (APHA 2005):
where c is the number of organisms counted, v′ the volume of concentrated sample/ml, v′′ the volume counted/ml, v″′ the volume of the grab sample/L.
Statistical analysis
Similarity index
Similarity between different stations was performed by using primer 5.
Diversity indices
Diversity indices, e.g., Shannon–Wiener, species richness, evenness, and Simpson, were carried out on data at selected sites by using Premier Program version 5.
-
a.
Shannon–Wiener index (H′):
The Shannon–Wiener index of species diversity was applied according to (Weber 1973) according to the following equation:
where ni represents the number of individuals of I species.
N represents the total number of individuals.
Diversity index between 0 and 3 means a medium pollution, and a diversity index > 3 means clean water (Wilhm 1972).
-
b.
Species richness index or Margalef’s diversity index (d):
It is expressed by simple ratio between total number of species (n) and total number of individuals (N). (Margalef 1958).
-
c.
Evenness index (J):
It was calculated according the Shannon index (Shannon and Wiener 1963), using the formula:
where S is the total number of species of each sample and H′max is the number of maximal theatric diversity.
-
d.
Simpson index:
The index of dominance (Simpson 1949) is the sum total of squares of the proportion of the species in the community and is expressed as follows:
where C is the index of dominance, ni the importance value for each species, N the total importance value.
Correlation coefficient
Correlation coefficient analysis was carried out using office Excel 2007 to show the relations between environmental parameters and the dominant group and species.
In addition, principal component analysis (PCA) between environmental and biological parameters was conducted by XL stat program 2019.
Results and discussion
Heavy metals
Pollution by heavy metals is a severe issue because of their toxicity, accumulation in biota, and inability to biodegrade in the environment. (Khadija et al. 2021) and industrial drainage threatens those sources. All heavy metals exhibit their toxic effects via metabolic interference and mutagenesis. They can enter into the water via drainage, atmosphere, soil erosion, and all human activities in different ways; these elements enter the biogeochemical cycle leading to toxicity in animals living in this water and then to humans consuming these animals (Pandey and Madhuri 2014). Industrial sector in Egypt consumes about 7% of the available water resources, and the industrial sector dispose of their waste, which is loaded with pollutants belonging to each industry, had a negative impact on the quality of wastewater (ElMassah 2018).
In this study, it was found that the average of heavy metals concentration follows the descending order Al > Fe > Mn > Zn > Ni > Co. This arrangement agreed with the studies of Abotalib et al. (2023) and Khallaf et al. (2021). This is because Al and Fe are the most common metals in Earth's crust. Adding to that, the higher values of Al and Mn are possible because of the decay of organic materials, agricultural wastes, fertilizers, insecticides, sewage, and illegal wastewater discharges (Khalaf et al. 2021).
As shown in (Table 2), the highest average value of aluminum occurred during autumn (0.33 mg/l), but the lowest one (0.18 mg/l) was listed during spring, agreeing with Khalaf et al. (2021). At all sampled sites, the highest value of aluminum (0.94 mg/l) was recorded at site 2 during autumn, but the lowest one (0.078 mg/l) occurred at site 5 during winter. With regard to sites, the maximum average value (0.49 mg/l) was noticed at El-Rahawy drain (site 2) which may have been attributed to the increase in discharged wastewater rich with aluminum from El-Rahawy drain, as it is considered the most abundant cocking bowels in Egypt. Lowest average of Al was noticed in Tamalay (site 4) in Monoufia, contradicting with (Authman 2008; 2011; Authman et al. 2008) who found that higher concentrations of Al were detected in some drainage canals water in Menoufia Province, Egypt.
Alnenaei and Authman (2010) reported that Al is released into aquatic ecosystems through the recycled Al industries emissions and the stations of water purification discharge that contains an enormous amount of Al sulfate, which is used as suspended solid particles coagulant.
According to Egyptian low no. 485, (2007), the permissible limit of Al is 0.2 mg/l, which was exceeded in both sites 2 and 6.
The highest average value of cobalt occurred during summer (0.0004 mg/l), but the lowest one (0.00023 mg/l) was listed during autumn. With regard to sites, the maximum average value (0.0005 mg/l) was noticed at site 6, but the minimum one (0.0002 mg/l) was recorded at site 3and site5. At all sampled sites, the highest value of cobalt (0.0014 mg/l) was recorded at site 6 during summer.
Cobalt is one of the most important transition metals which play double dealing in both harmful and beneficial impacts on human beings. The increased use of Co in many industries, such as petrochemical and dye industries, generates large quantities of effluent and thus contaminates water (Mahmud et al. 2016). It was noticed that the exact same industries dominated Kafr El-Zayat (site 6), which witnessed the highest Co average value. It was proved that a lot of physical and mental problems, such as vomiting, nausea, diarrhea, asthma, pneumonia, kidney congestion, skin degeneration, and weight loss, can occur due to excess Co in water (Rengaraj et al. 2002; Shibi and Anirudhan 2005; Naeem et al. 2009; Shahat et al. 2015). The permissible limits of cobalt allowed to be in drinking water is 0.01 mg/l, which was not exceeded in any of the studied areas.
The highest average of iron (0.26 mg/l) was recorded in summer, while the lowest average value (0.08 mg/l) was recorded in spring. With regard to sites, the highest average value (0.25 mg/l) was noticed at site 2, but the lowest one (0.13 mg/l) was recorded at site 5. Generally, the highest value of iron was recorded at site 3 (0.50 mg/l) in summer, but the lowest one was measured at site 6 during spring (0.02 mg/l).
It was rational that Fe would be in its highest level in summer, and at site 2, when and where the agriculture of rice thrives, as Fe is commonly reported from agricultural areas, especially those dominated by rice cultivation, where the pH of soil and water highly control the Fe uptake in rice fields (Gao et al. 2016; Muehe et al. 2013; Vatanpour et al. 2020). It is worth to be mentioned that the Nile Delta is dominated by rice cultivation with ∼1.5 million acres representing 32.7% of the total cultivated area in the Nile Delta (Tolba et al. 2020), and that site 2 is considered the largest area for rice agriculture in the whole Nile Delta (182,550 acres). The high level of Fe was also explained by Lasheen et al. (2008), who reported iron release from different types of water pipes used in Egypt namely polyvinyl chloride (PVC), polypropylene (PP), and galvanized iron (GI).
Fe levels were at the permissible levels in all sites according to Egypt’s Law No. 458 (2007) (0.3 mg/l). Only in El-Rahawy station (site 2) in winter, the limit was considered a borderline (0.36 mg/l).
Lowest level of Fe was recorded during spring. Similar results were found by (Mohamed et al. 2020). The low iron content in spring is possibly due to the consumption of iron by phytoplankton and oxidation of Fe2+ to Fe3+ and subsequent precipitation as hydroxide at high dissolved oxygen content (Ghallab 2000).
The highest average value of manganese occurred during winter (0.035 mg/l), but the lowest one (0.01 mg/l) was listed during the summer. With regard to sites, the maximum average value (0.04 mg/l) was noticed at site 2 due to the effect of El-Rahawy drain, but the minimum one (0.01 mg/l) was recorded at site 5. At all sampled sites, the highest value of manganese (0.089 mg/l) was recorded at site 2 during autumn, but the lowest was (0.005 mg/l) occurred at site 5 during autumn. The same seasonal results were found by (Khallaf et al. 2021), who stated that higher concentrations of Mn during winter than in summer may be attributed to the water's lower flow in winter, which could help in the heavy metals accumulation (Mohiuddin et al. 2011; Islam et al. 2014). Adding to that, the Mn contents lower values during summer due to the dissolution of Mn hydroxides and oxides to the overlying water under low dissolved oxygen values and high water temperatures (Elewa 1993).
The lowest average value of nickel (0.0026 mg/l) was recorded in autumn, but the highest average (0.0033 mg/l) was recorded in spring. The highest average value (0.0035 mg/l) was noticed at site 5, but the lowest one (0.0024 mg/l) was recorded at site 3. Generally, the highest value of nickel was recorded at site 5 (0.0058 mg/l) in spring. Increased industrialization is the major source of increment in environmental pollutants including Ni and their increased health hazards. There are two sources (Anthropogenic and natural release) for increased Ni in the environment and its increased exposure to humans. Ni is considered to be an important element for various vital body functions, but its increased exposure may lead to toxic levels in the human body (Diagomanolin et al. 2004; Haber et al. 2000; Scott-Fordsmand 1997). It was concluded that workplaces such as those related to welding and battery manufacturing that Ni concentration as a result of occupational exposure may vary widely from micrograms to milligrams of Ni (Bencko 1982). Leaching or corrosion processes are also the main reason by which Ni present in pipes and containers gets dissolved in drinking water and beverages. These processes may lead to daily oral Ni intake (Grandjean 1984a, 1984b).
Our results didn’t exceed the permissible levels of Ni by (WHO 2011) (0.07 mg/l) and by (Egypt’s Law No. 458 2007) (0.02 mg/l).
The highest average value of zinc occurred during autumn (0.016 mg/l), but the lowest one (0.007 mg/l) was listed during spring. With regard to sites, the maximum average value (0.013 mg/l) was noticed at site 1, but the minimum one (0.010 mg/l) was recorded at site 5. At all sampled sites, the highest value of zinc (0.026 mg/l) was recorded at site 2 during autumn, but the lowest one (0.003 mg/l) occurred at site 5 during autumn. These results came along with results by (Abdelhamid et al. 2013) and contradicted with results by (Mohamed et al. 2020).
Trace amounts of some metal ions, such as zinc (Zn) and cobalt (Co), are required by organisms as cofactors for enzymatic processes (Zhang et al. 2014; Kozlowski et al. 2009). However, an excess of these metals would cause serious problems in living organisms due to their high toxicity, carcinogenic, and bioaccumulation. (Zhang et al. 2014; Kozlowski et al. 2009; Sebastian and Srinivas 2015; Omraei et al. 2011). Zn is one of the most common pollutants for water, and due to its nonbiodegradability and acute toxicity, Zn-containing liquid and solid wastes are considered hazardous wastes. Anyway, our results didn’t exceed either WHO permissible limits (5 mg/l), or (Egypt’s Law No. 458 2007) (3 mg/l).
Biological study of zooplankton
Zooplankton occupy an important position in pelagic food webs, as they transfer energy produced through photosynthesis from phytoplankton to higher trophic levels (fish) consumed by humans (Sommer et al. 2002). They are also considered as remarkable detectors for pollution.
Zooplankton community were represented by 32 species in addition to 4 Meroplankton (larval stages). The highest number of species (21 species) was recorded during summer and spring. On the other hand, the lowest number of species was observed during autumn (16 species) and winter (12 species). Rotifera showed the highest number of species (25 species). It is followed by cladocera (4 species) and protozoa (2 species) in addition to other Meroplanktons (4 Taxa) (Table 3). (Khalifa and Bendary 2016) showed also that the largest zooplankton population was recorded during spring.
Five groups of zooplankton recorded, viz. Rotifera (1717 Org./l), Protozoa (552Org./l) Cladocera (54 Org./l), Nematoda (46 Org./l), and other Meroplankton (44 Org./l). Zooplankton communities were dominated by Rotifera followed by Protozoa, Cladocera, Nematoda, and other Meroplanktons contributing 71%, 23%, 2%, 2%, and 2%, respectively (Table 5, Fig. 2). Khalifa and Bendary (2016) recorded the dominance of Rotifers, Protozoa, and then Cladocera in their studied area. Sheir et al. (2020) partially agreed with our results as they showed a dominance of Protozoa, then Rotifers in their studied areas. Moreover, Mola et al. (2018) found that zooplankton communities were dominated by rotifers, followed by copepods, Cladocera, Meroplankton, and Protozoa, contributing, respectively. It is worth mentioning that these studies were conducted also in the Nile Delta of Egypt.
Highest average density of total zooplankton was recorded during spring (3090 Org./l), while the lowest annual average was recorded during summer to 1165 Org./l (Table 4, Fig. 3). Sheir et al. (2020) revealed that the highest density of zooplanktons was recorded in winter, which was our second highest season of zooplanktons, and the highest for Protozoa and Nematodes. This came along with Gulati (1978) who regarded temperature as one of the main factors controlling the growth and composition of zooplankton. In addition, winter was the favorable season for zooplankton to reproduce and increase in density in the lentic ecosystem (Sheir 2018).
As shown in (Table 5, Fig. 4), average number of total Rotifera was (1717 Org./l). The maximum average density was recorded during spring (2208 Org./l) while the minimum average density (843 Org./l) was recorded in the summer. Total rotifer recorded the maximum average density (3090 Org./l) at site 6, while the minimum one (657 Org./l) was recorded at site 2. The maximum average density of Rotifers was recorded during spring (2208 org./l), while the lowest was during summer (843 org./l). These results came along with (Fishar et al. 2019; Khalifa and Bendary 2016; Khalifa 2014).
The apparent dominance of rotifers in rivers may be due to their relatively short generation time compared to the larger crustacean zooplankton (Van Dijk and Van Zanten 1995 and Mola 2011). In addition, its simple parthenogenetic reproduction (Herzig 1983) which in favorable conditions results in high production rates often manifested as very high population densities (Andrew and Fizsimons 1992) and they are less vulnerable to fish predation (Allan 1976; Brook and Dodson 1965). Rotifers are also able to reproduce in a wide temperature range (Galkovskaja 1987), and the eutrophication condition of water affects the composition of zooplankton, shifting the dominance from large species as in Copepods to smaller species as in Rotifers (Emam 2006; El-Shabrawy 2000). The dominance of rotifers over other zooplankton groups in many tropical waters could also be explained due to high predation pressure by fish larvae on microcrustaceans (Nandini et al. 2015).
Philodina was the main dominant and abundant species of Rotifera community; it attained to an annual average of 358 Org./l organisms counting. Spring recorded the highest average density (455 Org./l) while the lowest one (144 Org./l) was detected during summer. On the other hand, the highest average count (542 Org./l) of the species was harvested from site 4, it decreased to the lowest average of 223 Org./l at site 3. Brachionus calyciflorus was considered the second dominant species of total rotifers with averages of 247 Org./l. It recorded the highest average density (827 Org./l) during spring, while in winter it recorded the lowest average density (28 Org./l), and it completely disappeared during autumn. The highest average densities (733 Org./l) were recorded at site 6, while the lowest average density was recorded at site 1 (17 Org./l). Polyathra vulgaris was the third dominant species of total Rotifera with an annual average of 242 Org./l (Table 23). It recorded its highest average density (500 Org./l) during autumn, while in spring, it recorded the lowest average density (126 Org./l), and it completely disappeared in summer. The highest average density of Polyathra vulgaris (533 Org./l) was recorded at site 1, while the minimum ones were listed at sites 2 (8 Org./l). These results came along with (Fishar et al. 2019; and Khalifa 2014). Keratella cochelaris was the fourth dominant species of total Rotifera with an annual average of 238 Org./l. It recorded its highest average density during winter (666 Org./l) in winter and recorded the lowest average density (6 Org./l) in summer. The highest average density of Keratella cochelaris (658 Org./l) was recorded at site 1, while the minimum ones were listed at sites 2 (17 Org./l). (Fishar et al. 2019) almost found the same Rotifers as their dominant genera were Proalides, Keratella, Brachionus, Trichocerca, Polyarthra, and Philodina.
According to Perbiche-Neves (2013) and Abd El-Mageed (2008), the high richness of Brachionidae indicates eutrophic conditions. (Kumari et al. 2008) described Keratella sp. and Brachionus sp. as pollution indicator species. El-Bassat (1995) reported the high existence of these genus at Delta Barrage was attributed to their ability to tolerate pollution. In addition, the study of Kumari et al. (2008) recorded rotifer as an indicator of water pollution and described Keratella sp. and Brachionus sp. as pollution indicator species.
In our study, Trachiounus elongata was the 5th most abundant species in Rotifera. It flourished in autumn with an average of (523 org/l) and reached the lowest average in winter (22 org/l). These results came in accordance with (Fishar et al. 2019) and with (Bedair 2006) who believed that this species preferred low temperature.
Most of the 5 dominant species abundance of Rotifera were found in sites (1 and 6), which indicated that these sites are most likely to be eutrophic, while the least abundance of almost all of these species was recorded in site (2), which may indicate again to the high pollution in El-Rahawy drain area. Many other species of Rotifera were found as listed in (Table 6).
Protozoa was the second most abundant group in zooplankton. Composition and distribution of Protozoa recorded the highest density of total Protozoans during winter (705 Org./l) and showed the lowest density (267 Org./l) during summer. It recorded the highest average density (1865 Org./l) at site 4, while the lowest average density recorded (17 Org./l) at sites 3. Protozoa was represented by two species (Vorticella spp and Sphenoderia). Sphenoderia has appeared only summer season with an average of (67 Org./l). It recorded an average of (100 Org./l) at site2. Vorticella sp. (Table 8) recorded the highest average density (536 Org./l) of total Protozoa. Its maximum density average recorded during winter (705 Org./l), while summer recorded the lowest average density of (200 Org./l). Regarding sites, the maximum density of Vorticella spp (1865 Org./l) appeared at site 4, while it recorded the minimum average density (17 Org./l) at site 3. Similar results were shown by Fishar et al. (2019) and Khalifa and Bendary (2016), who found that Protozoa was the second group of zooplankton during their study and that Vorticella sp. was the most abundant sp. among Protozoa. Emam, (2006) concluded that Protozoa are pollution tolerant group of zooplankton and attained its highest density in the polluted area, while Gideon et al. (2014) considered Vorticella campanula as one of the species that are indicators of high water pollution status. On the other hand, Fishar et al. (2019) didn’t agree with our seasonal distribution as they stated that the highest Protozoa recordings were found in summer while it sharply decreased in winter.
Cladocera was the third abundant group in zooplanktons in our study. Its maximum average density was recorded during spring (150 Org./l). Total Cladocera recorded the maximum average density (108 Org./l) at site 1 while recorded the minimum average density (17 Org./l) at sites 2, 4 (Table 5). Bosmina longirostris was considered as the main dominant species of total Cladocera with an annual average of 39 Org./l (Table 7). It showed its peak density of 89 Org./l during spring. During winter, it recorded the minimum average density (67 Org./l), while it completely disappeared during summer and autumn. Site 5 recorded the highest average number (67 Org./l), while sites 2 and 4 showed the lowest average one (17 Org./l).
Nematoda recorded the highest average density (144 Org./l) during winter, while it completely disappeared in summer; the lowest average density (13 Org./l) is recorded in autumn. According to sites, it was found that the highest average density was found at site 2 (102 Org./l), followed El-Rahawy site and still affected by sewage water, while site 4 showed the lowest average (8 Org./l) and completely disappeared in sites 1 and 5 (Table 5). Bouwman et al. (1984) stated that an abundance of nematodes occurs in contaminated environment and they are more tolerant to low oxygen content than other taxa.
Other forms of zooplanktons are calculated by the summation of Nauplius larvae, copipodite stage, harpacticoid stage, and insect larvae. Its maximum average density was recorded during spring (94 Org./L), while it recorded the minimum average density (11 Org./L) during winter. The highest density (125 Org./L) was shown at site 6, while the minimum average density (17 Org./L) was recorded at sites 1, 2, 3 (Table 5). The Nauplius larvae were more abundant than the adult Copepoda. It represented more than 75% of the total copepoda.
These results agreed with (Fishar et al. 2019; Mola et al. 2018; Gaber 2013) who found that the maximum peak of copepod was recorded during spring, and explanation as this result may be due to the abundance of Naupilii larvae and the copepodite stages during this period. In addition, this may be attributed to the effect of high water temperature which accelerates the copepods’ production in the presence of high nutrient concentrations. Moreover, (El-Bassat 2002) stated that the maximum abundance of this group was attributed to the high concentration of nutrients and high transparency. (Sheir et al. 2020) stated in their study that Arthropoda was the third population density (after Protozoa and Rotifera) of the collected zooplanktons during spring and summer seasons. (Manickam et al. 2015) outlined similar results and attributed that to temperature and availability of favorable food such as bacteria and suspended detritus where most planktonic arthropods are filter feeders, while (Waya et al. 2017) attributed the high abundance in rainfall seasons to that this is the entrance time of the nutrients through rainfall and runoff of water of agricultural lands.
Statistical analysis
Zooplankton may form an important component of the biological communities in large rivers due to their high abundance and their ability to cycle nutrients through the aquatic environment (Kobayashi et al. 1998; and Lehman 1980). The structure and function of the zooplankton community with regard to species composition and abundance are affected by several factors. These factors included the nature and availability of food resources, types of predatory interaction in the water environment, physical and chemical aspects of water, and anthropogenic changes (Sipaúba-Tavares et al. 2010).
Similarity index for zooplankton
A similarity index based on the two samples is calculated in the hope that it will indicate the degree of resemblance between the two ecological populations represented by the samples. If the resemblance is "high," the samples may be judged to come from the same population (Johnston 1976) (Table 8).
The similarity index between stations depending on zooplankton distribution in the studied sites is shown in cluster analysis (Table 9, Fig. 5). The highest similarity of (79.12 %) was observed between (Station 1) and (Station 5). Also, cluster analysis recorded high similarity between (Station 6) and (Station 5) being 69.27%, which may be attributed to its location at the northern part of the sites. On the other hand, the lowest similarity was observed between Stations 1 and 2 (40.44%).
In addition, site (6) showed the lowest similarity indices with all the other sites, which indicates that the environment of this location is different from the others.
Diversity indices for zooplankton
As shown in (Table 10), diversity indices were conducted depending on zooplankton species and zooplankton total number. The highest Richness Index values (2.75) were recorded at site 3 then site 2 (2.64), but the lowest one (2.17) occurred at site 1, at the same time, the Evenness Index showed the highest value (0.76) at site 5, but the lowest one (0.69) was recorded at site 2. Also, Shannon diversity index showed the maximum value (2.59) at site 6, but the lowest one showed at site 4 of 1.61. The highest Simpson index value (0.90) was recorded at site 6, but the lowest one (0.65) was recorded at site 4.
Ristau and Traunspurger (2011) mentioned the increase in species density, Shannon, richness, and evenness indices in the oligo and mesotrophic lakes. They explained these patterns as some species could tolerate different degrees of nutrient levels in the water. Also, they mentioned the distribution of some species were dependent on the water movement (lentic/lotic). Q
According to (Pyron 2010), species evenness is a description of the distribution of abundance across the species in a community. Species evenness is highest when all species in a sample have the same abundance. Evenness approaches zero as relative abundances vary. Site (6) recorded the highest evenness indices. And as Shannon indices are related to evenness indices, the highest Shannon indices were also recorded at site (6). That means that the highest zooplankton diversity was recorded in site 6, and all the species inhabiting this site have a high tendency to tolerate pollution.
PCA between zooplankton and heavy metals
The principal component analysis (PCA) was conducted between heavy metals and zooplankton (Fig. 6). Cobalt showed a significant positive correlation (at the same direction) with the most dominant zooplankton groups and species, especially total zooplankton, total rotifer, total Protozoa, Keratella cochlearis, Bosmina longirostris, total Copepoda, total Cladocera, and total other taxa).
Principal component analysis (PCA) diagram between zooplankton and heavy metals. Heavy metals (aluminum: Al, iron: Fe, manganese: Mn, zinc: Zn, nickel:Ni, and cobalt:Co) and the dominant zooplankton and their groups (total zooplankton:TZ, Keratella cochlearis: K.Coch, Brachionus angularis: B ang, Bosmina longirostris: Bos, nauplius larvae: Naup, nematoda: Nem, total cladocera: Tclad, total rotifera: TR, total others: Toth, total protozoa: TP, Vortcella sp.: vor, Polyathra vulgaris: Polyathra, Philodina sp.: phil, Brachionus quadridentatus: B quad, Keratella tropica: K tropi, Trichocerca elongata: tri)
PCA diagram showed significant negative correlations for the dominant zooplankton with the other heavy metals (Al, Fe, Mn Ni, Zn).
On the vice versa, nematoda and the rotifer Brachionus angularis recorded a positive correlation with the other heavy metals except cobalt. These results indicated that these species can tolerate the high concentrations of heavy metals. The present findings agreed with (Saad et al. 2015).
Conclusion
In conclusion, pollution affects the distribution of zooplanktons as shown in El-Rahawy station that had the highest level of pollution, and on the other hand, recorded the lowest degree of diversity of zooplanktons. More stringent measures must be followed in dealing with the problem of pollution resulting from drainage and intense industrial activities in the Nile Delta region, as it is the most crowded place in Egypt, and therefore, the impact of pollution will affect a larger percentage of the population.
References
Abd El-Mageed A (2008) Distribution and long-term historical changes of zooplankton assemblages in Lake Manzala (South Mediterranean Sea, Egypt). Egypt J Aqua Res 33(1):183–192
Abdel-Fatteh M, Abd-Elmabod S, Aldosari A, Elrys A, Mohamed E (2020) Multivariate analysis for assessing irrigation water quality: a case study of the Bahr Mouise Canal, eastern Nile Delta. Water 12:2537. https://doi.org/10.3390/w12092537
Abdelhamid AM, Gomaa AH, El-Sayed HGM (2013) Studies on some heavy metals in the River Nile water and fish at Helwan area. Egypt Egypt J Aquat Biol Fish 17(2):105–126
Abotalib AZ, Abdelhady AA, Heggy E, Salem SG, Ismail E, Ali A, Khalil MM (2023) Irreversible and Large-scale heavy metal pollution arising from increased damming and untreated water Reuse in the Nile Delta. Earth’s Future 11:e2022EF002987
Ali Z, Malik RN, Qadir A (2013) Heavy metals distribution and risk assessment in soils affected by tannery effluents. Chem Ecol 29:676–692. https://doi.org/10.1080/02757540.2013.810728
Allan JD (1976) Life history patterns in zooplankton. Am Nat 10:165–180
Alnenaei AE, Authman M (2010) Impact of human activities on aluminum contamination in the drainage canals in the Nile Delta. Egypt Toxicology Letters 196S(S114):P108–P112. https://doi.org/10.1016/j.toxlet.2010.03.403
Andrew TE, Fizsimous AG (1992) Seasonality, population dynamics and production of planktoic Rotifers in Lough Neagh, Northern Ireland. Hydrobiologia 246:147–164
APHA (2005) APHA: standard methods for the examination of water and wastes, 18th edn. American Public Health Association, Washington, DC
Armorek DR, Korman J (1993) The use of zooplankton in a biomonitoring program to detect lake acidification and recovery. Water Air Soil Poll 69(3–4):223–241
Authman MMN (2008) Oreochromis niloticus as a biomonitor of heavy metal pollution with emphasis on potential risk and relation to some biological aspects. Global Veterenaria 2(3):104–109
Authman MMN (2011) Environmental and experimental studies of aluminium toxicity on the liver of Oreochromis niloticus (Linnaeus 1758) fish. Life Sci J 8(4):764–776
Authman MMN, Bayoumy EM, Kenawy AM (2008) Heavy metal concentrations and liver histopathology of Oreochromis niloticus in relation to aquatic pollution. Glob Veterenaria 2(3):110–116
Badr EAE, Agrama AAE, Badr SAE (2011) Heavy metals in drinking water and human health. Egypt Nutr Food Sci 41(3):210–217
Bedair SM (2006) Environmental studies on zooplankton and phytoplankton in some polluted areas of the River Nile and their relation with feeding habit of fish. Ph. D. Thesis, Fac. Of Sci. Zagazig Uni
Bencko V (1982) Nickel: a review of its occupational and environmental toxicology. J Hyg Epidemiol Microbiol Immunol 27:237–247
Brkic Z, Kuhta M, Larva O, Gottstein S (2019) Groundwater and connected ecosystems: an overview of groundwater body status assessment in Croatia. Environ Sci Eur 31:75
Brooks JL, Dodson SI (1965) Prediction, body size and composition of plankton. Science 150:28–35
Brucet S, Boix D, Quintana XD, Jensen E, Nathansen LW, Trochine C, Jeppesena E (2010) Factors influencing zooplankton size structure at contrasting temperatures in coastal shallow lakes: implications for effects of climate change. Limnol Oceanogr 55(4):1697–1711
Carpenter SR, Kitchell JF (1993) The trophic cascade in lakes. Cambridge University Press, Cambridge, U.K.
Cuker BE (1997) Field experiment on the influence of suspended clay on the plankton of a small lake. Limnol Oceanogr 32:840–847
Dang Y, Liu Y, Sun Y, Yuan D, Liu X, Lu W, Tao X (2015) Bulk crystal growth of hybrid perovskite material CH 3 NH 3 PbI 3. CrystEngComm 17(3):665–670
Das S, Nag SK (2015) Deciphering groundwater quality for irrigation and domestic purposes—a case study in Suri I and II blocks, Birbhum District, West Bengal, India. J Earth Syst Sci 124:965–992. https://doi.org/10.1007/s12040-015-0583-8
Diagomanolin V, Farhang M, Ghazi-Khansari M, Jafarzadeh N (2004) Heavy metals (Ni, Cr, Cu) in the karoon waterway river, Iran. Toxicol Lett 151:63–67
Dodson S (1992) Predicting crustacean zooplankton species richness. Limnol Oceanogr 37:848–856
Dumont HJ (2009) A description of the Nile Basin and a synopsis of its history, ecology, biogeography, hydrology, and natural resources. In: Dumont HJ (ed) The Nile: origin, environments, limnology, and human use, pp 1–21
Egypt Decree No.458 (2007) Drinking water quality standards. Ministry of Health and Population in Arabic, Egypt
Eisler R (1988) Lead Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review; US Fish and Wildlife Service, Patuxent Wildlife Research Center
El Bouraie MM, Motawea EA, Mohamed GG, Yehia MM (2011) Water quality of Rosetta branch in Nile delta, Egypt. Suo 62(1):31–37
El-Amier YA, El-kawy Zahran MA, Al-mamory SH (2015) Assessment the Physicochemical characteristics of water and sediment in Rosetta Branch, Egypt. J Water Resource Prot 7:1075–1086
El-Bassat RA (1995) Ecological studies of zooplankton on the River Nile. M. Sc. Thesis, Fac. Of Sci., Suez canal Univ., p 199
El-Bassat RA (2002) Ecological studies on zooplankton communities with particular reference to free living protozoans at River Nile – Damietta Branch. Ph. D. Thesis, Wom. Coll. For Arts, Educ. & Sci., Ain Shams Univ
Elewa AS (1993) Distribution of Mn, Cu, Zn, and Cd in water, sediments and aquatic plants in River Nile and Aswan Reservoir. Egypt J Appl Sci 8(2):711–723
Elewa HH (2010) Potentialities of water resources pollution of the Nile River Delta, Egypt. Open Hydrol J 4:1–13
Elmassah S (2018) Industrial symbiosis within eco-industrial parks: sustainable development for Borg El-Arab in Egypt. Bus Strat Environ 27:884–892
El-Shabrawy GM (2000) Seasonal and spatial variation in zooplankton structure in Lake Nasser. Pelagic area of the main channel, Egypt. Egypt J Aquatic Biol Fish 4:61–84
Emam W (2006) Preliminary study on the impact of water pollution in El-Rahawy drain dumping in Rosetta Nile branch on zooplankton and benthic invertebrates. M. Sc. Thesis, Zoo. Dep. Fac. of Sci. Ain Shams Uni
Fishar MR, Mahmoud NH, El- Feqy FA, Khadiga MG, Gaber KMG (2019) Community Composition of Zooplankton in El-Rayah El-Behery, Egypt. Egypt J Aquatic Biol Fish 23(1):135–150
Foissner W, Berger H (1996) A user-friendly guide to the ciliates (Protozoa, Cilliophora) commonly, used by hydro biologists as bioindicators in rivers, lakes and waste waters, with notes on their ecology. Freshw Biol 35(2):375–482
Gaber KM (2013) Studies on the distribution and diversity of zooplankton in River Nile and its branches (Rosetta and Damietta branches), Egypt. M. Sc. Thesis, Fac. Sci., Al-Azhar Univ., Cairo, p 198
Gabr ME, Soussa H (2023) Assessing surface water uses by water quality index: application of Qalyubia Governorate, Southeast Nile Delta. Egypt Appl Water Sci 13:181. https://doi.org/10.1007/s13201-023-01994-3
Galkovskaja GA (1987) Planktonic rotifers and temperature. Hydrobiologia 147:307–317
Gao L, Chang J, Chen R, Li H, Lu H, Tao L, Xiong J (2016) Comparison on cellular mechanisms of iron and cadmium accumulation in rice: Prospects for cultivating Fe-rich but Cd-free rice. Rice 9(1):1–12. https://doi.org/10.1186/s12284-016-0112-7
Ghallab MH (2000) Some physical and chemical changes on the River Nile downstream of Delta barrage at El-Rahawy drain. M.Sc. Thesis. Fac. Sci. Ain Shams Univ., Egypt
Gideon AA, Mirela P, Maria C, Constantin O, Palela M, Bahrim G (2014) Biological and physico-chemical evaluation of the eutrophication potential of a highly rated temperate water body in South-Eastern Romania. J Env Eco 5(2):108–129
Goher ME, Ali MHH, El-Sayed SM (2019) Heavy metals contents in Nasser Lake and the Nile River, Egypt: an overview. Egypt J Aquatic Res 45(3):301–312
Grandjean P (1984a) Human exposure to nickel. IARC Sci Publ., pp 469–85
Grandjean P (1984b) Lead poisoning: hair analysis shows the calendar of events. Hum Toxicol 3:223–228
Gulati RD (1978) The ecology of common planktonic crustacea of the freshwaters in the Netherlands. Hydrobiologia 59(2):101–112
Haber L, Erdreicht L, Diamond G, Maier A, Ratney R, Zhao Q, Dourson M (2000) Hazard identification and dose response of inhaled nickel-soluble salts. Regul Toxicol Pharmacol 31:210–230
Hassan AS, Abubakar IB, Musa A, Limanchi MT (2017) Water quality investigation by physicochemical parameters of drinking water of selected areas of Kureken Sani, Kumbotso local government area of Kano. Int J Min Process Extractive Metall 2(5):83. https://doi.org/10.11648/j.ijmpem.20170205.14
Hegazy D, Abotalib AZ, El-Bastaweesy M, El-Said MA, Melegy A, Garamoon H (2020) Geo-environmental impacts of hydrogeological setting and anthropogenic activities on water quality in the Quaternary aquifer southeast of the Nile Delta. Egypt J Afr Earth Sci 172:103947. https://doi.org/10.1016/j.jafrearsci.2020.103947
Helal HA (1981) Studies on the Zooplankton of Dametta Branch of the River Nile North of El-Mansoura. Mansoura University, Zoology Department, p 462
Herzig A (1983) Comparative studies on the relationship between temperature and duration of embryonic development of rotifers. Hydrobiologia 104:237–246
Islam MS, Han S, Ahmed MK, Masunaga S (2014) Assessment of trace metal contamination in water and sediment of some rivers in Bangladesh. J Water Environ Technol 12(2):109–121. https://doi.org/10.2965/jwet.2014.109
Johnston JW (1976) Similarity Indices I: What Do They Measure? Pacific northwest laboratory operated by Battelle for the energy research and development administration Under Contract E Y-76-C-06–7830
Khadija D, Hicham A, Rida A, Hicham E, Nordine N, Najlaa F (2021) Surface water quality assessment in the semi-arid area by a combination of heavy metal pollution indices and statistical approaches for sustainable management. Environ Challenges 5:100230
Khalifa NS (2014) Population dynamics of rotifera in Ismailia Canal. Egypt J Bio Envion Sci 4(2):58–67
Khalifa NS, Bendary RE (2016) Composition and Biodiversity of zooplankton and Macrobenthic populations in El-Rayah El-Menoufy. Egypt Inter J App Envion Sci 11(2):683–700
Khalil MKH, Radwan AM, El-Moselhy KHM (2007) Distribution of phosphorus fractions and some of heavy metals in surface sediments of Burullus lagoon and adjacent Mediterranean Sea. Egypt J Aquat Res 33(1):277–289
Khallaf EA, Authman MMN, Alne-na-ei AA (2021) Aluminum, Chromium and Manganese in Sediments of Bahr Shebeen Nilotic Canal, Egypt: Spatial and Temporal Distribution, Pollution Indices and Risk Assessment. Egypt J Aquatic Biol Fish 25(1):983–1015
Kobayashi TRJ, Shiel P, Gibbs PI, Dixon PI (1998) Freshwater zooplankton in the Hawkesbury-Nepean River: comparison of community structure with other rivers. Hydrobiologia 377:133–145
Koste VG (1978) Dramatic play in childhood: rehearsal for life
Kozlowski H, Janicka-Klos A, Brasun J, Gaggelli E, Valensin D, Valensin G (2009) Copper, iron, and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation). Coord Chem Rev 253(21–22):2665–2685
Kumari P, Dhadse S, Chaudhari PR, Wate SR (2008) A biomonitoring of plankton to assess quality of water in the lakes of Nagpur city. In: Sengupta M, Dalwani R (eds). Proc. of Taal. The 12th World Lake Conference, pp 160–164
Lasheen MR, Sharaby CM, El-Kholy NG, Elsherif IY, El-Wakeel ST (2008) Factors influencing lead and iron release from some Egyptian drinking water pipes. J Hazard Mater 160(2–3):675–680
Lehman JT (1980) Nutrient cycling as an interface between Algae and grazers in freshwater communities. In: Kerfot WC (ed) Evolution and ecology of zooplankton Communities, pp 251–263
Mahmud HN, Obidul Huq AK, Yahya R (2016) The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: a review. RSC Adv 6:14778
Manickam N, Bhavan PS, Santhanam P, Muralisankar T, Srinivasan V, Vijayadevan K, Bhuvaneswari R (2015) Biodiversity of freshwater zooplankton community and physicochemical parameters of Barur Lake, Krishnagiri District, Tamilnadu, India. Malaya J Biosci 2(1):1–12
Margalef R (1958) Information Theory in Ecology. Gen Syst 3:36–71
Mohamed MF, Ahmed NM, Fathy YM, Abdelhamid IA (2020) Impact of heavy metals on Oreochromis niloticus fish and using electrophoresis as bio-indicator for invironmental pollution of Rosetta branch, River Nile, Egypt. Eur Chem Bull 9(2):48–61
Mohammed AM, Refaee A, El-Din GK (2022) Hydro-chemical characteristics and quality assessment of shallow groundwater under intensive agriculture practices in arid region. Qena Egypt Appl Water Sci. https://doi.org/10.1007/s13201-022-01611-9
Mohiuddin KM, Ogawa Y, Zakir HM, Otomo K, Shikazono N (2011) Heavy metals contamination in water and sediments of an urban river in a developing country. Int J Environ Sci Technol 8(4):723–736
Mola HR (2011) Seasonal and spatial distribution of Brachionus (Pallas, 1966; Eurotatoria: Monogonanta: Brachionidae), a bioindicator of eutrophication in lake El-Manzalah, Egypt. Biol Med 3(2):60–69
Mola HRA, Shaldoum FMA, Alhussieny AM (2018) Diversity of planktonic and epiphytic microinvertebrates associated with the macrophyte Eichhornia crassipes (Mart) in River Nile at El-Qanater El-Khiria region, Egypt. J Egypt Acad Soc Environ Dev 19(1):117–132
Muehe EM, Adaktylou IJ, Obst M, Zeitvogel F, Behrens S, Planer-Friedrich B (2013) Organic carbon and reducing conditions lead to cadmium immobilization by secondary Fe mineral formation in a pH-neutral soil. Environ Sci Technol 47(23):13430–13439. https://doi.org/10.1021/es403438n
Murugan N, Murugavel P, Kodarkar MS (1998) Cladocera: the biology, classification, identification and ecology. Indian Association of Aquatic Biologists (IAAB), Hyderabad
Naeem A, Saddique M, Mustafa S, Tasleem S, Shah K, Waseem M (2009) Removal of Co2+ ions from aqueous solution by cation exchange sorption onto NiO. J Hazard Mater 172(1):124–128
Nandini S, Ramírez-García P, Sarma SSS (2015) Water quality indicators in Lake Xochimilco, Mexico: zooplankton and Vibrio cholera. Journal of Limnology, Under Publication
Nikiel CA, Eltahir EA (2021) Past and future trends of Egypt’s water consumption and its sources. Nat Commun 12(1):4508
Omraei M, Esfandian H, Katal R, Ghorbani M (2011) Study of the removal of Zn(II) from aqueous solution using polypyrrole nanocomposite. Desalination 271(1–3):248–256
Pandey G, Madhuri S (2014) Heavy metals causing toxicity in animals and fishes. Res J Anim Et Fish Sci 2:17–23
Pennak RW (1978) Freshwater invertebrates of United States, 2nd edn. Wiley, New York, p 803
Perbiche-Neves G, Fileto C, Laçoportinho J, Troguer A, Serafim-Júnior M (2013) Relations among planktonic rotifers, cyclopoid copepods, and water quality in two Brazilian reservoirs. Lat Am J Aquat Res 41(1):138–149
Pyron M (2010) Characterizing communities. Nat Educ Knowl 3(10):39
Radwan M, El-Sadek A (2008) Water quality assessment of irrigation and drainage canals in Upper Egypt, Proc. Twelfth Int. Conf. on Water Technology (IWTC12), Alexandria, Egypt, pp 1239–1251
Rengaraj S, Moon SH (2002) Kinetics of adsorption of Co(II) removal from water and wastewater by ion exchange resins. Water Res 36:1783–1793
Ristau K, Traunspurger W (2011) Relation between nematode communities and trophic state in southern Swedish lakes. Hydrobiologia 663:121–133
Ruttner-Kolisko A (1974) Planktonic rotifers. Biol Taxon Binnengewasser Suppl 26:146
Saad AA, Emam WM, Mola HRA, Omar HM (2015) Effect of pollution on macrobenthic invertebrates in some localities along the River Nile at Great Cairo, Egypt. Egypt J Aquat Biol Fish 19(2):1–11
Scott-Fordsmand JJ (1997) Toxicity of nickel to soil organisms in Denmark editor editors: reviews of environmental contamination and toxicology. Springer, pp 1-34.
Sebastian J, Srinivas D (2015) Factors influencing catalytic activity of Co–Zn double-metal cyanide complexes for alternating polymerization of epoxides and CO2. Appl Catal A 506:163–172
Shahat A, Awual MR, Naushad M (2015) Functional ligand anchored nanomaterial based facial adsorbent for cobalt(II) detection and removal from water samples. Chem Eng J 271:155–163
Shannon CE, Wiener W (1963) The mathematical theory of communication. University Illinois Press, Urbana
Sheir SK (2018) Diversity of zooplankton communities at Bahr Shebeen Nilotic canal, El-Menoufia, Egypt: environmental fluctuations or chemical pollution effects? Egypt J Zool 69:175–190
Sheir SK, Osman GY, Abd Elhafez AR, Mohamad AH (2020) Distribution and abundance patterns of freshwater zooplanktons at different water habitats of Bahr Shebeen Nilotic Canal, Egypt. Egypt J Aquatic Biol Fish 24(4):165–179
Shibi I, Anirudhan T (2005) Adsorption of Co(II) by a carboxylate-functionalized polyacrylamide grafted lignocellulosics. Chemosphere 58:1117
Shiel RJ, Koste W (1992) Rotifera from Australian inland waters: VIII: trichocercidae (Monogononta). Trans R Soc South Australia 116(1–2):1–27
Simpson EH (1949) Measurement of diversity. Nature 163:688
Sipaúba-Tavares LH, Millan RN, Santeiro RM (2010) Characterization of a plankton community in a fish farm. Acta Limnol Bras 22(1):60–69
Sladeček V (1983) Rotifers as indicator of water quality. Hydrobiologia 100:169–201
Sommer U, Stibor H, Katechakis A, Sommer F, Hansen T (2002) Pelagic food web configurations at different levels of nutrient richness and their implications for the ratio fish production:primary production. Hydrobiologia 484:11–20
Steinberg DK, Condon RH (2009) Zooplankton of the York River. J Coast Res 10057:66–79
Tolba RA, El-Shirbeny MA, Abou-Shleel SM, El-Mohandes MA (2020) Rice acreage delineation in the Nile Delta based on thermal signature. Earth Syst Environ 4(1):287–296. https://doi.org/10.1007/s41748-019-00132-x
Van Dijk GM, Van Zanten B (1995) Seasonal changes in zooplankton abundance in the Lower Rhine during 1987–1991. Hydrobiologia 52:29–38
Vatanpour N, Feizy J, Talouki HH, Es’haghi Z, Scesi L, Malvandi AM (2020) The high levels of heavy metal accumulation in cultivated rice from the Tajan river basin: Health and ecological risk assessment. Chemosphere 245:125639. https://doi.org/10.1016/j.chemosphere.2019.125639
Waya RK, Limbu SM, Ngupula GW, Mwita CJ, Mgaya YD (2017) Temporal patterns in phytoplankton, zooplankton and fish composition, abundance and biomass in Shirati Bay, Lake Victoria, Tanzania. Lakes Reserv 22:19–42
Weber CI (1973) Biological field and laboratory methods for measuring the quality of surface water and effluents. Environmental monitoring series, Office of Res. Devi. Useda, Cincinnati, Ohio
WHO (2011. Guidelines for Drinking-Water Qualityeditor^editors: World Health Organisation
Wilhm J (1972) Graphic and mathematical analysis of biotic communities in polluted streams. Ann Rev Entomol 17:223–252
Yan ND, Keller W, Somers KM, Pawson TW (1996) Recovery of crustacean zooplankton communities from acid and metal contamination: comparing manipulated and reference lakes. Can J Fish Aquat Sci 53:1301–1327
Zhang Y, Chi H, Zhang W, Sun Y, Liang Q, Gu Y, Jing R (2014) Highly efficient adsorption of copper ions by a pvp-reduced graphene oxide based on a new adsorptions mechanism. Nano-Micro Lett 6:80
Acknowledgements
Thanks to all authors, Professor El-Sayed T.E. Rizk, Professor Shehata E. Elewa, Dr. Mai L. Younis, Olfat MohamedAbo-Elfotouh, and Dr. Hesham R.A. Mola for collecting samples, dissecting, methodology, and writing the original draft, reviewing, and editing.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: E-STER and SEE studied conception and design, OMA-E and HRAM conducted the data collection, MLY, HRAM, and OMA-E made the analysis and interpretation of results, MLY and HRAM made figures, and MLY drafted manuscript. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
I declare that the authors have no competing interests as defined by Applied Water Science, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Younis, M.L., Rizk, ES.T.E., Elewa, S.E. et al. Seasonal evaluation of heavy metals and zooplankton distribution and their co-relationship in the Rosetta branch area of the Nile Delta in Egypt. Appl Water Sci 14, 73 (2024). https://doi.org/10.1007/s13201-024-02121-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02121-6