Abstract
Vitamin K antagonists (VKA) is the primary anticoagulant in most settings of Sub-Saharan Africa. Understanding the quality of anticoagulation services in the continent is vital in optimising the intended benefits. This study assessed the quality of anticoagulation and associated factors among VKA-treated patients in nine SSA countries. We conducted a retrospective cohort study of randomly selected patients on anticoagulation from 20 clinics in Botswana, the Democratic Republic of Congo, Ethiopia, Gambia, Ghana, Mozambique, Nigeria, Tanzania, and South Africa. Eligible participants were those on VKAs for at least three months and with at least four international normalised ratios (INR) results in 2019–2021. We report the proportion of INR values in the therapeutic range, time-in-therapeutic range (TTR) using the Rosendaal method, and the proportion of patients with TTR ≥ 65% (optimal anticoagulation). The mean age was 51.1(16.1) years, and 64.2% were women. The most common indications for VKA included venous thromboembolism (29.6%), prosthetic valves (26.7%) and atrial fibrillation/flutter (30.1%). We analysed 6743 INR tests from 1011 participants, and of these, 48.5% were sub-therapeutic, 34.1% therapeutic, and 17.4% were supratherapeutic relative to disease-specific reference ranges. TTR was calculated for 660 patients using 4927 INR measurements. The median (interquartile range [IQR]) TTR was 35.8(15.9,57.2) %. Optimal anticoagulation control was evident in 19.2% of participants, varying from 2.7% in Tanzania to 23.1% in Ethiopia. The proportion of patients with TTR ≥ 65% was 15,4% for prosthetic heart valves, 21.1% for venous thromboembolism and 23.7% for atrial fibrillation or flutter. Countries with universal health coverage had higher odds of optimal anticoagulation control (adjusted odds ratio (aOR) 1.79, 95% confidence interval [CI], 1.15– 2.81, p = 0.01). Patients on VKAs for different therapeutic indications in SSA had suboptimal TTR. Universal health coverage increased the odds of achieving TTR by 79%. The evidence calls for more intensive warfarin management strategies in SSA, including providing VKA services without out-of-pocket payments.
Similar content being viewed by others
Highlights
-
The study of nine countries in SSA provided a snapshot of the quality of VKA anticoagulation in daily clinical practice for a region with similar health system challenges.
-
The analysis noted a suboptimal quality of anticoagulation for different therapeutic VKA indications across selected countries.
-
The median TTR was 35.8.% (recommended minimum 65%), and only 80.8% of participants had a suboptimal quality of anticoagulation.
-
Countries with universal health coverage had higher odds of optimal anticoagulation control.
Introduction
Vitamin K anticoagulants (VKAs) have been widely used for decades for treating and preventing thromboembolic complications of venous thromboembolism (VTE), atrial fibrillation (AF), and valvular heart disease [1]. Furthermore, VKAs are the only recommended anticoagulant treatment for patients with rheumatic heart disease-associated atrial fibrillation and mechanical heart valves [2]. However, VKA use can be challenging given the narrow therapeutic window, unpredictable response, and multiple interactions with other drugs and diets [3, 4]. Therefore, to attain the maximal therapeutic benefits of VKA while minimising bleeding complications, warfarin therapy must be tightly controlled and maintained within a narrow therapeutic range based on INR [5]. The recommended INR targets are 2.0–3.0 for VTE and AF and 2.5–3.5 for mechanical prosthetic valves [6, 7]. The safety and efficacy of warfarin depend on the extent to which anticoagulant regimens maintain patients in these therapeutic ranges. The average percentage of the time in the therapeutic range (TTR) and the percentage of INR values in the therapeutic range are ways to assess the quality of anticoagulation related to thrombotic and bleeding complications [5, 8]. Guideline-recommended thresholds for optimal anticoagulation are TTR ≥ 65% for the British National Institute for Health and Care Excellence and TTR ≥ 70% for the European Society of Cardiology [9, 10]. Achieving these thresholds has been challenging in most settings, leaving a substantial proportion of warfarin-treated patients sub-optimally anticoagulated and with an increased stroke, bleeding, and mortality risk [11,12,13,14,15]. In the Global Anticoagulant Registry in the FIELD (GARFIELD) study, the proportion of patients with TTR ≥ 65% was low, varying from 16.7% in Asia to 49.4% in Europe [13]. The situation is worse in SSA, with the proportion of patients with optimal anticoagulation control as low as 15%. [16] The long travel distance to access the few unreliable centralised VKA services, leading to the low frequency of INR monitoring, out-of-pocket expenses for VKA services and other health system-related problems that affect the accessibility of VKA services, significantly impact anticoagulation control in these settings [13, 16,17,18]. The resulting suboptimal quality of anticoagulation is a significant concern in SSA, given the high burden of rheumatic heart disease-associated AF and mechanical heart valve transplantation, for which VKA is the preferred oral anticoagulation strategy. Even for therapeutic indications that non-vitamin K oral anticoagulants (NOACs) are possible substitutes, VKAs remain the primary anticoagulant as the uptake of NOACs is limited by the high cost and accessibility in African settings. Consequently, VKAs could remain the primary anticoagulant in SSA for decades and efforts to achieve optimal VKA anticoagulation are crucial [13]. Evidence suggests that VKA at stable optimal anticoagulation has comparable efficacy and safety to NOACs [19,20,21]. The current study aimed to describe the quality of anticoagulation control among patients using VKA in nine SSA countries.
Methods
Study design, setting and patients
This retrospective cohort study extracted data from adults 18 years or older at eight outpatient clinics from Nigeria, four from South Africa, two from Tanzania and one each from Botswana, the Democratic Republic of Congo (DRC), Ethiopia, Gambia, Ghana and Mozambique, [22]. These countries were conveniently chosen based on the availability and willingness of the investigators to participate in this investigator-initiated study. In each country, clinics were selected based on the availability of anticoagulation services and laboratories for INR tests. Eligible patients received care from clinics chosen for at least three months and had at least four INR results.
Sampling of participants and sample size
We applied a simple random sampling approach using random numbers generated by Excel/Stata to identify and select case records in clinics with more than or equal to 100 patients. All patients meeting the criteria were selected in clinics with fewer than 100 patients. We needed a pooled minimum sample size of 800 to estimate the suboptimal anticoagulation prevalence of 41% with a margin of error of 3.5% on a two-sided alpha level of 0.05 [18].
Data collection procedures
Patient information was extracted from medical charts and electronic medical records. The information included demographic data (age, gender) indications, type and duration of VKA use and coexisting medical conditions. Other data were the international normalised ratio (INR) values, dates of INR testing and corresponding warfarin dosages for the 12-month duration during the study period.
Assessment of anticoagulation control
We determined the level of anticoagulation using the Rosendaal and the Percent of INR in the therapeutic range methods [5, 8]. With the Rosendaal linear interpolation technique, we calculated the time-in-therapeutic range (TTR) in patients with at least four INR measurements whose consecutive INR testing time points were separated by 56 days or less [8]. We considered TTR less than 65% suboptimal anticoagulation control [23, 24]. The mean TTR for the cohort was the average of all TTRs, unadjusted for follow-up time included in the calculation. As there were centres where INR testing was done at intervals of more than 56 days, the anticoagulation control level was also calculated using the per cent of INR in the therapeutic range method by dividing the total number of INRs in the range for the cohort by the number of INRs [5, 25,26,27].. For both ways, the therapeutic INR range was 2.0–3.0 for venous thromboembolism and cardiac arrhythmia and 2.5 – 3.5 for mechanical prosthetic valves [7]. We used the results of the Rosendaal method for the bivariate and multivariate analysis of factors associated with anticoagulation control.
Patient and public involvement
We did not directly involve patients in the study design, recruitment, and conduct.
Statistical analysis
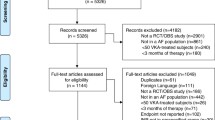
Data were entered into the Research Electronic Data Capture (REDCap) system hosted by the University of Botswana. We performed analyses using Stata V.14 (Stata Corp, College Station, Texas, USA). We used percentages to summarise categorical variables. Means and SD or medians and IQR were used to summarise continuous variables. We categorised deep vein thrombosis, pulmonary embolus, hepatic and splenic thrombosis, inferior vena cava thrombosis and cerebral venous thrombosis as venous thromboembolism (VTE). Intracardiac thrombus, Ischaemic stroke, dilated cardiomyopathy, antiphospholipid syndrome, peripheral arterial disease, thoracic aortic aneurysm, valvular heart disease, thrombophlebitis, carotid stent, femoral-saphenous junction blood stasis was classified as “other” indications for VKA. Countries with few participants, such as the Democratic Republic of Congo, Gambia, and Mozambique, were classified as “other”. Bivariate logistic regression explored factors associated with anticoagulation control. We further performed multivariable logistic regression models using clinically meaningful independent variables (indication for anticoagulation, medical service payment category, age groups and gender). Adjusted odds ratios (ORs), 95% confidence intervals (CIs), and p-values were documented. A 2-sided p-value < 0.05 was considered statistically significant (Fig. 1).
Results
The study included 1011 patients whose mean age was 51 years (Table 1). Most participants (64%) were females, half were from Nigeria and South Africa, and half had an out-of-pocket payment for their medical. Warfarin was prescribed in 99.3% of the patients (n = 1004). The most common VKA indications for warfarin were venous thromboembolism, atrial fibrillation, and mechanical heart valves.
Anticoagulation control
We analysed 6743 INR tests from all the 1011 participants, with each patient having a median (IQR) of 6(4,9) tests. Of these tests, 48.5% were subtherapeutic, 34.1% therapeutic, and 17.4% were supratherapeutic relative to disease-specific reference ranges (Fig. 2).
The time in therapeutic range (TTR)
Of the 1011 participants, 660(65.3%) were eligible for the TTR assessment. The median (IQR) observation period was 118 (84,174) days. The median (IQR) TTR was 35.7(15.9, 57.3) %, ranging from 0% in Nigeria to 44.6% in Ghana (Table 2). The proportion of patients with optimal anticoagulation was 19.2%, varying from 2.7% in Tanzania to 23.1% in Ethiopia.
Factors associated with anticoagulation control
Countries with universal health coverage had higher odds of optimal anticoagulation control (adjusted odds ratio (aOR) 1.79, 95% confidence interval [CI], 1.15– 2.81, p = 0.01) (Table 3).
Discussion
We sought to determine the anticoagulation control among patients on VKA in SSA. Data from our study indicate that the proportions of INR values in the therapeutic range, the median TTR and the proportion of patients with TTR ≥ 65% are all low. In all nine countries, the median TTR values were below 65%, ranging from 0 to 44.6%. In addition, we consistently observed poor anticoagulation control in patients with all VKA indications (mechanical heart valves, atrial fibrillation, and venous thromboembolism). For those with suboptimal control, under-anticoagulation is more frequent than over-anticoagulation. Universal health coverage was more likely associated with optimal anticoagulation control. Our analysis also showed a lower frequency of INR measurements among the study participants.
The high burden of suboptimal anticoagulation control in the current study is consistent with the findings in similar studies in SSA, where the TTR values ranged from 13.7% to 47% and the proportion of patients with TTR ≥ 65% as low as 15% [16, 28]. Our findings are consistent with the global GARFIELD study, which also reported a low proportion of patients with TTR ≥ 65%, varying from 16.7% in Asia to 49.4% in Europe [13]. Therefore, real-world data, including ours, indicate that optimal anticoagulation is often not achieved in routine clinical practice. This apparent suboptimal anticoagulation in VKA-treated individuals is concerning, given the increased mortality risk of as much as fourfold when TTR falls below 30% [15]. Our findings strengthen the argument for improving anticoagulation quality, particularly in SSA, where VKAs are the primary anticoagulants [13]. Evidence suggests that VKA at stable optimal anticoagulation has comparable efficacy and safety to NOACs [19,20,21].
The relatively high proportion of sub-therapeutic INRs is consistent with a previous meta-analysis and meta-regression conclusion, which reported predominantly under-anticoagulated in patients on VKA anticoagulation [14]. Although most studies typically report 25–36% of INRs below the range, the proportion of sub-therapeutic INRs in our cohorts was almost 50% [13, 14, 29,30,31,32]. Our findings not only depict suboptimal anticoagulation control but also that when patients were out of range, they were more likely to be sub-therapeutic and at an increased risk of thrombosis than supratherapeutic with an increased risk of bleeding. This is a concern as subtherapeutic anticoagulation may cause up to a 16-fold increase in the rate of thromboembolism [33]. Though the reasons are unclear, subtherapeutic anticoagulation is likely related to a lack of dedicated anticoagulation clinics and less stringent monitoring, VKA non-adherence, dosage interruption for several reasons, a recent dose reduction in response to a previously recorded high INR value and clinicians' using low dosage due to fears of inducing a major haemorrhage [33]. Regardless of the reason, our results call for efforts to reduce or eliminate sub-therapeutic anticoagulation to improve patient outcomes.
Better anticoagulation control has been attained in clinical trials performed in the SSA [2]. In the Investigation of Rheumatic AF Treatment Using VKA, Rivaroxaban or Aspirin Studies (INVICTUS) trial that involved several African countries, 56.1% to 65.3% of INR tests were in the therapeutic range during the four years of follow-up, rising from 33.2% before the trial. The INVICTUS study reported a lower rate of a composite of stroke, systemic embolism or myocardial infarction, or death in patients with rheumatic heart disease–associated atrial fibrillation on warfarin than rivaroxaban therapy. The improved quality of anticoagulation is a significant determinant of thrombotic and bleeding events in patients receiving a vitamin K antagonist and likely influenced outcomes in the INVICTUS trial. Although these results show that optimal quality of anticoagulation can be achieved in resource-limited countries, the trial's rigorous follow-up and strict inclusion criteria make it difficult to extrapolate the results to real-life VKA-treated patients [2]. Due to frequent anticoagulation monitoring, patients in the VKA arm had frequent physician interactions, which could have resulted in better clinical outcomes. Our study analysed anticoagulation data routinely collected from different levels of healthcare and likely reflects the accurate picture of the quality of anticoagulation in non-trial clinical sub-Saharan African settings. Putting these results into perspective, the benefits seen in the INVICTUS trial are difficult to replicate in sub-Saharan Africa, where time, distance, economic or other access-to-care issues impede access to anticoagulation care.
Our analyses noted an association between optimal quality of anticoagulation and universal health coverage. This finding is consistent with previous evidence linking out-of-pocket expenses for VKA services and suboptimal quality of the anticoagulation [13, 16, 18]. Owing to the underfunding of the health systems in Africa, patients travel a long distance to access the few unreliably running centralised VKA services [17].
Limitations
The limitations of this study are those typical of a retrospective design. Although the analysis of variables in medical charts and electronic records was limited by missed information, the extracted information provided an accurate picture of the quality of anticoagulation in daily clinical practice. The convenient inclusion of sites and investigators may have potentially selected more motivated investigators and participants, with a consequential overestimation of the anticoagulation control. The distribution of the clinics in selected countries may not have yielded a representative sample as they were predominantly in urban settings. However, most anticoagulation services in SSA are often centralised, caring for urban and rural patients who often travel long distances to access these services[17]. The small number of participants in some countries and clinics may also have affected the representativeness of our sample and the generalisation of the findings in such settings. However, the quality of anticoagulation management in our analysis is consistent with that reported in other studies in SSA. Therefore, our results can be extrapolated to most SSA settings. Although data entries from different sites may have been prone to errors, site investigators were responsible for supervising and ensuring correct data entry. In addition, the REDCap dataset included customised validation rules to ensure that valid data are entered.
Conclusion
In conclusion, patients on VKAs for different therapeutic indications in SSA are frequently outside the therapeutic INR range and tend to be sub-therapeutic rather than over-anticoagulated. Anticoagulation care in settings without universal health is associated with suboptimal anticoagulation control. The evidence calls for efforts to improve the region’s anticoagulation control among patients in sub-Saharan Africa, including providing VKA services free of charge.
References
Wardrop D, Keeling D (2008) The story of the discovery of heparin and warfarin. Br J Haematol 141(6):757–763. https://doi.org/10.1111/j.1365-2141.2008.07119.x
Connolly SJ, Karthikeyan G, Ntsekhe M et al (2022) Rivaroxaban in rheumatic heart disease-associated atrial fibrillation. N Engl J Med 387(11):978–988. https://doi.org/10.1056/NEJMoa2209051
De Caterina R, Husted S, Wallentin L et al (2013) Vitamin K antagonists in heart disease: current status and perspectives (Section III). Position paper of the ESC Working Group on Thrombosis-Task Force on Anticoagulants in Heart Disease. Thromb Haemost 110(6):1087–1107. https://doi.org/10.1160/th13-06-0443
Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E (2004) The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126(3):204–233. https://doi.org/10.1378/chest.126.3_suppl.204S
Kaatz S (2008) Determinants and measures of quality in oral anticoagulation therapy. J Thromb Thrombolysis 25(1):61–66. https://doi.org/10.1007/s11239-007-0106-9
Hirsh J, Fuster V, Ansell J, Halperin JL (2003) American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. J Am Coll Cardiol 41(9):1633–1652. https://doi.org/10.1016/s0735-1097(03)00416-9
Hirsh J, Poller L, Deykin D, Levine M, Dalen JE (1989) Optimal therapeutic range for oral anticoagulants. Chest 95(2 Suppl):5s–11s. https://doi.org/10.1378/chest.95.2_Supplement.5S
Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E (1993) A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 69(3):236–239
National Institute for Health and Care Excellence Atrial fibrillation: diagnosis and management (Clinical guideline196). 2021. https://www.nice.org.uk/guidance/ng196/chapter/Recommendations#stroke-prevention.
Hindricks G, Potpara T, Dagres N et al (2020) 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 42(5):373–498. https://doi.org/10.1093/eurheartj/ehaa612
van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ (2006) Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest 129(5):1155–1166. https://doi.org/10.1378/chest.129.5.1155
Sarawate C, Sikirica MV, Willey VJ, Bullano MF, Hauch O (2006) Monitoring anticoagulation in atrial fibrillation. J Thromb Thrombolysis 21(2):191–198. https://doi.org/10.1007/s11239-006-4968-z
Haas S, Ten Cate H, Accetta G et al (2016) Quality of vitamin K antagonist control and 1-year outcomes in patients with atrial fibrillation: a global perspective from the GARFIELD-AF Registry. PLoS ONE 11(10):e0164076. https://doi.org/10.1371/journal.pone.0164076
Mearns ES, White CM, Kohn CG et al (2014) Quality of vitamin K antagonist control and outcomes in atrial fibrillation patients: a meta-analysis and meta-regression. Thromb J 12:14. https://doi.org/10.1186/1477-9560-12-14
Van Den Ham HA, Klungel OH, Leufkens HG, Van Staa TP (2013) The patterns of anticoagulation control and the risk of stroke, bleeding and mortality in patients with non-valvular atrial fibrillation. J Thromb Haemost 11(1):107–115. https://doi.org/10.1111/jth.12041
Mwita JC, Francis JM, Oyekunle AA, Gaenamong M, Goepamang M, Magafu M (2018) Quality of anticoagulation with Warfarin at a Tertiary Hospital in Botswana. Clin Appl Thromb Hemost 24(4):596–601. https://doi.org/10.1177/1076029617747413
Barth DD, Zühlke LJ, Joachim A, Hoegger T, Mayosi BM, Engel ME (2015) Effect of distance to health facility on the maintenance of INR therapeutic ranges in rheumatic heart disease patients from Cape Town: no evidence for an association. BMC Health Serv Res 15:219–219. https://doi.org/10.1186/s12913-015-0890-4
Semakula JR, Mouton JP, Jorgensen A et al (2020) A cross-sectional evaluation of five warfarin anticoagulation services in Uganda and South Africa. PLoS ONE 15(1):e0227458. https://doi.org/10.1371/journal.pone.0227458
Piccini JP, Hellkamp AS, Lokhnygina Y et al (2014) Relationship between time in therapeutic range and comparative treatment effect of rivaroxaban and warfarin: results from the ROCKET AF trial. J Am Heart Assoc 3(2):e000521. https://doi.org/10.1161/JAHA.113.000521
Wallentin L, Yusuf S, Ezekowitz MD et al (2010) Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 376(9745):975–983. https://doi.org/10.1016/S0140-6736(10)61194-4
Wallentin L, Lopes RD, Hanna M et al (2013) Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation 127(22):2166–2176. https://doi.org/10.1161/CIRCULATIONAHA.112.142158
Mwita JC, Damasceno A, Chillo P et al (2022) Vitamin K-dependent anticoagulant use and level of anticoagulation control in sub-Saharan Africa: protocol for a retrospective cohort study. BMJ Open 12(2):e057166. https://doi.org/10.1136/bmjopen-2021-057166
Connolly SJ, Pogue J, Eikelboom J et al (2008) Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 118(20):2029–2037. https://doi.org/10.1161/circulationaha.107.750000
Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM (2009) Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 124(1):37–41. https://doi.org/10.1016/j.thromres.2008.09.016
Singer DE, Hellkamp AS, Yuan Z et al (2015) Alternative calculations of individual patient time in therapeutic range while taking warfarin: results from the ROCKET AF trial. J Am Heart Assoc 4(3):e001349. https://doi.org/10.1161/JAHA.114.001349
Parbhoo P, Jacobson B (2019) A comparison between TTR and FIR as a measure of the quality of anticoagulation in patients with atrial fibrillation. Wits J Clin Med 1(1):23–30
Schmitt L, Speckman J, Ansell J (2003) Quality assessment of anticoagulation dose management: comparative evaluation of measures of time-in-therapeutic range. J Thromb Thrombolysis 15(3):213–216. https://doi.org/10.1023/B:THRO.0000011377.78585.63
Tadesse TA, Tegegne GT, Yadeta D, Chelkaba L, Fenta TG (2022) Anticoagulation control, outcomes, and associated factors in long-term-care patients receiving warfarin in Africa: a systematic review. Thromb J 20(1):58. https://doi.org/10.1186/s12959-022-00416-9
Ansell J, Hollowell J, Pengo V, Martinez-Brotons F, Caro J, Drouet L (2007) Descriptive analysis of the process and quality of oral anticoagulation management in real-life practice in patients with chronic non-valvular atrial fibrillation: the international study of anticoagulation management (ISAM). J Thromb Thrombolysis 23(2):83–91. https://doi.org/10.1007/s11239-006-9022-7
Samsa GP, Matchar DB, Goldstein LB et al (2000) Quality of anticoagulation management among patients with atrial fibrillation: results of a review of medical records from 2 communities. Arch Intern Med 160(7):967–973. https://doi.org/10.1001/archinte.160.7.967
Chiquette E, Amato MG, Bussey HI (1998) Comparison of an anticoagulation clinic with usual medical care: anticoagulation control, patient outcomes, and health care costs. Arch Intern Med 158(15):1641–1647. https://doi.org/10.1001/archinte.158.15.1641
Chamberlain MA, Sageser NA, Ruiz D (2001) Comparison of anticoagulation clinic patient outcomes with outcomes from traditional care in a family medicine clinic. J Am Board Fam Pract 14(1):16–21
Rose AJ, Ozonoff A, Grant RW, Henault LE, Hylek EM (2009) Epidemiology of subtherapeutic anticoagulation in the United States. Circulation 2(6):591–597. https://doi.org/10.1161/CIRCOUTCOMES.109.862763
Acknowledgements
The authors thank all participating hospital staff for their support with this study.
Funding
Open access funding provided by University of Botswana. This research received no specific grant from any public, commercial, or not-for-profit sector funding agency.
Author information
Authors and Affiliations
Contributions
JCM contributed to the conception, design of the work, acquisition, analysis, and interpretation of data for the work and wrote the first draft of the work. AD, KC, PC, OO, AO, JMF, DJG, RA, FA, JMM, MCJ, ET, KT, KID, KN, AYY, WPM, AD, AAF, ET, FK, KF, KK, FLJ, CFO, TAT, STT, CEN, OO, RA and KC contributed to the design of the work, acquisition, interpretation of data for the work, and revised the draft critically for important intellectual content. KC and CP contributed to analysing and interpreting data for the work. All authors reviewed and edited the work and approved its final version. JCM is responsible for the overall content as the guarantor.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
Botswana's Ministry of Health and Wellness granted ethical approval for the University of Botswana/Princess Marina as a coordinating centre (HPDME13/8/1). The authors also obtained necessary ethical approvals from their respective institutional review boards – Muhimbili National Hospital, Tanzania (MNH/TRCU/Perm/2021/012); the University of Cape Town, South Africa (HREC:047/2021); the University of the Witwatersrand Johannesburg, South Africa(R14/49); Catholic University of Allied Sciences Bugando, Tanzania (CREC/445/2020/); Federal Medical Centre, Umuahia, Nigeria (FMC/QEH/G/596/VOL.10/485); University of Ibadan College of Medicine (UI/EC/20/0349; Korle Bu Teaching Hospital Institutional Review Board ( KBCH/MD/G3/20); the University of Port Harcourt Teaching Hospital (UPTH/ADM/90/5.II/VOL XI/959; the University of Nigeria Teaching Hospital, Nigeria (UNTH/CSA/329/VOL.5/09; Federal Teaching Hospital Gombe, Nigeria (NHREC/25/10/2013); Amino Kano is teaching Hospital Nigeria (AKTH/MAC/SUB/12A/P-3/VI/2989; Les Cliniques Universitaires de Kinshasa (213/CNES/BN/PMMF/2020); University of Uyo Teaching Hospital; College of Health Sciences, Addis Ababa University, Ethiopia (115/20/IM and Faculty of Medicine, University Eduardo Mondlane, Mozambique, the Gambia Government/MRCG Joint Ethics Committee ( 22803). Written informed consent, however, was waived since the study collected data from existing records and did not engage living human subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mwita, J.C., Francis, J.M., Pillay, C. et al. Anticoagulation control among patients on vitamin K antagonists in nine countries in Sub-Saharan Africa. J Thromb Thrombolysis 57, 613–621 (2024). https://doi.org/10.1007/s11239-023-02928-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-023-02928-1