Abstract
Background
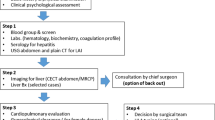
Living donor liver transplantation using hepatic steatosis-improved grafts mitigates donor shortage. Herein, we aimed to evaluate the safety and feasibility of right-lobe adult-to-adult living donor liver transplantation using grafts improved through donor weight loss.
Methods
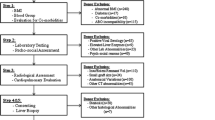
In this retrospective study conducted in a single institution in the Republic of Korea, we reviewed the medical records of living liver donors who lost ≥ 10% of their body weight to improve steatosis before right lobe donation between January 2015 and December 2020. Overall, 1040 right-lobe donors were included, with 150 and 890 donors in the weight loss and control (non-steatosis) groups, respectively.
Results
We performed 1:1 individual matching using the greedy matching method, by which 124 patients were included in each group. The median period from the date of the first visit to donation was 113 (interquartile range: 78–184) days in the weight loss group. As body weight changed from 82.8 ± 13.7 kg to 70.8 ± 11.8 kg (p < 0.0001), body mass index also improved from 27.8 ± 3.9 kg/m2 to 23.8 ± 3.1 kg/m2 (p < 0.0001). No significant between-group differences existed in the postoperative laboratory data for living donors and recipients. The incidence of postoperative complications in donors was comparable between the groups (control group, 9.7%; weight loss group, 13.7%; p = 0.3185). The graft and recipient survival rates were comparable between the groups (p = 1.000).
Conclusion
Weight loss through diet and exercise significantly could improve hepatic steatosis in living donor candidates for liver transplantation, with the surgical outcomes in recipients and donors being equivalent to those in recipients and non-steatotic donors.



Similar content being viewed by others
Data availability
All data included in this study are available upon request by contact with the corresponding author.
References
Soejima Y, Shimada M, Suehiro T, Kishikawa K, Yoshizumi T, Hashimoto K, et al. Use of steatotic graft in living-donor liver transplantation. Transplantation. 2003;76:344–48. https://doi.org/10.1097/01.TP.0000071205.52835.A4
Bhangui P, Sah J, Choudhary N, Gautam D, Gupta V, Srinivasan T, et al. Safe use of right lobe live donor livers with up to 20% macrovesicular steatosis without compromising donor safety and recipient outcome. Transplantation. 2020;104:308–16. https://doi.org/10.1097/TP.0000000000002847
Yoon YI, Song GW, Lee SG, Park GC, Hwang S, Kim KH, et al. Safe use of right lobe living donor livers with moderate steatosis in adult-to-adult living donor liver transplantation: a retrospective study. Transpl Int. 2021;34:872–81. https://doi.org/10.1111/tri.13859
Miller CM, Durand F, Heimbach JK, Kim-Schluger L, Lee SG, Lerut J, et al. The international liver transplant society guideline on living liver donation. Transplantation. 2016;100:1238–43. https://doi.org/10.1097/TP.0000000000001247
Chu MJ, Dare AJ, Phillips AR, Bartlett AS. Donor hepatic steatosis and outcome after liver transplantation: a systematic review. J Gastrointest Surg. 2015;19:1713–24. https://doi.org/10.1007/s11605-015-2832-1
Rose JT, Vargas P, Seay T, Pesch AJ, Williams T, Sites A, et al. Lose weight to donate: development of a program to optimize potential donors with hepatic steatosis or obesity for living liver donation. Transplant Direct. 2021;7:e702. https://doi.org/10.1097/TXD.0000000000001161
Hwang S, Lee SG, Jang SJ, Cho SH, Kim KH, Ahn CS, et al. The effect of donor weight reduction on hepatic steatosis for living donor liver transplantation. Liver Transpl. 2004;10:721–25. https://doi.org/10.1002/lt.20172
Oshita A, Tashiro H, Amano H, Kobayashi T, Onoe T, Ide K, et al. Safety and feasibility of diet-treated donors with steatotic livers at the initial consultation for living-donor liver transplantation. Transplantation. 2012;93:1024–30. https://doi.org/10.1097/TP.0b013e31824c9e25
Doyle A, Adeyi O, Khalili K, Fischer S, Dib M, Goldaracena N, et al. Treatment with Optifast reduces hepatic steatosis and increases candidacy rates for living donor liver transplantation. Liver Transpl. 2016;22:1295–300. https://doi.org/10.1002/lt.24495
Chung JH, Ryu JH, Yang KH, Choi BH, Park Y, Lee TB, et al. Efficacy and safety of weight reduction of the donor in hepatic steatosis for living donor liver transplantation. Ann Transplant. 2020;25:e923211
Trakroo S, Bhardwaj N, Garg R, Modaresi EJ. Weight loss interventions in living donor liver transplantation as a tool in expanding the donor pool: a systematic review and meta-analysis. World J Gastroenterol. 2021;27:3682–92. https://doi.org/10.3748/wjg.v27.i24.3682
Pamecha V, Patil NS, Parthasarathy K, Sinha PK, Mohapatra N, Rastogi A, et al. Expanding donor pool for live donor liver transplantation: utilization of donors with non-alcoholic steatohepatitis after optimization. Langenbecks Arch Surg. 2022;407:1575–84. https://doi.org/10.1007/s00423-022-02444-5
Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708–15
Park HJ, Kim KW, Kwon HJ, Lee S, Kim DW, Moon HH, et al. CT-based visual grading system for assessment of hepatic steatosis: diagnostic performance and interobserver agreement. Hepatol Int. 2022;16:1075–84. https://doi.org/10.1007/s12072-022-10373-0
Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA, NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–20
Fujii Y, Kawamura N, Zaitsu M, Watanabe M, Goto R, Kamiyama T, et al. Outcome of living-donor liver transplantation using grafts from donors treated for fatty liver. Ann Transplant. 2020;25:e920677
Bian H, Hakkarainen A, Lundbom N, Yki-Järvinen H. Effects of dietary interventions on liver volume in humans. Obesity (Silver Spring). 2014;22:989–95. https://doi.org/10.1002/oby.20623
Lewis MC, Phillips ML, Slavotinek JP, Kow L, Thompson CH, Toouli J. Change in liver size and fat content after treatment with Optifast very low calorie diet. Obes Surg. 2006;16:697–701. https://doi.org/10.1381/096089206777346682
Chu KK, Wong KH, Chok KS. Expanding indications for liver transplant: tumor and patient factors. Gut Liver. 2021;15:19–30. https://doi.org/10.5009/gnl19265
Broering DC, Sterneck M, Rogiers X. Living donor liver transplantation. J Hepatol. 2003;38(Suppl 1):S119–S35. https://doi.org/10.1016/s0168-8278(03)00009-6
Müllhaupt B, Dimitroulis D, Gerlach JT, Clavien PA. Hot topics in liver transplantation: organ allocation—extended criteria donor—living donor liver transplantation. J Hepatol. 2008;48(Suppl 1):S58–S67. https://doi.org/10.1016/j.jhep.2008.01.013
Goldaracena N, Sapisochin G, Spetzler V, Echeverri J, Kaths M, Cattral MS, et al. Live donor liver transplantation with older (≥50 years) versus younger (<50 years) donors: does age matter? Ann Surg. 2016;263:979–85. https://doi.org/10.1097/SLA.0000000000001337
Egawa H, Teramukai S, Haga H, Tanabe M, Fukushima M, Shimazu M. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 2008;47:143–52. https://doi.org/10.1002/hep.21928
Song GW, Lee SG, Moon DB, Ahn CS, Hwang S, Kim KH, et al. Dual-graft adult living donor liver transplantation: an innovative surgical procedure for live liver donor pool expansion. Ann Surg. 2017;266:10–18. https://doi.org/10.1097/SLA.0000000000001776
McCormack L, Dutkowski P, El-Badry AM, Clavien PA. Liver transplantation using fatty livers: always feasible? J Hepatol. 2011;54:1055–62. https://doi.org/10.1016/j.jhep.2010.11.004
Doyle MB, Vachharajani N, Wellen JR, Anderson CD, Lowell JA, Shenoy S, et al. Short- and long-term outcomes after steatotic liver transplantation. Arch Surg. 2010;145:653–60. https://doi.org/10.1001/archsurg.2010.119
Nagai S, Fujimoto Y, Kamei H, Nakamura T, Kiuchi T. Mild hepatic macrovesicular steatosis may be a risk factor for hyperbilirubinaemia in living liver donors following right hepatectomy. Br J Surg. 2009;96:437–44. https://doi.org/10.1002/bjs.6479
El-Badry AM, Breitenstein S, Jochum W, Washington K, Paradis V, Rubbia-Brandt L, et al. Assessment of hepatic steatosis by expert pathologists: the end of a gold standard. Ann Surg. 2009;250:691–97. https://doi.org/10.1097/SLA.0b013e3181bcd6dd
Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol. 2013;58:1007–19. https://doi.org/10.1016/j.jhep.2012.11.021
Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36:1011–14. https://doi.org/10.1002/jmri.23741
Danis N, Weeks SR, Kim A, Baghdadi A, Ghadimi M, Kamel IR, et al. Noninvasive risk stratification for nonalcoholic fatty liver disease among living liver donor candidates: a proposed algorithm. Liver Transpl. 2022;28:670–77. https://doi.org/10.1002/lt.26365
Acknowledgements
None.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Conception and design: Y-IY, S-GL. Administrative support: K-HK, C-SA, D-BM. Provision of study materials or patients: SH. Collection and assembly of data: T-YH, G-WS, D-HJ, G-CP. Data analysis and interpretation: Y-IY. Manuscript writing: All authors. Final approval of manuscript: S-GL.
Corresponding author
Ethics declarations
Conflict of interest
All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoon, YI., Lee, SG., Hwang, S. et al. Safety of right liver donation after improving steatosis through weight loss in living donors: a retrospective study. Hepatol Int (2024). https://doi.org/10.1007/s12072-024-10641-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12072-024-10641-1