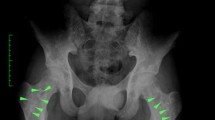
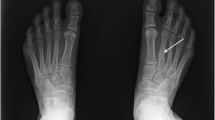
Abstract
Osteopetrosis describes several types of rare sclerosing bone dysplasias of varying clinical and radiographic severity. The classic autosomal dominant subtype emerges most often in adolescence but can present from infancy through adulthood. The autosomal recessive osteopetrosis, or “malignant infantile osteopetrosis,” presents in infancy with a grimmer prognosis, though the autosomal dominant forms (often mislabeled as “benign”) actually can have life-threatening consequences as well. Often osteopetrosis is detected due to skeletal findings on radiographs performed to evaluate injury or as an incidental finding during evaluation for illness. Given the varied phenotypic severity and presentations at different ages, radiologists play an integral role in the care of these patients both in diagnosis and in clinical evaluation and monitoring. A deeper understanding of the underlying genetic basis of the disease can aid in the radiologist in diagnosis and in anticipation of unique complications. An overview of current clinical management is also discussed.
Graphical abstract












Similar content being viewed by others
References
Albers-Schonberg HE (1904) X-ray images of rare bone disease [Rontgenbilder einer seltenen Knockenerkrankung]. Münchener Medizinische Wochenschrift 51:365–368
Karshner RG (1926) Osteopetrosis. Am J Roentgenol 16:405–419
Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH (2013) Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Reviews Endocrinol 9:522–536
Mortier GR, Cohn DH, Cormier-Daire V, Hall C, Krakow D, Mundlos S, Nishimura G, Robertson S, Sangiorgi L, Savarirayan R, Sillence D, Superti-Furga A, Unger S, Warman ML (2019) Nosology and classification of genetic skeletal disorders: 2019 revision. Am J Med Genet 179:2393–2419
Stenbeck G (2002) Formation and function of the ruffled border in osteoclasts. Semin Cell Dev Biol 13:285–292
Moreira CA, Dempster DW, Baron R (2019) Anatomy and ultrastructure of bone – histogenesis, growth and remodeling. In: Feingold KR (ed) Endotext. MDText.com, Inc., South Dartmouth (MA)
Stauber T, Wartosch L, Vishnolia S, Schulz A, Kornak U (2023) CLCN7, a gene shared by autosomal recessive and autosomal dominant osteopetrosis. Bone 168:116639
Pillai NR, Aggarwal A, Orchard P (2022) Phenotype-autosomal recessive osteopetrosis. Bone 165:116577
Waguespack SG, Hui SL, DiMeglio LA, Econs MJ (2007) Autosomal dominant osteopetrosis: clinical severity and natural history of 94 subjects with a chloride channel 7 gene mutation. J Clin Endocrinol Metabolism 92:771–778
Polgreen LE, Imel EA, Econs MJ (2023) Autosomal dominant osteopetrosis. Bone 170:116723
Bollerslev J, Andersen PE Jr. (1988) Radiological, biochemical and hereditary evidence of two types of autosomal dominant osteopetrosis. Bone 9:7–13
Jodeh W, Katz A, Hart M, Niziolek P, Alam I, Ing S, Polgreen LE, Imel EA, Econs MJ (2024) Autosomal dominant osteopetrosis (ADO) caused by a missense variant in the TCIRG1 gene. J Clin Endocrinol Metabol. Advanced online publication
Keats TE, Anderson MW (2012) Atlas of normal roentgen variants that may simulate disease. Elsevier, Philadelphia, p 189
Williams HJ, Davies AM, Chapman S (2004) Bone within a bone. Clin Radiol 59:132–144
Grodum E, Gram J, Brixen K, Bollerslev J (1995) Autosomal dominant osteopetrosis: bone mineral measurements of the entire skeleton of adults in two different subtypes. Bone 16:431–434
Stark Z, Savarirayan R (2009) Osteopetrosis. Orphanet J Rare Dis 4:5
Loria-Cortes R, Quesada-Calvo E, Cordero-Chaverri C (1977) Osteopetrosis in children: a report of 26 cases. J Pediatr 91:43–47
Spinnato P, Pedrini E, Petrera MR, Zarantonello P, Trisolino G, Sangiorgi L, Carpezano M, Crombe A, Tetta C (2022) Spectrum of Skeletal Imaging Features in Osteopetrosis: inheritance pattern and Radiological associations. Genes 13:1965
Wu CC, Econs MJ, DiMeglio LA, Insogna KL, Levine MA, Orchard PJ, Miller WP, Petryk A, Rush ET, Shoback DM, Ward LM, Polgreen LE (2017) Diagnosis and management of osteopetrosis: consensus guidelines from the osteopetrosis working group. J Clin Endocrinol Metabolism 102:3111–3123
Ladd LM, Imel EA, Niziolek PJ, Liu Z, Warden SJ, Liang Y, Econs MJ (2021) Radiographic imaging, densitometry and disease severity in autosomal dominant osteopetrosis type 2. Skeletal Radiol 50:903–913
Walia H, Jain R, Nirwan R, Bansal RK, Gupta GN (2013) Osteopetrosis: trephine biopsy an essential tool. Int J Students Res 3:45–47
Rauch F (2005) Bone growth in length and width: the Yin and Yang of bone stability. J Musculoskelet Neuronal Interact 5:194–201
Calder AD, Arulkumaran S, D’Arco F (2022) Imaging in osteopetrosis. Bone 165:116560
Chu K, Snyder R, Econs MJ (2006) Disease status in autosomal dominant osteopetrosis type 2 is determined by osteoclastic properties. J Bone Miner Res 21:1089–1097
Whyte MP (2005) Misinterpretation of osteodensitometry with high bone density: BMD Z > or = + 2.5 is not normal. J Clin Densitometry 8:1–6
Arruda M, Coelho MCA, Moraes AB, de Paula Paranhos-Neto F, Madeira M, Farias MLF, Neto LV (2016) Bone Mineral density and microarchitecture in patients with autosomal dominant osteopetrosis: a report of two cases. J Bone Miner Res 31:657–662
Bollerslev J, Grontved A, Andersen PE Jr (1988) Autosomal dominant osteopetrosis: an otoneurological investigation of the two radiological types. Laryngoscope 98:411–413
Al-Tamimi YZ, Tyagi AK, Chumas PD, Crimmins DW (2008) Patients with autosomal-recessive osteopetrosis presenting with hydrocephalus and hindbrain posterior fossa crowding. J Neurosurgery: Pediatr 1:103–106
Dozier TS, Duncan IM, Klein AJ, Lambert PR, Key J, Lyndon L (2005) Otologic manifestations of malignant osteopetrosis. Otology Neurotology 26:762–766
Akdulum I, Gurun E, Tiken R, Aydemir AB, Boyunaga OL (2021) Optic canal diameters according to age in the pediatric population. J Pediatr Ophthalmol Strabismus 58:319–323
Capo V, Abinun M, Villa A (2022) Osteoclast rich osteopetrosis due to defects in the TCIRG1 gene. Bone 165:116519
Steward CG (2003) Neurological aspects of osteopetrosis. Neuropathol Appl Neurobiol 29:87–97
Key J, Lyndon L, Rodriguiz RM, Willi SM, Wright NM, Hatcher HC, Eyre DR, Cure JK, Griffin PP, Ries WL (1995) Long-term treatment of osteopetrosis with recombinant human interferon gamma. N Engl J Med 332:1594–1599
Nguyen A, Miller WP, Gupta A, Lund TC, Schiferl D, Lam LSK, Arzumanyan Z, Orchard PJ, Polgreen LE (2022) Open-label pilot study of interferon gamma–1b in patients with non-infantile osteopetrosis. JBMR Plus 6:e10597
Imel EA, Liu Z, Acton D, Coffman M, Gebregziabher N, Tong Y, Econs MJ (2019) Interferon gamma-1b does not increase markers of bone resorption in autosomal dominant osteopetrosis. J Bone Miner Res 34:1436–1445
Alam I, Gray AK, Acton D, Gerard-O’Riley RL, Reilly AM, Econs MJ (2015) Interferon gamma, but not calcitriol improves the osteopetrotic phenotypes in ADO2 mice. J Bone Miner Res 30:2005–2013
Hashemi Taheri AP, Radmard AR, Kooraki S, Behfar M, Pak N, Hamidieh AA, Ghavamzadeh A (2015) Radiologic resolution of malignant infantile osteopetrosis skeletal changes following hematopoietic stem cell transplantation. Pediatr Blood Cancer 62:1645–1649
Shapiro G, Fishleder J, Stepensky P, Simanovsky N, Goldman V, Lamdan R (2020) Skeletal changes after hematopoietic stem cell transplantation in osteopetrosis. J Bone Miner Res 35:1645–1651
Orchard P, Fasth AL, Le Rademacher J, He W, Boelens JJ, Horwitz EM, Al-Seraihy A, Ayas M, Bonfim CM, Boulad F, Lund T, Buchbinder DK, Kapoor N, O’Brien TA, Perez MAD, Veys PA, Eapen M (2015) Hematopoietic stem cell transplantation for infantile osteopetrosis. Blood 126:270–276
Maurizi A (2022) Experimental therapies for osteopetrosis. Bone 165:116567
Funding
We would like to acknowledge funding under NIAMS/NIH R01AR077869 and R01AR084202.
Author information
Authors and Affiliations
Contributions
MNM drafted the initial manuscript and collected imaging examples.
EAI provided clinical expertise in the drafting of the manuscript.
MFA conceived the manuscript idea and supervised its drafting and revisions.
All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McLuckey, M.N., Imel, E.A. & Forbes-Amrhein, M.M. Osteopetrosis in the pediatric patient: what the radiologist needs to know. Pediatr Radiol (2024). https://doi.org/10.1007/s00247-024-05899-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00247-024-05899-4